Abstract
Purpose
Cancer stem cells (CSCs) are held accountable for the progress of head and neck squamous cell carcinoma (HNSCC). In the presented study, the authors evaluated the prognostic value of CSC markers in two particular HNSCC cohorts.
Methods
This two cohort study consisted of 85 patients with advanced stage HNSCC, treated with primary radio(chemo)therapy (pRCT), and 95 patients with HNSCC, treated with surgery and partially adjuvant radio(chemo)therapy. Overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) were assessed. Samples were assessed for the expression of different molecular stem cell markers (ALDH1, BCL11B, BMI‑1, and CD44).
Results
In the pRCT cohort, none of the baseline patient and tumor features exhibited a statistically significant relation with survival in either the cohort or the human papillomavirus (HPV)-stratified subcohorts. High expression of BMI‑1 significantly decreased OS and DFS, while high expression of CD44 decreased all modes of survival. Multivariate analysis showed significant prognostic influence for all tested CSC markers, with high BMI‑1 and CD44 decreasing survival (BMI-1: OS, DFS, DSS; CD44: OS, DFS) and high ALDH1 and BCL11B showing a beneficial effect on survival (ALDH1: OS, DFS; BCL11B: OS, DSS). In the surgical cohort, classical prognosticators such as HPV status, R1 resection, and nodal status in HPV-negative HNSCC played a significant role, but the tested CSC markers showed no significant effect on prognosis.
Conclusion
Although validation in independent cohorts is still needed, testing for CSC markers in patients with advanced or late stage HNSCC might be beneficial, especially if many comorbidities exist or disease is irresectable. The findings might guide the development and earlier use of targeted therapies in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell cancer (HNSCC) is one of the most prevalent cancers worldwide [1]. Risk factors include tobacco and betel nut use, alcohol consumption, as well as combined abuse of tobacco and alcohol, frequent mouthwash use, and exposure to human papillomavirus (HPV) [2]. Of the mentioned risk factors, HPV exposure seems to cause an increasing fraction of HNSCC incidence worldwide, including Germany [3, 4].
Clinical outcome is dependent on tumor size, locoregional spread, resection margins, extracapsular extension, lymphovascular invasion, and distant metastasis [5, 6]. Alongside TNM classification, HPV status can serve as a marker to predict survival probabilities, which eventually led to the development of a new TNM classification system for p16-positive oropharyngeal carcinomas [6,7,8].
Despite improvements in diagnosis and treatment of HNSCC over the past decades, long-term survival has improved only marginally [5, 9, 10]. To improve survival in HNSCC patients, investigations into the underlying molecular and phenotypic changes associated with tumorigenesis, disease progression, and metastasis are necessary.
More recent studies have shown that survival of cancer stem cells (CSCs; also known as tumor-initiating cells) may contribute to tumor progression, metastasis, and recurrence of HNSCC [11,12,13]. Moreover, CSCs may contribute to conventional therapy resistance and are responsible for tumor progression and recurrence [13].
BMI‑1, ALDH1, and CD44 are amongst the most common and most studied CSC markers in HNSCC [14]. BMI‑1 (B-lymphoma Moloney murine leukemia virus insertion region-1) acts in the self-renewal ability of stem cells. High expression of BMI‑1 in cancer was related to epithelial–mesenchymal transition (EMT) and poor prognosis [14, 15]. ALDH1 (aldehyde dehydrogenase 1) is important for the maintenance and differentiation of stem cells [16]. High ALDH1 expression was associated with advanced tumor stage, tumor size, and lymph node metastasis [17]. CD44 is a surface glycoprotein and a common CSC marker in several human tumor entities [18]. In oral cancer, high expression CD44 was linked to adverse outcome [19]. In strong co-expression to BMI‑1, BCL11B (B-cell lymphoma/leukemia 11B) was identified as an additional CSC marker in HNSCC that is otherwise observed in both embryogenesis and tumor suppression [14, 20].
While there is already some data on the correlation with disease characteristics such as tumor size, lymph node metastasis, and grading, the correlation of CSC marker expression and prognosis of survival is not well studied. In this study, the authors therefore tested the prognostic value of CSC markers in distinct patient cohorts with mostly advanced HNSCC.
Material and methods
Patients
In a single-center retrospective two cohort study, 184 patients were included. One cohort (primary radio[chemo]therapy cohort, pRCT cohort) included 89 patients. Continuous cases were included that were (1) diagnosed with histopathologically confirmed advanced stage head and neck squamous cell carcinoma between November 1998 and May 2012, and (2) treated with primary radiotherapy or pRCT. All diagnostic and treatment procedures (3) had to be performed in the same tertiary referral center. Patients that (1) received pRCT in palliative intent with reduced planned radiation dose and with (2) missing clinical data or (3) retracted informed consent were excluded. Further important reasons for exclusion included (4) insufficient amounts of tumor tissue, (5) damaged specimen or failure of immunohistochemistry control stainings.
The other cohort (surgical cohort) included 95 patients that were (1) diagnosed with head and neck squamous cell carcinoma between August 2001 and June 2015 and (2) treated with surgical resection. Of these patients, 72 also underwent adjuvant radiotherapy or radiochemotherapy. All diagnostic and treatment procedures (3) had to be performed in the same tertiary referral center. Many patients needed to be excluded due to (1) missing informed consent. Also patients with (2) relapse at diagnosis, (3) distant spread at diagnosis, or (4) previous radiation therapy for any cause before surgical treatment, (5) missing clinical data, as well as (6) insufficient amounts of tumor tissue, (7) damaged specimen or failure of immunohistochemistry control stainings were excluded.
Clinical data, including risk factors such as smoking and drinking habits, as well as data regarding disease stage and therapy, were obtained from the medical documentation of the patients. Histologic samples were collected and analyzed as described below.
In the pRCT cohort, out of 89 patients in total, HPV testing showed valid results in 88 patients and molecular testing of all of the tested markers could be obtained in the histologic samples of 85 patients. Detailed patient characteristics of these 85 patients are described in Table 1. In the surgical cohort, HPV and molecular testing could be obtained in all 95 patients. Patient characteristics of the 95 patients of the surgical cohort are described in Table 2. All data is displayed for the entire sample set regardless of the HPV status and separately for the HPV-distinct subcohorts.
All clinical samples were obtained with written informed consent during routine surgery or biopsy based on the approval by the ethics committee of the local medical faculty and in compliance with the World Medical Association Declaration of Helsinki.
Histological samples and immune staining
Specimens were formalin-fixed and paraffin-embedded. Antigen expression was assessed in tissue microarrays (TMAs) of biopsies from primary tumor specimens and represented the average of two to four punches of 1.5 mm diameter, taken randomly from different regions within each specimen. P16 status as a surrogate marker of HPV infection will be referred to as HPV status in the following (p16-specific antibodies: cs56330, Santa Cruz Biotechnology) [21, 22]. Immunohistochemistry (IHC) was performed using ALDH1-, BCL11B-, BMI-1-, and CD44-specific antibodies (ALDH1: ab52492, abcam; BCL11B: HPA049117, Sigma Aldrich; BMI-1: #6964, Cell Signaling Technology; CD44: #3570, Cell Signaling Technology).
In order to quantify and compare expression levels, immunohistochemistry score (IHC scores) were applied to all stainings. IHC scores represent the product of the percentages of positive cells and the staining intensity scored from negative (0), low (1), intermediate (2), to strong (3). They have a range of 0–300 (Fig. 1a–d).
ALDH1, BCL11B, BMI‑1, and CD44 expression in head and neck squamous cell cancer (HNSCC) and distribution in the two study cohorts. Shown are examples of ALDH1 (a), BCL11B (b), BMI-1 (c), and CD44 (d) expression ranging from none (0), weak (1), and intermediate (2) to strong (3) in HNSCC (200 ×). ALDH1, BCL11B, BMI‑1, and CD44 staining is red-brown, while nuclei and cytoplasm are counter-stained with hemalaun (blue). e Immunohistochemistry (IHC) scores combine proportions and intensities of staining as described in “Material and methods” for the four reported stem cell markers ALDH1 (cytoplasmic expression), BCL11B (nuclear), BMI‑1 (nuclear), and CD44 (cytoplasmic). Per definition, IHC scores could reach values between 0 and 300. The plots reflect the frequency distribution of the IHC scores
Clinical endpoints, survival analysis, and statistical analysis
Overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) were chosen as clinical endpoints (in months). OS was calculated from the date of diagnosis to the point of death by any cause, DFS to the first observation of any recurrence or death, and DSS to the date of HNSCC-related death. In the absence of an event, patients were censored at the date of the last follow-up visit.
Survival data were analyzed by log rank test and visualized as Kaplan-Meier plots. To define dichotomous cut-off values for continuous IHC variables, an online tool (http://molpath.charite.de/cutoff/) was applied [23]. Univariate and multivariate analyses were conducted using Cox regression. A p-value < 0.05 was considered statistically significant. All further statistical procedures were conducted using SPSS, version 12.0 (IBM).
Results
All 85 patients in the pRCT cohort were treated with primary radiotherapy or pRCT after diagnosis of advanced stage HNSCC. The 95 patients in the surgical cohort received surgical treatment with or without adjuvant radiotherapy. Detailed patient characteristics, risk factors, disease staging, treatment, and post-treatment follow-up including follow-up time of both cohorts are shown in Tables 1 and 2. Representative stainings and patterns of stem cell marker distributions are displayed in Fig. 1. The most abundant expression was detected for CD44, whereas it was absent for ALDH1 in most tumors. Deviations between the two study cohorts were most pronounced for BMI‑1 (p < 0.001, Mann-Whitney U test), less distinct for BCL11B (p = 0.016), and absent for ALDH1 (p = 0.922) and CD44 (p = 0.892). IHC staining was positively correlated in particular between BC11B and BMI‑1 in both study cohorts; however, the correlation coefficients did not exceed 0.5 in either case. Weaker positive correlations were observed between ALDH1 and BMI‑1 (pRCT cohort only) and between BMI‑1 and CD44 (both cohorts) (Table 3).
Baseline features and molecular markers in the pRCT cohort
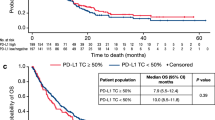
First, univariate Cox regression analysis was performed for baseline patient and tumor features with respect to OS, DFS, and DSS. Age was used as a continuous variable, while the other parameters are dichotomous (Supplemental Table 1). None of the baseline features exhibited a statistically significant relation with either OS, DFS, or DSS in the entire sample set (see Supplemental Table 1) or in the subsets stratified by HPV status (all p > 0.05). In particular, HPV status had no significant effect on survival (OS: p 0.38, DFS: p 0.39, DSS: p 0.27; log rank; Fig. 2a).
Association of human papillomavirus (HPV) status with clinical outcome in the surgical cohort and the primary radio(chemo)therapy (pRCT) cohort and association of single ALDH1, BCL11B, BMI‑1, and CD44 IHC scores with clinical outcome in the pRCT cohort. a Kaplan-Meier curves for the endpoints overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) in head and neck squamous cell cancer (HNSCC) patients stratified according to HPV status (HPV-positive versus HPV-negative). p-Values (log-rank test) are indicated. b Kaplan-Meier curves for OS, DFS, and DSS in HNSCC patients stratified into groups with low and high expression of ALDH1, BCL11B, BMI‑1, and CD44. p-Values (log-rank test) and the applied optimized threshold for the classification of patient subgroups are indicated for the entire pRCT cohort
Thereafter, univariate Cox regression analysis was performed for the four investigated IHC parameters stained. For each staining parameter cut-off (CO), values were determined each for OS, DFS, and DSS. Analyses were each done for the entire pRCT cohort and, using the same COs, for the HPV-distinct subcohorts (Table 4). In the single marker analyses of the pRCT cohort, high expression of BMI‑1 was associated with a significantly decreased OS and DFS and a tendency to decreased DSS (OS: p 0.031, DFS: p 0.048, DSS: p 0.077; log rank). High expression of CD44 was associated with all types of survival (OS: p 0.008, DFS: p 0.007, DSS: p 0.036; log rank). High expression of ALDH1 showed a tendency to increased OS and DFS (OS: p 0.091, DFS: p 0.084; log rank). For the single marker analysis of BCL11B, no significant differences were observed (Table 4; Fig. 2b).
In the HPV-positive subcohort, no significant differences were observed between high and low marker expression. High expression of CD44 showed a tendency to decreased OS and DFS (OS: p 0.055, DFS: p 0.051; log rank; Table 4; Fig. 3a). In the HPV-negative subcohort, no significant differences were observed between high and low marker expression. High expression of BMI‑1 showed a tendency to decreased OS and DFS (OS: p 0.081, DFS: p 0.090; log rank; Table 4; Fig. 3b).
Association of single ALDH1, BCL11B, BMI‑1, and CD44 immunohistochemistry scores with clinical outcome in the human papillomavirus (HPV)-stratified primary radio(chemo)therapy (pRCT) subcohorts. Kaplan-Meier curves for overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) in head and neck squamous cell carcinoma patients stratified into groups with low and high expression of ALDH1, BCL11B, BMI‑1, and CD44. p-Values (log-rank test) and the applied optimized threshold for the classification of patient subgroups are indicated for the HPV-positive subcohort (a) and the HPV-negative subcohort (b)
To assess covariate effects in the pRCT cohort, a multivariable Cox regression model comprising patient-, tumor-, and therapy-related features as well as the four IHC parameters (each stratified by the optimized threshold with regard to either OS, DFS, and DSS) was conducted. Of the patient-, tumor-, and therapy-related features, only concomitant chemotherapy was associated with favorable outcome (p 0.035, Table 5). In the HPV-stratified subcohorts, no significant associations were observed. For the four tested CSC markers, multivariable analysis showed significant prognostic influence of BMI‑1, with CD44 decreasing survival (BMI-1: OS, DFS, and DSS; CD44: OS, DFS and DSS), and ALDH1 and BCL11B supporting survival (ALDH1: OS, and DFS; BCL11B: OS, and DSS) (Table 5). In a multivariable analysis of the four tested CSC markers only, similar results were found for the whole pRCT cohort (Supplemental Table 2). For the HPV-negative subcohort, high expression of BMI‑1 was associated with significantly decreased OS. In the HPV-positive subcohort, OS was reduced with high expression of BMI‑1; DFS was decreased with high expression of CD44 and low expression of ALDH1 (Supplemental Table 2).
Baseline features and molecular markers in the surgical cohort
Accordingly, univariate Cox regression analysis was performed for baseline patient and tumor features with respect to OS, DFS, and DSS, in the surgical cohort. Age was used as a continuous variable, while the other parameters are dichotomous (Table 6).
In the surgical cohort, the authors found a significant reduction of survival in patients with R1-resection (OS: p 0.0002, DFS: p 0.006, DSS: p 0.004; log rank). Also, DFS was significantly decreased in patients with no HPV association; OS only tended to decrease (DFS: p 0.008, OS: p 0.066; log rank; Table 6; Fig. 2a). In the HPV-negative subcohort, node metastasis significantly decreased both OS and DSS (OS: p 0.042, DSS: p 0.040; log rank; Table 6).
Thereafter, univariate Cox regression analysis was performed for the four investigated IHC parameters stained, accordingly to the pRCT cohort. For each staining parameter, CO values were determined each for OS, DFS, and DSS, using the same COs for all (sub)cohorts. None of the four investigated IHC parameters exhibited a statistically significant relation with either OS, DFS, or DSS in the entire sample set (Supplemental Table 3) or in the subsets stratified by HPV status (all p > 0.05). Therefore, no multivariable Cox regression model was conducted in the surgical cohort.
Discussion
In HNSCC, classical prognosticators include age, comorbidities, performance status, frailty, and advanced tumor stage [24], as well as nodal status [25], especially extracapsular extension [26], and close or involved margin resection [26, 27]. Especially in advanced stage cancer, data and knowledge on prognosis are very limited [28]. For some time, HPV association has been a known factor in HNSCC prognosis [29, 30]. Therefore, the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S) proposed an alternative staging system that eventually led to the independent HPV-positive oropharyngeal cancer classification in the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer TNM classification [8]. The data presented here support this hypothesis, but only in the surgical cohort.
While in the surgical cohort R status, HPV status, and nodal status were prognostic survival markers (worse prognosis after R1 resection, in HPV−, and HPV− N+ disease), these factors, and especially HPV status, were not relevant factors for prognosis in the pRCT cohort. In early stage cancer, prognosis may be driven by cancer biology such as tumor stage, histological grade, and mutation status, whereas prognostic variables in patients with advanced cancer tend to consist of patient-related factors such as performance status and cancer anorexia/cachexia [31]. In the current study, the prognostic indifference of HPV status in the pRCT cohort might be explained by a selection bias of relatively sick patients with a high number of relevant comorbidities and low performance status. Unfortunately, robust data on performance status was not available in most of the older cases.
Interestingly, the authors did not see any significant effect of the tested CSC markers in the surgical cohort. A possible explanation is too short a follow-up time. One other reason might be a rather small sample size being responsible for no significance in the univariate analyses of the tested molecular markers, especially in the surgical cohort. In general, there might be a relevant selection bias in the surgical cohort, since many primarily identified patients needed to be excluded as described in “Material and methods”. Regardless, the molecular CSC markers had a significant influence in the pRCT cohort with a comparable sample size and an only slightly longer follow-up time. Although TNM staging seems similar in both cohorts, the two cohorts cannot be considered fully comparable since co-morbidities and especially non-resectability might be strong confounders for survival. In this retrospective investigation, it was inconclusive in some cases what exactly triggered the therapy decision, especially in the pRCT cohort. Presumably, overall prognosis in the pRCT cohort is a priori worse than in the surgical cohort. Therefore, results of the cohorts must be seen independently from each other. In general, the retrospective manner of the investigation and missing validation in independent cohorts are limitations of the study design.
Recently, the number of molecular markers linked to survival increased considerably [26]. The identification of new markers in cancer is crucial to develop better forecasts on prognosis and to find new therapy options [5, 7, 19, 32]. In this field of research, CSC markers are very prominent, although some are linked to a better and some to a worse prognosis, whereas the literature is controversial for some markers [12, 13, 33, 34].
Of the CSC markers, CD44 is of the highest clinical relevance. It was demonstrated that expression of CD44 isoforms in HNSCC is differently associated with advanced T stage, regional and distant metastasis, and radiation failure, which suggests an involvement of CD44 in HNSCC tumor cell proliferation and migration [35]. In the present surgical cohort, different CSC marker expression did not alter survival prognosis, but in the pRCT cohort, high CD44 expression was associated with poorer survival (OS, DFS, and DSS). These results support the findings that identified high levels of CD44 mRNA, CD44 protein, and SCL3A2 mRNA expression as prognosticators for local recurrence in HNSCC after adjuvant RCT [36]. These findings have been validated for an independent patient cohort with locally advanced HNSCC after adjuvant RCT and for a cohort of HNSCC patients receiving pRCT [36, 37]. CD44 levels have also been correlated with clinical response to radiotherapy and may predict local recurrence in patients with early-stage laryngeal cancers and local recurrence and progression-free survival in oropharyngeal SCC [38, 39]. While CD44 plays an emerging role in HNSCC prognostication, the development of an anti-CD44 immunoconjugate bivatuzumab mertansine for therapeutic use in patients with advanced HNSCC was terminated after the immunoconjugate resulted in skin toxicity [40]. In oropharyngeal SCC patients, low CD44 expression levels in combination with HPV positivity were found to be positively associated with 3‑year DFS and OS after undergoing different therapy regimens [41].
The polycomb complex protein BMI‑1 is frequently overexpressed in HNSCC and increased BMI‑1 expression was associated with cervical node metastasis, Ki-67 abundance, and reduced OS and also served as an independent prognostic factor for patient outcomes in oral cavity SCC [42]. While some studies could not predict survival from BMI‑1 expression [43, 44], other studies demonstrated decreased radiosensitivity and increased probability for distant metastases in an experimental setting [45]. The data presented here demonstrate different perspectives on BMI‑1 in HNSCC: While it was not associated with survival in the surgical cohort, BMI‑1 was a strong and negative prognosticator in the pRCT cohort. Frequently, BMI‑1 has been linked to other CSC markers such as ALDH1 and BCL11B [20, 45, 46].
ALDH family proteins belong to the most studied CSC markers in HNSCC. These proteins are increased under cisplatin treatment and radiotherapy and mediate CSC survival in HNSCC [14]. Targeting of ALDH+ cells decreased tumor burden and sensitized HNSCC cells for cisplatin treatment [47]. ALDH1 was well correlated with tumor size, lymph node metastasis, and histopathological grading in HNSCC, being predominantly found in more aggressive tumors and higher tumor stage [14, 17, 48]. ALDH1 and other proteins from the ALDH family belong to the most prevalent markers to identify CSC in HNSCC [34]. ALDH1 expression was linked to treatment resistance, CSC-like properties, higher circulating myeloid-derived suppressor cells, and poor prognosis as well as angiolympathic invasion in oral cavity SCC [49, 50]. Although in the literature high ALDH1 expression was mostly correlated to worse prognosis [51, 52], the authors could not show an effect on survival in their surgical cohort. In the pRCT cohort, high ALDH1 even correlated with better outcome in the multivariate analysis and tended to do so in univariate analysis.
Strongly linked to BMI‑1, BCL11B was suggested as a marker to identify CSCs in HNSCC [20]. This link is supported by the authorsʼ findings in the correlation analysis of the tested CSC markers. Predominantly studied in T‑cell malignancies, high BCL11B expression indicated a favorable outcome in patients [20, 53]. Similarly, the authors could show in their data that high expression of BCL11B was protective in the multivariate analysis of the CSC markers in the pRCT cohort.
Although all of the markers are discussed as identifying CSCs, it is possible that the authorsʼ data show a differential perspective on the four tested markers regarding prognosis in the pRCT cohort. One explanation might be a heterogeneity in CSCs, with different types or states of CSCs promoting disease progression and others slowing it down. The present data suggest that CD44 and BMI‑1 are linked to poorer prognosis, whereas ALDH1 and BCL11B are linked to tendentially better prognosis.
All studies on clinical correlations of the expression of CSC markers, including the present study, have only limited scope to consider intratumoral heterogeneity as well as tumor/metastasis heterogeneity. Experimental data suggest that expression profiles of CSC and EMT markers may vary within tumors and during metastasizing [54]. Innovative approaches are warranted in order to take into account these aspects in the future. In the age of precision medicine and increasing numbers of approaches in HNSCC treatment, CSC markers, such as ALDH, CD44, BMI‑1, and Sox2, as well as c‑Met and EGFR, yield opportunities to further stratify prognosticators and gain therapy targets [34, 55]. The authorsʼ data emphasize the role of interdisciplinary clinical conferences especially in advanced stage patients with comorbidities to thoroughly identify separate prognostic features and search for alternate targeted therapy approaches.
Conclusion
This two cohort study is the first to show remarkable differences for prognosticators in HNSCC between patients that received either surgical treatment, with or without adjuvant therapy, or were treated with pRCT .
In the pRCT cohort of advanced stage HNSCC patients, CSC markers had a differential effect on survival, while HPV status had no significant influence. Of the CSC markers, BMI‑1 and CD44 indicated a significantly worse prognosis on survival. However, ALDH1 and BCL11B appear to have protective properties in HNSCC survival.
In a cohort with mostly advanced stage HNSCC patients that were suitable for and treated with surgery and with or without adjuvant radio(chemo)therapy, classical prognostic factors such as HPV status, R1 resection, and nodal status in HPV-negative HNSCC played a significant role in survival prognosis, but the tested CSC markers showed no significant effect on survival prognosis.
In conclusion, testing for CSC markers might be beneficial in patients with advanced or late stage HNSCC, especially if many comorbidities exist or disease is inoperable. In these cases, and if the findings can be validated in independent cohorts, early molecular testing in interdisciplinary clinical conferences might be beneficial, e.g., for earlier application of targeted therapies.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA A Cancer Journal for Clinicians 69(1):7–34. https://doi.org/10.3322/caac.21551
Spitz MR (1994) Epidemiology and risk factors for head and neck cancer. Semin Oncol 21(3):281–288
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–386. https://doi.org/10.1002/ijc.29210
Reuschenbach M, Tinhofer I, Wittekindt C, Wagner S, Klussmann JP (2019) A systematic review of the HPV-attributable fraction of oropharyngeal squamous cell carcinomas in Germany. Cancer Med. https://doi.org/10.1002/cam4.2039
Leemans CR, Braakhuis BJ, Brakenhoff RH (2011) The molecular biology of head and neck cancer. Nat Rev Cancer 11(1):9–22. https://doi.org/10.1038/nrc2982
Hess J (2017) Predictive factors for outcome and quality of life in HPV-positive and HPV-negative HNSCC. Recent Results Cancer Res 206:233–242. https://doi.org/10.1007/978-3-319-43580-0_18
Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, Rieke D, Endhardt K, Fang P, Bragelmann J, DeBoer R, El-Dinali M, Aktolga S, Lei Z, Tan P, Rozen SG, Salgia R, Weichselbaum RR, Lingen MW, Story MD, Ang KK, Cohen EE, White KP, Vokes EE, Seiwert TY (2015) Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res 21(4):870–881. https://doi.org/10.1158/1078-0432.Ccr-14-2481
O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, Lee N, Riaz N, Pei X, Koyfman SA, Adelstein D, Burkey BB, Friborg J, Kristensen CA, Gothelf AB, Hoebers F, Kremer B, Speel EJ, Bowles DW, Raben D, Karam SD, Yu E, Xu W (2016) Development and validation of a staging system for HPV-related oropharyngeal cancer by the international collaboration on oropharyngeal cancer network for staging (ICON-S): a multicentre cohort study. Lancet Oncol 17(4):440–451. https://doi.org/10.1016/s1470-2045(15)00560-4
Gibson MK, Forastiere AA (2006) Reassessment of the role of induction chemotherapy for head and neck cancer. Lancet Oncol 7(7):565–574. https://doi.org/10.1016/s1470-2045(06)70757-4
Rodrigo JP, Suarez C, Ferlito A, Devaney KO, Petruzzelli GJ, Rinaldo A (2003) Potential molecular prognostic markers for lymph node metastasis in head and neck squamous cell carcinoma. Acta Otolaryngol 123(1):100–105
Rosen JM, Jordan CT (2009) The increasing complexity of the cancer stem cell paradigm. Science 324(5935):1670–1673. https://doi.org/10.1126/science.1171837
Zhang Z, Filho MS, Nor JE (2012) The biology of head and neck cancer stem cells. Oral Oncol 48(1):1–9. https://doi.org/10.1016/j.oraloncology.2011.10.004
Major AG, Pitty LP, Farah CS (2013) Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int 2013:319489. https://doi.org/10.1155/2013/319489
Curtarelli RB, Goncalves JM, Dos Santos LGP, Savi MG, Nor JE, Mezzomo LAM, Rodriguez Cordeiro MM (2018) Expression of cancer stem cell biomarkers in human head and neck carcinomas: a systematic review. Stem Cell Rev 14(6):769–784. https://doi.org/10.1007/s12015-018-9839-4
Kurihara K, Isobe T, Yamamoto G, Tanaka Y, Katakura A, Tachikawa T, Nomura T (2016) Correlation of BMI1 and ZEB1 expression with epithelial–mesenchymal transition in gingiva squamous cell carcinoma. J Oral Maxillofac Surg Med Pathol 28(5):462–469. https://doi.org/10.1016/j.ajoms.2016.03.008
Clark DW, Palle K (2016) Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann Transl Med 4(24):518–518. https://doi.org/10.21037/atm.2016.11.82
Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ (2015) Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget 6(11):8807–8821. https://doi.org/10.18632/oncotarget.3556
Orian-Rousseau V (2015) CD44 acts as a signaling platform controlling tumor progression and metastasis. Front Immunol. https://doi.org/10.3389/fimmu.2015.00154
Mascolo M, Ilardi G, Romano MF, Celetti A, Siano M, Romano S, Luise C, Merolla F, Rocco A, Vecchione ML, De Rosa G, Staibano S (2012) Overexpression of chromatin assembly factor‑1 p60, poly(ADP-ribose) polymerase 1 and nestin predicts metastasizing behaviour of oral cancer. Histopathology 61(6):1089–1105. https://doi.org/10.1111/j.1365-2559.2012.04313.x
Ganguli-Indra G, Wasylyk C, Liang X, Millon R, Leid M, Wasylyk B, Abecassis J, Indra A (2009) CTIP2 expression in human head and neck squamous cell carcinoma is linked to poorly differentiated tumor status. PLoS One 4(4):e5367. https://doi.org/10.1371/journal.pone.0005367
Langendijk JA, Psyrri A (2010) The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: implications for treatment strategies and future clinical studies. Ann Oncol 21(10):1931–1934. https://doi.org/10.1093/annonc/mdq439
Ragin CCR, Taioli E, Weissfeld JL, White JS, Rossie KM, Modugno F, Gollin SM (2006) 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer 95:1432. https://doi.org/10.1038/sj.bjc.6603394
Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, Denkert C (2012) Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One 7(12):e51862. https://doi.org/10.1371/journal.pone.0051862
Coca-Pelaz A, Halmos GB, Strojan P, de Bree R, Bossi P, Bradford CR, Rinaldo A, Vander Poorten V, Sanabria A, Takes RP, Ferlito A (2019) The role of age in treatment-related adverse events in patients with head and neck cancer: a systematic review. Head Neck. https://doi.org/10.1002/hed.25696
Talmi YP, Takes RP, Alon EE, Nixon IJ, Lopez F, de Bree R, Rodrigo JP, Shaha AR, Halmos GB, Rinaldo A, Ferlito A (2018) Prognostic value of lymph node ratio in head and neck squamous cell carcinoma. Head Neck 40(5):1082–1090. https://doi.org/10.1002/hed.25080
Budach V, Tinhofer I (2019) Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: a systematic review. Lancet Oncol 20(6):e313–e326. https://doi.org/10.1016/s1470-2045(19)30177-9
Mitchell DA, Kanatas A, Murphy C, Chengot P, Smith AB, Ong TK (2018) Margins and survival in oral cancer. Br J Oral Maxillofac Surg 56(9):820–829. https://doi.org/10.1016/j.bjoms.2018.06.021
Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Vigano A, Larkin P, De Conno F, Hanks G, Kaasa S (2005) Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the steering committee of the European association for palliative care. Journal of clinical oncology : official journal of the American society of. Clin Oncol 23(25):6240–6248. https://doi.org/10.1200/jco.2005.06.866
Psyrri A, Boutati E, Karageorgopoulou S (2011) Human papillomavirus in head and neck cancers: biology, prognosis, hope of treatment, and vaccines. Anticancer Drugs 22(7):586–590. https://doi.org/10.1097/CAD.0b013e328344ec44
Syrjanen S (2010) The role of human papillomavirus infection in head and neck cancers. Ann Oncol 21(7):vii243–245. https://doi.org/10.1093/annonc/mdq454
Hui D (2015) Prognostication of survival in patients with advanced cancer: predicting the unpredictable? Cancer Control 22(4):489–497. https://doi.org/10.1177/107327481502200415
Collins FS, Varmus H (2015) A new initiative on precision medicine. N Engl J Med 372(9):793–795. https://doi.org/10.1056/NEJMp1500523
Reid P, Marcu LG, Olver I, Moghaddasi L, Staudacher AH, Bezak E (2019) Diversity of cancer stem cells in head and neck carcinomas: the role of HPV in cancer stem cell heterogeneity, plasticity and treatment response. Radiother Oncol 135:1–12. https://doi.org/10.1016/j.radonc.2019.02.016
Peitzsch C, Nathansen J, Schniewind SI, Schwarz F, Dubrovska A (2019) Cancer stem cells in head and neck squamous cell carcinoma: identification, characterization and clinical implications. Cancers. https://doi.org/10.3390/cancers11050616
Wang SJ, Wong G, de Heer AM, Xia W, Bourguignon LY (2009) CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 119(8):1518–1530. https://doi.org/10.1002/lary.20506
Linge A, Lock S, Gudziol V, Nowak A, Lohaus F, von Neubeck C, Jutz M, Abdollahi A, Debus J, Tinhofer I, Budach V, Sak A, Stuschke M, Balermpas P, Rodel C, Avlar M, Grosu AL, Bayer C, Belka C, Pigorsch S, Combs SE, Welz S, Zips D, Buchholz F, Aust DE, Baretton GB, Thames HD, Dubrovska A, Alsner J, Overgaard J, Baumann M, Krause M (2016) Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clinical cancer research : an official journal of the American association for. Cancer Res 22(11):2639–2649. https://doi.org/10.1158/1078-0432.Ccr-15-1990
Linge A, Lock S, Krenn C, Appold S, Lohaus F, Nowak A, Gudziol V, Baretton GB, Buchholz F, Baumann M, Krause M (2016) Independent validation of the prognostic value of cancer stem cell marker expression and hypoxia-induced gene expression for patients with locally advanced HNSCC after postoperative radiotherapy. Clin Transl Radiat Oncol 1:19–26. https://doi.org/10.1016/j.ctro.2016.10.002
de Jong MC, Pramana J, van der Wal JE, Lacko M, Peutz-Kootstra CJ, de Jong JM, Takes RP, Kaanders JH, van der Laan BF, Wachters J, Jansen JC, Rasch CR, van Velthuysen ML, Grenman R, Hoebers FJ, Schuuring E, van den Brekel MW, Begg AC (2010) CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res 16(21):5329–5338. https://doi.org/10.1158/1078-0432.Ccr-10-0799
Motegi A, Fujii S, Zenda S, Arahira S, Tahara M, Hayashi R, Akimoto T (2016) Impact of expression of CD44, a cancer stem cell marker, on the treatment outcomes of intensity modulated radiation therapy in patients with oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 94(3):461–468. https://doi.org/10.1016/j.ijrobp.2015.11.019
Spiegelberg D, Nilvebrant J (2017) CD44v6-targeted imaging of head and neck squamous cell carcinoma: antibody-based approaches. Contrast Media Mol Imaging 2017:2709547. https://doi.org/10.1155/2017/2709547
Nasman A, Nordfors C, Grun N, Munck-Wikland E, Ramqvist T, Marklund L, Lindquist D, Dalianis T (2013) Absent/weak CD44 intensity and positive human papillomavirus (HPV) status in oropharyngeal squamous cell carcinoma indicates a very high survival. Cancer Med 2(4):507–518. https://doi.org/10.1002/cam4.90
Li Z, Wang Y, Yuan C, Zhu Y, Qiu J, Zhang W, Qi B, Wu H, Ye J, Jiang H, Yang J, Cheng J (2014) Oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab Invest 94(12):1431–1445. https://doi.org/10.1038/labinvest.2014.123
Fan Z, Li M, Chen X, Wang J, Liang X, Wang H, Wang Z, Cheng B, Xia J (2017) Prognostic value of cancer stem cell markers in head and neck squamous cell carcinoma: a meta-analysis. Sci Rep 7:43008. https://doi.org/10.1038/srep43008
Lundberg M, Renkonen S, Haglund C, Mattila PS, Leivo I, Hagstrom J, Makitie AA (2016) Association of BMI‑1 and p16 as prognostic factors for head and neck carcinomas. Acta Otolaryngol 136(5):501–505. https://doi.org/10.3109/00016489.2015.1122227
Yu CC, Lo WL, Chen YW, Huang PI, Hsu HS, Tseng LM, Hung SC, Kao SY, Chang CJ, Chiou SH (2011) Bmi‑1 regulates snail expression and promotes metastasis ability in head and neck squamous cancer-derived ALDH1 positive cells. J Oncol. https://doi.org/10.1155/2011/609259
Nor C, Zhang Z, Warner KA, Bernardi L, Visioli F, Helman JI, Roesler R, Nor JE (2014) Cisplatin induces Bmi‑1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 16(2):137–146. https://doi.org/10.1593/neo.131744
Kim J, Shin JH, Chen CH, Cruz L, Farnebo L, Yang J, Borges P, Kang G, Mochly-Rosen D, Sunwoo JB (2017) Targeting aldehyde dehydrogenase activity in head and neck squamous cell carcinoma with a novel small molecule inhibitor. Oncotarget 8(32):52345–52356. https://doi.org/10.18632/oncotarget.17017
Hildebrand LC, Carvalho AL, Lauxen IS, Nor JE, Cerski CT, Sant’Ana Filho M (2014) Spatial distribution of cancer stem cells in head and neck squamous cell carcinomas. J Oral Pathol Med 43(7):499–506. https://doi.org/10.1111/jop.12169
Tsai MS, Chen WC, Lai CH, Chen YY, Chen MF (2017) Epigenetic therapy regulates the expression of ALDH1 and immunologic response: relevance to the prognosis of oral cancer. Oral Oncol 73:88–96. https://doi.org/10.1016/j.oraloncology.2017.08.007
Ortiz RC, Lopes NM, Amor NG, Ponce JB, Schmerling CK, Lara VS, Moyses RA, Rodini CO (2018) CD44 and ALDH1 immunoexpression as prognostic indicators of invasion and metastasis in oral squamous cell carcinoma. J Oral Pathol Med 47(8):740–747. https://doi.org/10.1111/jop.12734
Dong Y, Ochsenreither S, Cai C, Kaufmann AM, Albers AE, Qian X (2017) Aldehyde dehydrogenase 1 isoenzyme expression as a marker of cancer stem cells correlates to histopathological features in head and neck cancer: a meta-analysis. PLoS ONE 12(11):e187615. https://doi.org/10.1371/journal.pone.0187615
Zhou C, Sun B (2014) The prognostic role of the cancer stem cell marker aldehyde dehydrogenase 1 in head and neck squamous cell carcinomas: a meta-analysis. Oral Oncol 50(12):1144–1148. https://doi.org/10.1016/j.oraloncology.2014.08.018
Bartram I, Gokbuget N, Schlee C, Heesch S, Fransecky L, Schwartz S, Stuhlmann R, Schafer-Eckhart K, Starck M, Reichle A, Hoelzer D, Baldus CD, Neumann M (2014) Low expression of T‑cell transcription factor BCL11b predicts inferior survival in adult standard risk T‑cell acute lymphoblastic leukemia patients. J Hematol Oncol 7:51. https://doi.org/10.1186/s13045-014-0051-y
Ihler F, Gratz R, Wolff HA, Weiss BG, Bertlich M, Kitz J, Salinas G, Rave-Frank M, Canis M (2018) Epithelial-mesenchymal transition during metastasis of HPV-negative pharyngeal squamous cell carcinoma. Biomed Res Int 2018:7929104. https://doi.org/10.1155/2018/7929104
Baumeister P, Hollmann A, Kitz J, Afthonidou A, Simon F, Shakhtour J, Mack B, Kranz G, Libl D, Leu M, Schirmer MA, Canis M, Belka C, Zitzelsberger H, Ganswindt U, Hess J, Jakob M, Unger K, Gires O (2018) High expression of EpCAM and sox2 is a positive prognosticator of clinical outcome for head and neck carcinoma. Sci Rep 8(1):14582. https://doi.org/10.1038/s41598-018-32178-8
Funding
The authors received no specific funding for this work.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: Mark Jakob, Julia Kitz; study concepts and design: Mark Jakob, Kariem Sharaf, Markus Schirmer, Julia Kitz, Martin Canis; literature research: Kariem Sharaf, Mattis Bertlich, Friedrich Ihler; clinical studies: Mark Jakob, Friedrich Ihler, Frank Haubner, Martin Canis; experimental studies/data analysis: Markus Schirmer, Julia Kitz, Kariem Sharaf, Martin Leu, Stefan Küffer; statistical analysis: Markus Schirmer, Kariem Sharaf; manuscript preparation: Kariem Sharaf, Markus Schirmer, Friedrich Ihler, Mattis Bertlich; manuscript editing: Mark Jakob, Frank Haubner, Martin Canis, Julia Kitz.
Corresponding authors
Ethics declarations
Conflict of interest
Any financial or personal relationships with other people or organizations that could inappropriately influence (bias) the work, are disclosed by all authors. M. Jakob, K. Sharaf, M. Schirmer, M. Leu, S. Küffer, M. Bertlich, F. Ihler, F. Haubner, M. Canis and J. Kitz declare that they have no competing interests.
Ethical standards
All clinical samples were obtained with written informed consent during routine surgery or biopsy based on the approval by the ethics committee of the local medical faculty (Ethikkomission der Medizinischen Fakultät der Universität Göttingen; #7/4/2012) and in compliance with the WMA Declaration of Helsinki.
Additional information
M. Jakob and K. Sharaf contributed equally to the manuscript.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author.
Caption Electronic Supplementary Material
66_2020_1653_MOESM1_ESM.docx
Supplementary Table 1: Univariate Cox regression analysis for baseline patient and tumor features in the pRCT cohort. Supplementary Table 2: Multivariable Cox regression model with the four IHC parameters in the pRCT cohort. Supplementary Table 3: Univariate Cox regression analysis for the four investigated IHC parameters in the surgical cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jakob, M., Sharaf, K., Schirmer, M. et al. Role of cancer stem cell markers ALDH1, BCL11B, BMI-1, and CD44 in the prognosis of advanced HNSCC. Strahlenther Onkol 197, 231–245 (2021). https://doi.org/10.1007/s00066-020-01653-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01653-5