Abstract
Aim
The goal was to evaluate feasibility, side effects and biochemical no evidence of disease (bNED) after stereotactic body radiation therapy (SBRT) delivered on 5 consecutive days for localized prostate cancer (PC).
Methods
The study was approved by the ethical committee and started in March 2014. Inclusion criteria were age ≤85 years, WHO performance status ≤2, histologically proven adenocarcinoma, low–intermediate risk, no previous surgery (except transurethral resection of the prostate), and a pre-SBRT International Prostatic Symptoms Score of 0–7. The radiotherapy regimen consisted of 35 Gy for low-risk and 37.5 Gy for intermediate-risk PC in 5 consecutive fractions.
Results
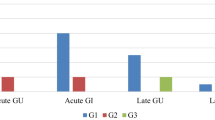
At the time of the analysis, 52 patients were recruited to the study (median age 73 years, range 55–83 years; median follow-up 34 months, range 12–49 months; 34 patients low-risk and 18 intermediate risk). The median initial prostate-specific antigen (PSA) was 5.9 ng/ml (range 1.8–15.7). Acute genitourinary (GU) toxicity was G0 (grade 0) 36/52 (69%), G1 11/52 (21%), G2 5/52 (10%), while acute rectal (GI) toxicity was G0 43/52 (83%), G1 8/52 (15%), G2 1/52 (2%). No acute toxicity ≥G3 was recorded. At the time of analysis late GU and GI toxicities were as follows: GU-G0 43/52 (83%), GU-G1 7/52 (13%), GU-G2 2/52 (4%); GI-G0 48/52 (92%), GI-G1 2/52 (4%), GI-G2 2/52 (4%). No late toxicities ≥G3 were recorded. bNED was 98%. One patient with intermediate PC had distant progression.
Conclusions
Accelerated SBRT for low-intermediate PC is feasible and well tolerated with comparable oncological outcome as described for other series with the same RT technique but treatment delivery on every other day. Longer follow-up is needed to the assess late toxicity profile and long-term clinical outcome.
Zusammenfassung
Fragestellung
Ziel war die Evaluation von Durchführbarkeit, Nebenwirkungen und biochemischer Kontrolle (bNED) der extrakraniellen Körperstereotaxie (SBRT) bei lokalisiertem Prostatakarzinom (PC) an 5 aufeinanderfolgenden Tagen.
Methoden
Nach positivem Ethikvotum begann die Patientenrekrutierung im März 2014. Einschlusskriterien waren: Alter ≤85 Jahre, WHO-Performance-Status ≤2, histologisch nachgewiesenes Adenokarzinom, niedrige bis intermediäre Risikokonstellation, keine vorherige Operation (außer transurethrale Prostataresektion) und ein prä-SBRT International Prostatic Symptoms Score von 0–7. Das Fraktionierungsschema war 35 Gy bei niedrigem und 37,5 Gy bei intermediärem Risiko an 5 aufeinanderfolgenden Tagen.
Ergebnisse
Zum Zeitpunkt der Analyse wurden 52 Patienten im Rahmen der Phase-II-Studie behandelt (mittleres Alter 73 Jahre, Spanne 55–83 Jahre; medianes Follow-up 34 Monate, Spanne 12–49 Monate; 34 Patienten mit niedrigem Risiko, 18 Patienten mit intermediärem Risiko). Das mediane initiale prostataspezifische Antigen (PSA) betrug 5,9 ng/ml (Spanne 1,8–15,7 ng/ml). Akute urogenitale Nebenwirkungen (GU) waren: G0 36/52 (69%), G1 11/52 (21%), G2 5/52 (10%). Die akute rektale (GI) Toxizität war: G0 43/52 (83%), G1 8/52 (15%), G2 1/52 (2%). Es gab keine akute Grad-3-Toxizität. Zum Zeitpunkt der Analyse waren die späten GU- und GI-Toxizitäten: GU-G0 43/52 (83%), GU-G1 7/52 (13%), GU-G2 2/52 (4%); GI-G0 48/52 (92%), GI-G1 2/52 (4%), GI-G2 2/52 (4%). Es lagen keine Spätnebenwirkungen ≥G3 vor. bNED war 98%. Ein Patient mit intermediärem Risiko hatte einen distanten Progress.
Schlussfolgerung
Eine akzelerierte SBRT bei PC mit niedrigem bis intermediärem Risiko ist durchführbar und gut verträglich, mit vergleichbaren onkologische Ergebnisse wie in anderen Studien unter Anwendung der gleichen RT-Technik, aber Bestrahlungen an alternierenden Tagen. Eine längere Nachbeobachtung ist notwendig, um Spätnebenwirkungsprofil und klinische Langzeitergebnisse zu beurteilen.

Similar content being viewed by others
References
Attard G, Parker C, Eeles RA et al (2016) Prostate cancer. Lancet 387:70–82
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 25:16–27
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update. Eur Urol 65:124–137
Pollack A, Zagars GK, Starkschall G et al (2002) Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 53:1097–1105
Peeters ST, Heemsbergen WD, Koper PC et al (2006) Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24:1990–1996
Zelefsky MJ, Yamada Y, Fuks Z et al (2008) Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 71:1028–1033
Alongi F, Fogliata A, Navarria P et al (2012) Moderate hypofractionation and simultaneous integrated boost with volumetric modulated arc therapy (RapidArc) for prostate cancer. Report of feasibility and acute toxicity. Strahlenther Onkol 188:990–996
Höcht S, Aebersold DM, Albrecht C et al (2017) Hypofractionated radiotherapy for localized prostate cancer. Strahlenther Onkol 193:1–12. https://doi.org/10.1007/s00066-016-1041-5 (Epub 2016 Sep 14. Review. Erratum in: Strahlenther Onkol. 2016 192(11):830)
Zietman AL, DeSilvio ML, Slater JD et al (2005) Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294:1233–1239
Kuban DA, Tucker SL, Dong L et al (2008) Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67–74
Dearnaley DP, Jovic G, Syndikus I et al (2014) Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomized controlled trial. Lancet Oncol 15:464–473
Dearnaley D, Syndikus I, Mossop H et al (2016) Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5‑year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 17:1047–1060
King CR, Brooks JD, Gill H, Presti JC Jr (2012) Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 82:877–882
Musunuru HB, Quon H, Davidson M et al (2016) Dose-escalation of five-fraction SABR in prostate cancer: toxicity comparison of two prospective trials. Radiother Oncol 118:112–117
National Comprehensive Cancer Network (NCCN) (2017) Clinical Practice Guidelines in Oncology. Prostate cancer, Version 2.2017. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 1 Dec 2017
Alongi F, Cozzi L, Arcangeli S et al (2013) Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol 8:171
D’Agostino G, Franzese C, De Rose F et al (2016) High-quality linac-based stereotactic body radiation therapy with flattening filter free beams and volumetric modulated arc therapy for low-intermediate risk prostate cancer. A mono-institutional experience. Clin Oncol (r Coll Radiol) 28:e173–e178
Zeng GG, McGowan TS, Larsen TM et al (2008) Calcifications are potential surrogates for prostate localization in image-guided radiotherapy. Int J Radiat Oncol Biol Phys 72:963–966
Roach M 3rd, Hanks G, Thames H Jr et al (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965–974
De Bari B, Arcangeli S, Ciardo D et al (2016) Extreme hypofractionation for early prostate cancer: biology meets technology. Cancer Treat Rev 50:48–60
Ahmad S, Vlachaki MT, Teslow TN et al (2005) Impact of setup uncertainty in the dosimetry of prostate and surrounding tissues in prostate cancer patients treated with Peacock/IMRT. Med Dosim 30:1–7
Hossain S, Xia P, Huang K et al (2010) Dose gradient near target-normal structure interface for nonisocentric CyberKnife and isocentric intensity-modulated body radiotherapy for prostate cancer. Int J radiat Oncol Biol Phys 78:58–63
Kang JK, Cho CK, Choi CW et al (2011) Image-guided stereotactic body radiation therapy for localized prostate cancer. Tumori 97:43–48
Ruggieri R, Naccarato S, Stavrev P et al (2015) Volumetric-modulated arc stereotactic body radiotherapy for prostate cancer: dosimetric impact of an increased near-maximum target dose and of a rectal spacer. Br J Radiol 88:20140736
Friedland JL, Freeman DE, Masterson-McGary ME et al (2009) Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat 8:387–392
Katz AJ, Santoro M, Ashley R et al (2010) Stereotactic body radiotherapy for organ-confined prostate cancer. Bmc Urol 10:1
Anwar M, Weinberg V, Chang AJ et al (2014) Hypofractionated SBRT versus conventionally fractionated EBRT for prostate cancer: comparison of PSA slope and nadir. Radiat Oncol 9:42–51
Park YH, Choi IY, Yoon SH et al (2015) Prostate-specific antigen kinetics after primary stereotactic body radiation therapy using Cyberknife for localized prostate cancer. Prostate Int 3:6–9
Kole TP, Chen LN, Obayomi-Davies O et al (2015) Prostate specific antigen kinetics following robotic stereotactic body radiotherapy for localized prostate cancer. Acta Oncol 54:832–838
De Bari B, Daidone A, Alongi F (2015) Is high dose rate brachytherapy reliable and effective treatment for prostate cancer patients? A review of the literature. Crit Rev Oncol Hematol 94:360–370
Critz FA, Williams WH, Benton JB et al (2000) Prostate specific antigen bounce after radioactive seed implantation followed by external beam radiation for prostate cancer. J Urol 163:1085–1089
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Alongi, R. Mazzola, A. Fiorentino, S. Corradini, D. Aiello, V. Figlia, F. Gregucci, R. Ballario, S. Cavalleri and R. Ruggieri declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Alongi, F., Mazzola, R., Fiorentino, A. et al. Phase II study of accelerated Linac-based SBRT in five consecutive fractions for localized prostate cancer. Strahlenther Onkol 195, 113–120 (2019). https://doi.org/10.1007/s00066-018-1338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1338-7