Abstract
Purpose:
To date, no valid imaging modality exists for early response prediction to neoadjuvant radiochemotherapy in carcinoembryonic-antigen-(CEA)-expressing rectal cancers (UICC stages II and III). It is hypothesized that the uptake of an anti-CEA antibody is directly related to the number of viable tumor cells and may be quantified by immuno-positron emission tomography (immuno-PET). Therefore, we evaluated a novel pretargeting system using TF2, a humanized bispecific trivalent monoclonal antibody (mAb), directed against CEA and the IMP-288-peptide, a hapten for binding radiometals for imaging. Uptake and kinetics of the pretargeting system were investigated in vitro prior to and after irradiation.
Methods:
TF2 was labeled with 131I and IMP-288 with 111InCl3. The colorectal cancer cell lines HT29, SW480, and T84 with known varying CEA expression were incubated (≤72 hours) with 131I-TF2 or the TF2-111In-IMP-288 pretargeting system. Parallel cultures were irradiated with 2–10 Gy high-energy photons. Tracer uptake, proliferation, apoptosis, and CEA-RNA expression of cancer cells were investigated.
Results:
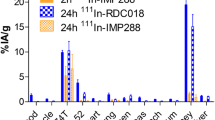
The uptake of tracers was dependent on CEA expression and cell count of the cell lines (uptake/106 cells: 0.3% in HT29, 1.5% in SW480, and 14% in T84, p < 0.001). The TF2-111In-IMP-288 pretargeting system showed a higher uptake after 4 and 72 hours compared to 131I-TF2 in parallel cultures. Only in one cell line (SW480) an increased apoptosis after irradiation could be detected. Irradiation increased dose dependently both the specific uptake of 131I-TF2 and of the TF2-111In-IMP-288 system (4-fold in HT29 and T84 after 10 Gy (72 hours), p < 0.001). These results were CEA-mRNA independent.
Conclusion:
This novel pretargeting system allows the quantitative analysis of CEA-expressing colorectal cancer cells and represents a promising tool for evaluation of tumor cell viability after irradiation.
Zusammenfassung
Hintergrund:
Derzeit gibt es beim CEA-exprimierenden Rektumkarzinom (der UICC-Stadien II und III) keine valide Diagnostik zur frühzeitigen Response-Beurteilung auf eine neoadjuvante Radiochemotherapie. Allerdings wird vermutet, daß mit einer Immuno-Positronen-Emissions-Tomographie (Immuno-PET) und einem anti-CEA-Antikörper die Anzahl viabler Tumorzellen nach erfolgter Radiatio quantifizierbar ist. Demzufolge wurde von unserer Arbeitsgruppe zur Etablierung eines Immuno-PETs ein neues Pretargeting-System evaluiert. Dieses Pretargeting-System besteht aus dem humanisierten bispezifischen trivalenten monoklonalen Antikörper (TF2), der an CEA sowie an das radioaktiv markierte Hapten-Peptid IMP-288 bindet. Wir untersuchten in vitro die Bindung und Kinetik dieses Pretargeting-Systems vor und nach Radiatio.
Methoden:
TF2 wurde mit 131I und IMP-288 mit 111InCl3 markiert. Die Inkubation (≤72 h) der kolorektalen Tumorzelllinien HT29, SW480 und T84 mit unterschiedlicher CEA-Expression erfolgte mit 131I-TF2 und dem TF2-111In-IMP-288-Pretargeting-System. Parallelkulturen wurden mit 2–10 Gy hochenergetischer Photonen bestrahlt. Neben der Bindung der markierten Substanzen wurden die Proliferation, Apoptose und CEA-RNA-Expression der Tumorzellen untersucht.
Ergebnisse:
Der Tracer-Uptake korrelierte sowohl mit der CEA-Expression als auch der Zellzahl der jeweiligen Zelllinie (Uptake/106 Zellen: 0,3% in HT29, 1,5% in SW480 und 14% in T84; p < 0,001). Das Pretargeting-System wurde nach 4 h und 72 h stärker gebunden als 131I-TF2 in Parallelkulturen. Lediglich in einer Zelllinie (SW480) konnte eine erhöhte Apoptoserate nach Bestrahlung nachgewiesen werden. Die Radiatio erhöhte dosisabhängig die spezifische Aufnahme des Pretargeting-Systems (4fach in HT29 und T84 nach 10 Gy (72 h), p < 0,001). Diese Ergebnisse waren CEA-mRNA-unabhängig.
Schlussfolgerung:
Das neue Pretargeting-System erlaubt eine quantitative Analyse der CEA-Expression kolorektaler Tumorzellen und ist eine vielversprechende Methode zur Evaluation der Tumorzellviabilität nach Radiatio.
Similar content being viewed by others
References
Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 2009;250:730–9.
Calcagno AM, Chewning KJ, Wu CP, et al. Plasma membrane calcium ATPase (PMCA4): a housekeeper for RT-PCR relative quantification of polytopic membrane proteins. BMC Mol Biol 2006;7:29.
Calvo FA, Domper M, Matute R, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 2004;58:528–35.
Chen CC, Lee RC, Lin JK, et al. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum 2005;48:722–8.
Cicinnati VR, Shen Q, Sotiropoulos GC, et al. Validation of putative reference genes for gene expression studies in human hepatocellular carcinoma using real-time quantitative RT-PCR. BMC Cancer 2008;8:350.
Fantini J, Rognoni JB, Culouscou JM, et al. Induction of polarized apical expression and vectorial release of carcinoembryonic antigen (CEA) during the process of differentiation of HT29-D4 cells. J Cell Physiol 1989;141:126–34.
Furuta M, Hasegawa M, Hayakawa K, et al. Rapid rise in FDG uptake in an irradiated human tumour xenograft. Eur J Nucl Med 1997;24:435–8.
Goldenberg DM, Chatal JF, Barbet J, et al. Cancer Imaging and Therapy with Bispecific Antibody Pretargeting. Update Cancer Ther 2007;2:19–31.
Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest 2005;23: 338–51.
Guerra L, Niespolo R, Di Pisa G, et al. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging 2009 Dec 22 (Epub ahead of print); DOI: 10.1007/s00261-009-9594-8
Hicks RJ, Ware RE, Lau EW. PET/CT: will it change the way that we use CT in cancer imaging? Cancer Imaging 2006;6:S52–62.
Higashi T, Fisher SJ, Brown RS, et al. Evaluation of the early effect of local irradiation on normal rodent bone marrow metabolism using FDG: preclinical PET studies. J Nucl Med 2000;41:2026–35.
Hoffer PB, Lathrop K, Bekerman C, et al. Use of 131-I-CEA antibody as a tumor scanning agent. J Nucl Med 1974;15:323–7.
Janssen MH OM, van Stiphout RG, Buijsen J, et al. Evaluation of early metabolic responses in rectal cancer during combined radiochemotherapy or radiotherapy alone: sequential FDG-PET-CT findings. Radiother Oncol 2010;94:151–55.
Karacay H, Sharkey RM, Gold DV, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med 2009;50:2008–16.
Lahaye MJ, Engelen SM, Kessels AG, et al. USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: predictive criteria. Radiology 2008;246:804–11.
Larbouret C, Robert B, Linard C, et al. Radiocurability by targeting tumor necrosis factor-alpha using a bispecific antibody in carcinoembryonic antigen transgenic mice. Int J Radiat Oncol Biol Phys 2007;69:1231–7.
Liersch T, Langer C, Jakob C, et al. [Preoperative diagnostic procedures in locally advanced rectal carcinoma (>or =T3 or N+). What does endo- luminal ultrasound achieve at staging and restaging (after neoadjuvant radiochemotherapy) in contrast to computed tomography?]. Chirurg 2003;74:224–34.
Liersch T, Meller J, Bittrich M, et al. Update of carcinoembryonic antigen radioimmunotherapy with (131)I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group. Ann Surg Oncol 2007;14:2577–90.
Liersch T, Meller J, Kulle B, et al. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results. J Clin Oncol 2005;23:6763–70.
Liersch T, Rothe H, Ghadimi BM, et al. [Individualizing treatment for locally advanced rectal cancer]. Chirurg 2009;80:281–93.
Marquardt F, Rödel F, Capalbo G, et al. Molecular targeted treatment and radiation therapy for rectal cancer. Strahlenther Onkol 2009;185:371–8.
MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 2007;243:132–9
McBride WJ, Zanzonico P, Sharkey RM, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med 2006;47:1678–88.
Meller B, Deisting W, Wenzel BE, et al. Increased radioiodine uptake of thyroid cell cultures after external irradiation. Strahlenther Onkol 2006;182:30–6.
Ohira H, Kubota K, Ohuchi N, et al. Comparison of intratumoral distribution of 99mTc-MIBI and deoxyglucose in mouse breast cancer models. J Nucl Med 2000;41:1561–8.
Park JW, Lim SB, Kim DY, et al. Carcinoembryonic antigen as a predictor of pathologic response and a prognostic factor in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and surgery. Int J Radiat Oncol Biol Phys 2009;74:810–7.
Paskeviciute B, Bolling T, Brinkmann M, et al. Impact of (18)F-FDG-PET/CT on staging and irradiation of patients with locally advanced rectal cancer. Strahlenther Onkol 2009;185:260–5.
Pelosi E, Deandreis D. The role of 18F-fluoro-deoxy-glucose positron emission tomography (FDG-PET) in the management of patients with colorectal cancer. Eur J Surg Oncol 2007;33:1–6.
Rades D, Wolff C, Nadrowitz R, et al. Radioactive EGFR antibody cetuximab in multimodal cancer treatment: stability and synergistic effects with radiotherapy. Int J Radiat Oncol Biol Phys 2009;75:1226–31.
Riemann B, Konemann S, Popping D, et al. Early effects of irradiation on [(123)I]-IMT and [(18)F]-FDG uptake in rat C6 glioma cells. Strahlenther Onkol 2004;180:434–41.
Rödel C, Arnold D, Hipp M, et al. Phase I–II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:1081–6.
Rödel C, Grabenbauer GG, Papadopoulos T, et al. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol 2003;21:3098–104.
Rödel C, Liersch T, Hermann RM, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 2007;25:110–7.
Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688–96.
Rödel C, Sauer R, Fietkau R. [The role of magnetic resonance imaging to select patients for preoperative treatment in rectal cancer]. Strahlenther Onkol 2009;185:488–92.
Rossi EA, Goldenberg DM, Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A 2006;103:6841–6.
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40.
Sauer R, Fietkau R, Wittekind C, et al. Adjuvant versus neoadjuvant radiochemotherapy for locally advanced rectal cancer. A progress report of a phase-III randomized trial (protocol CAO/ARO/AIO-94). Strahlenther Onkol 2001;177:173–81.
Sharkey RM, McBride WJ, Karacay H, et al. A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res 2003;63:354–63.
Tan E, Gouvas N, Nicholls RJ, et al. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol 2009;18:15–24.
Weiss C, Arnold D, Dellas K, et al. Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: a pooled analysis of three prospective phase I–II trials. Int J Radiat Oncol Biol Phys 2010;78:472–8.
Wolff HA, Gaedcke J, Jung K, et al. High-grade acute organ toxicity during preoperative radiochemotherapy as positive predictor for complete histopathologic tumor regression in multimodal treatment of locally advanced rectal cancer. Strahlenther Onkol 2010;186:30–5.
Yamamoto T, Nishizawa S, Maruyama I, et al. Acute effects of stereotactic radiosurgery on the kinetics of glucose metabolism in metastatic brain tumors: FDG PET study. Ann Nucl Med 2001;15:103–9.
Yoon SM, Kim DY, Kim TH, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2007;69:1167–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meller, B., Rave-Fränck, M., Breunig, C. et al. Novel Carcinoembryonic-Antigen-(CEA)-Specific Pretargeting System to Assess Tumor Cell Viability after Irradiation of Colorectal Cancer Cells. Strahlenther Onkol 187, 120–126 (2011). https://doi.org/10.1007/s00066-010-2191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-010-2191-5