Abstract
Purpose:
To evaluate the prevalence of acute nausea and emesis during concurrent chemoradiotherapy (CRT) with emphasis on the influence of patient- and treatment-related risk factors and prophylactic antiemetic medication.
Patients and Methods:
A total of 335 patients treated with different intravenous standard chemoradiotherapy protocols in the inpatient setting were included in this retrospective study. Acute nausea and emesis, scored according to the CTC (version 3.0) criteria, were evaluated during 821 chemotherapy cycles. Side effects were correlated with patient-, tumor-, and treatment-related parameters.
Results:
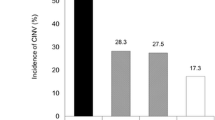
Overall, at least one episode of acute nausea occurred in 48% of the patients and at least one episode of vomiting occurred in 25% of patients. The emetogenic level of the applied chemotherapy protocol was the most significant risk factor for developing nausea and emesis (p < 0.0001). The site of irradiation – namely the thorax (p = 0.0110) and head and neck (p = 0.0415) – was also confirmed as a risk factor. Patient-related parameters, e.g., female gender (p = 0.0003), young age (< 40 years; p = 0.0029), weight loss > 5% (p = 0.0004), and the presence of a percutaneous endoscopic gastrostomy (PEG; p = 0.0071), were associated with higher rates of nausea and emesis, while a history of alcohol abuse showed a protective effect (p = 0.0553). In high emetogenic chemotherapy protocols, prophylaxis with 5-HT3 antagonist plus dexamethasone was superior to 5-HT3 antagonist alone (p = 0.0383).
Conclusion:
Future studies should evaluate more effective prophylaxis protocols in CRT in order to reduce the high rates of nausea and emesis.
Zusammenfassung
Zielsetzung:
Das Auftreten von akuter Übelkeit und Erbrechen unter simultaner Radiochemotherapie (CRT) sollte in Hinblick auf patienten- und therapiebezogene Parameter sowie antiemetische Prophylaxe untersucht werden.
Material und Methode:
335 Patienten, die mit unterschiedlichen intravenösen Standard-Radiochemotherapie-Protokollen stationär behandelt worden waren, konnten in diese retrospektive Studie eingeschlossen werden. Übelkeit und Erbrechen, basierend auf den CTC-Kriterien (Version 3.0), wurden während insgesamt 821 Chemotherapie-Zyklen untersucht. Die Nebenwirkungen wurden mit patienten-, tumor- und therapiebezogenen Parametern korreliert.
Ergebnisse:
Mindestens eine Episode von Übelkeit trat bei 48% der Patienten auf; 25% hatten mindestens einmaliges Erbrechen. Das emetogene Potential des verwendeten Chemotherapie-Protokolls war der signifikanteste Risikofaktor für das Auftreten von Übelkeit und Erbrechen (p < 0,0001). Die Bestrahlungsregion, namentlich Thorax (p = 0,0110) und Kopf/Hals (p = 0,0415), konnte auch als Risikofaktor bestätigt werden. Patientenbezogene Parameter wie weibliches Geschlecht (p = 0,0003) und jüngeres Alter (< 40 Jahre) (p = 0,0029) sowie Gewichtsabnahme von mehr als 5% (p = 0,0004) und das Vorliegen einer PEG-Sonde (p = 0,0071) waren mit verstärkter Übelkeit und Erbrechen verbunden, während Alkoholabusus einen protektiven Effekt zeigte (p = 0,0553). Bei hoch emetogenen Chemotherapie-Protokollen war die Prophylaxe mit der Kombination aus 5-HT3-Antagonist plus Dexamethason einem 5-HT3-Antagonisten allein überlegen (p = 0,0383).
Schlussfolgerung:
Künftige Studien sollten effektivere Prophylaxe-Protokolle bei simultaner Radiochemotherapie untersuchen, um die hohen Raten an Übelkeit und Erbrechen zu reduzieren.
Similar content being viewed by others
References
Blackstock A, Ayala D, Squire S, et al. A reduction in chemoradiation induced nausea and vomiting (CRINV) with prophylactic aprepitant/5HT-3/ Dexamethasone therapy during upper abdominal chemoradiation. Int J Radiat Oncol Biol Phys 2009;75:520(abs).
Campos D, Pereira JR, Reinhardt RR, et al. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol 2001;19:1759–67.
Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 2007;15:497–503.
Dunst J, Debus J, Rudat V, et al. Neoadjuvant capecitabine combined with standard radiotherapy in patients with locally advanced rectal cancer: mature results of a phase II trial. Strahlenther Onkol 2008;184:450–6.
Enblom A, Bergius-Axelsson B, Steineck,G. One third of patients with radiotherapy- induced nausea consider their antiemetic treatment insufficient. Support Care Cancer 2009;17:23–32.
Feyer P, Maranzano E, Molassiotis A, et al. Radiotherapy-induced nausea and vomiting (RINV): antiemetic guidelines. Support Care Cancer 2005;13:122–8.
Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 1999;17:2971–94.
Griffin AM, Butow PN, Coates AS, et al. On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 1996;7:189–95.
Herrstedt J, Koeller JM, Roila F, et al. Acute emesis: moderately emetogenic chemotherapy. Support Care Cancer 2005;13:97–103.
Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997;15:103–9.
Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – The aprepitant protocol 052 study group. J Clin Oncol 2003;21:4112–9.
Horiot JC, Aapro M. Treatment implications for radiation-induced nausea and vomiting in specific patient groups. Eur J Cancer 2004;40:979–87.
Kim K, Chie EK, Jang JY, et al. Ramosetron for the prevention of nausea and vomiting during 5-fluorouracil-based chemoradiotherapy for pancreaticobiliary cancer. Jpn J Clin Oncol 2009;39:111–5.
Kris MG, Hesketh PJ, Herrstedt J, et al. Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer 2005;13:85–96.
Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 2006;24:2932–47.
Maranzano E, De Angelis V, Pergolizzi S, et al. A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol;94:36–41.
Monroe AT, Reddy SC, Gibbs GL, et al. Factors associated with radiationinduced nausea and vomiting in head and neck cancer patients treated with intensity modulated radiation therapy. Radiother Oncol 2008;87:188–94.
Morita M, Kuwano H, Ohno S, et.al. Antiemetic effect of ramosetron during hyperthermo-chemo-radiotherapy for esophageal cancer. Anticancer Res 2000;20:3631–6.
Naeim A, Dy SM, Lorenz KA, et al. Evidence-based recommendations for cancer nausea and vomiting. J Clin Oncol 2008;26:3903–10.
National Comprehensive Cancer Network: Antiemesis. (http://www.nccn. org/professionals/physician_gls/PDF/antiemesis.pdf).
Patyanik M, Nemeskeri C, Poti Z, et al. Concomitant radiochemotherapy of cervical cancer: is it justified to reduce the dosage of cisplatin? Strahlenther Onkol 2009;185:582–7.
Prevention of chemotherapy- and radiotherapy-induced emesis: results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Ann Oncol 1998;9:811–9.
Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol 1988;6:1746–52.
Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004;90:1905–11.
Tinkl D, Grabenbauer GG, Golcher H, et al. Downstaging of pancreatic carcinoma after neoadjuvant chemoradiation. Strahlenther Onkol 2009;185:557–66.
Tribius S, Kronemann S, Kilic Y, et al. Radiochemotherapy including cisplatin alone versus cisplatin +5-fluorouracil for locally advanced unresectable stage IV squamous cell carcinoma of the head and neck. Strahlenther Onkol 2009;185:675–81.
Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822–30.
de Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol 2003;21:4105–11.
de Wit R, Herrstedt J, Rapoport B, et al. The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomised, placebo-controlled phase III clinical trials. Eur J Cancer 2004;40:403–10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraunholz, I., Grau, K., Weiß, C. et al. Patient- and Treatment-Related Risk Factors for Nausea and Emesis during Concurrent Chemoradiotherapy. Strahlenther Onkol 187, 1–6 (2011). https://doi.org/10.1007/s00066-010-2196-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-010-2196-0