Abstract
Purpose
Individual regions of the Alberta Stroke Programme Early CT Score (ASPECTS) may contribute differently to the clinical symptoms in large vessel occlusion (LVO). Here, we investigated whether the predictive performance on clinical outcome can be increased by considering specific ASPECTS subregions.
Methods
A consecutive series of patients with LVO affecting the middle cerebral artery territory and subsequent endovascular treatment (EVT) between January 2015 and July 2020 was analyzed, including affected ASPECTS regions. A multivariate logistic regression was performed to assess the individual impact of ASPECTS regions on good clinical outcome (defined as modified Rankin scale after 90 days of 0–2). Machine-learning-driven logistic regression models were trained (training = 70%, testing = 30%) to predict good clinical outcome using i) cumulative ASPECTS and ii) location-specific ASPECTS, and their performance compared using deLong’s test. Furthermore, additional analyses using binarized as well as linear clinical outcomes using regression and machine-learning techniques were applied to thoroughly assess the potential predictive properties of individual ASPECTS regions and their combinations.
Results
Of 1109 patients (77.3 years ± 11.6, 43.8% male), 419 achieved a good clinical outcome and a median NIHSS after 24 h of 12 (interquartile range, IQR 4–21). Individual ASPECTS regions showed different impact on good clinical outcome in the multivariate logistic regression, with strongest effects for insula (odds ratio, OR 0.56, 95% confidence interval, CI 0.42–0.75) and M5 (OR 0.53, 95% CI 0.29–0.97) regions. Accuracy (ACC) in predicting good clinical outcome of the test set did not differ between when considering i) cumulative ASPECTS and ii) location-specific ASPECTS (ACC = 0.619, 95% CI 0.58–0.64 vs. ACC = 0.629, 95% CI 0.60–0.65; p = 0.933).
Conclusion
Cumulative ASPECTS assessment in LVO remains a stable and reliable predictor for clinical outcome and is not inferior to a weighted (location-specific) ASPECTS assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain imaging is a key criterion to select patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO) for endovascular therapy (EVT). In the presence of LVO affecting the middle cerebral artery (MCA) territory, early signs of ischemia are usually quantified by applying the Alberta Stroke Programme Early CT Score (ASPECTS), subdividing the MCA territory into 10 different predefined regions [1]. ASPECTS has widely been used as selection criterion for studies in ischemic stroke [2], however, in clinical practice there is no uniformly defined cut-off for treatment decisions in individual patients. Even though lower ASPECTS are commonly associated with a poorer chance for a good outcome, patients with supposedly low baseline ASPECTS (< 6) can still achieve good functional outcome after complete recanalization [3]. Likewise, it was also observed that patients with a relatively high ASPECTS might achieve a poor long-term clinical outcome, even despite successful recanalization. This circumstance inevitably results in patients being denied treatment due to an ASPECTS that is supposedly too low, even though they could still benefit from EVT.
A potential explanation for this seemingly apparent contradiction may be that the involvement of specific anatomical areas in ischemic stroke is associated with poorer functional outcome, e.g., the primary motor cortex or areas linked to language processing [4]; however, all 10 ASPECTS areas are considered as equally important. Additional factors that limit the information value of the ASPECTS are the disregard of the different sizes of the ASPECTS regions as well as the laterality of stroke, which also has been shown to play a crucial role in outcome prediction [5]. To sharpen the prognostic value of ASPECTS, previous studies suggested a differing impact for some ASPECTS subregions on predicting EVT outcomes [6, 7]; however, the clinical value of a weighted, location-specific ASPECTS as compared to the established cumulative ASPECTS has not yet been investigated.
In the present study, we compared the performance of the cumulative vs. location-specific ASPECTS with differential weighting of the affected regions for predicting EVT outcome in AIS.
Methods
Patients and Study Design
We reviewed consecutive patients with AIS undergoing EVT between January 2015 and July 2020 from a prospectively compiled database of a tertiary care university hospital to identify all patients with LVO affecting the MCA territory. Clinically and procedure-related parameters of patients are presented in Table 1.
Because of its retrospective character, additional written informed consent was waived by the local ethics committee.
Imaging, Endovascular Treatment and Clinical Assessment
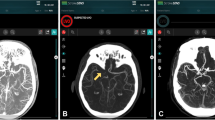
All patients received non-contrast cranial computed tomography (CT) supplemented by CT angiography on admission and subsequent EVT. Patients with a suspected time window of more than 6 h since the onset of the stroke additionally received CT perfusion. ASPECTS, including its subregions, were evaluated visually by a board-certified radiologist with 8 years of experience (UN) in consensus with automated analysis by e‑ASPECTS (Brainomix, Oxford, UK) on 1 mm slices [8, 9]. Baseline imaging was acquired at admission and the decision for endovascular treatment as well as the administration and dosing of rtPA was individually made for each patient based on a consensus between the treating neurologist and neurointerventionalist, following national and international guidelines. Success of recanalization was evaluated by applying the modified thrombolysis in cerebral infarction (mTICI, with mTICI 2b or 3 representing complete recanalization) scale by the treating neurointerventionalist [10]. Routine follow-up cranial CT or magnetic resonance imaging (MRI) was performed within 18–36 h or earlier in cases of clinical deterioration and visually assessed by follow-up ASPECTS. Clinical symptoms were assessed according to the National Institutes of Health Stroke Scale (NIHSS) score on admission and 24 h after EVT, as well as modified Rankin Scale (mRS) at 90 days after onset by certified neurologists blinded to the intervention. A NIHSS score of 42 was assigned to patients who died during the initial hospitalization.
Statistics
Statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Descriptive statistics of clinical and imaging data as mean (± standard deviation), n (%) or median (interquartile range, IQR) were calculated, accordingly (see Table 1).
A multivariate logistic regression model was built using each ASPECTS region as binary value, as well as onset-to-imaging time as continuous value, as independent parameters, and good clinical outcome (defined as mRS of 0–2 after 90 days) as dependent variable. This experiment served as a preliminary investigation of whether different effects of the individual regions on clinical outcome, as observed in other publications, were derivable in our patient cohort.
Multivariate logistic regression models were built using each ASPECTS region as binary value for the outcomes of i) mRS 0–1 after 90 days, ii) mRS 0–2 after 90 days, iii) mRS 0–3 after 90 days, iv) mRS 4–6 after 90 days and v) mRS 6 after 90 days. For all models, the logistic regression coefficients of each ASPECTS region were extracted and then applied to each region as a weighting factor to derive an adjusted ASPECTS (aASPECTS). To ensure comparability between the original ASPECTS (oASPECTS) and the aASPECTS, the scale of the aASPECTS was normalized to 10 points. Univariate logistic regression models for both i) oASPECTS and ii) aASPECTS were then performed for the respective outcome, in which the weighting factors were initially obtained, and effects on the outcome measured by odds ratios and their corresponding 95% confidence intervals of the models were compared.
Details about the comparison of cumulative and location-specific ASPECTS using machine-learning driven predictive models on clinical outcome can be found in the online-only supplemental material.
Supplemental Analyses
Additional analyses were performed to investigate the predictive implications of ASPECTS subregions and their combinations. For this purpose, additional binary as well as linear outcomes on the mRS and NIHSS spectrum were analyzed using multivariate logistic and linear regressions as well as machine-learning approaches, including the comparison of different machine-learning algorithms. Furthermore, subgroup analyses were performed to assess the effects of laterality as well as complete recanalization. Details of these additional analyses are provided in the online-only supplemental material.
Results
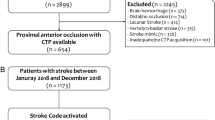
A total of 1109 patients met the inclusion criteria (see Fig. 1 for a flowchart of patient selection). The median NIHSS was 16 (IQR 11–21) on admission and 12 (IQR 4–21) after 24 h. In 576 cases (51.9%), the proximal M1 segment was occluded, while in 288 cases (26.0%) either distal M1 or proximal M2 occlusions were present. Complete recanalization was achieved in 543 patients (49.0%), 90 days after treatment, the median mRS was 3 (IQR 2–5), with 415 patients (37.4%) achieving a good clinical outcome, 545 patients (49.1%) had an unfavorable outcome (mRS 4–6), with a total of 245 fatal cases (22.1%). An overview of clinically and procedure-related parameters of patients is presented in Table 1.
Predicting Clinical Outcome Using Logistic Regression Models
Multivariate regression with all ASPECTS subregions for good clinical outcome showed strongest effects for insula (OR 0.56, 95% CI 0.42–0.75) and M5 (OR 0.53, 95% CI 0.29–0.97). An overview of the adjusted effects of all regions on good as well as poor clinical outcome can be found in Table 2. Here, the insular cortex as well as the M5 region showed significant associations to good clinical outcome.
Results of the comparison of location-specific ASPECTS, applying regional weighting factors derived by logistic regression, and the cumulative ASPECTS on good clinical outcome are summarized in supplemental Table 3.
Predicting Clinical Outcome Using Machine-learning Models
Overall accuracy to predict good clinical outcome using a generalized linear model did not show a difference between i) cumulative ASPECTS (ACC = 0.619, 95% CI 0.58–0.64) and ii) location-specific ASPECTS (ACC = 0.629, 95% CI 0.60–0.65; p = 0.933). The results of the main machine-learning approach are summarized in Table 4 and Fig. 2.
Supplemental Results
The results of the additional analyses are presented in the online-only supplemental results. In brief, more extensive analyses, including the evaluation of various binarized and linear clinical outcomes (comprising the continuous spectrum of mRS and NIHSS and degree of recanalization as well as effects of laterality, see supplemental Tables S1–S3), as well as the application and comparison of machine-learning models (see supplemental Tables S4 and S5), showed no prognostic superiority using location-specific ASPECTS compared with cumulative ASPECTS.
Discussion
Reliable assessment of infarct size and the associated neurological relevance is crucial for outcome prediction in both clinical studies and clinical practice. The ASPECTS has been used as established marker for evaluation of acute infarcts in multiple trials and daily practice. As ASPECTS does not weight individual brain regions according to their functional relevance or consider laterality, several previous studies attempted to improve the prognostic meaning by a location-specific approach [6].
Here, we assessed the original ASPECTS and several weighted variations for their prognostic performance in a large uniform stroke cohort. Our findings demonstrate that consideration of specific ASPECTS subregions does not improve overall prediction of clinical outcome. Although we were able to confirm differences in the impact of individual ASPECTS regions on the clinical outcome after EVT, seemingly these effects were not strong enough to translate into a more accurate prediction that would outperform the traditional cumulative ASPECTS.
Specifically, we found strongest effects for the insula followed by varying involvement of cortical areas (mostly M5 region) in our statistical approaches, including binary and linear outcomes for mRS after 90 days, as well as for NIHSS after 24 h using regression and machine-learning approaches. Our results are in line with the findings of previous studies that used advanced imaging techniques such as CT perfusion or diffusion-weighted imaging on MRI to assess the topographic correlation of infarct areas with poor clinical outcome after EVT [6]. The functional significance of the affected areas and their corresponding predictive importance has been highlighted in these studies. Compared to ASPECTS derived from non-contrast CT images, ASPECTS based on MR imaging and CT perfusion have even higher reliability and reproducibility, underscoring our findings [11]. In these analyses, the insula was the only region to be consistently linked to poor clinical outcome [5, 12,13,14], explained by its importance in recovery from aphasia or paralysis and by its association with autonomic functions [4, 15, 16]. Generally, infarct involvement of the insula indicates proximal occlusion of the MCA, which has been shown to be associated with worse clinical outcome and grade of recanalization than distal MCA occlusions with primarily cortical involvement [17].
However, our second most important ASPECTS area for poor clinical outcome was the peripheral M5 region. Likewise, this finding is reinforced by the results of previous studies that used advanced imaging modalities, which showed that the M5 region was the second most affected region after the insula [13, 14, 18]. Further studies have also been able to demonstrate the strong effect of the M5 region in non-contrast CT [19]. Additionally, in an analysis that investigated regional collateral status, it was reported that the collateral status of the M5 region was the only significant predictor of good functional outcome in comparison to all other cortical ASPECTS regions [20]. Here, good collateral supply appears to have a protective function, as the lateral cortical surface (M5) controls higher cortical functions such as language processing [21].
In addition to the individual contribution of the specific ASPECTS regions to the clinical outcome, the combinations of affected regions are of great importance; however, there are few data available in the existing literature about this topic. As expected from our initial results, the combination of both M5 and insula regions were found to be subset with the strongest two-way interactions terms with respect to the clinical outcome of all included patients. In a subgroup analysis of patients with poor baseline ASPECTS (< 6), strongest interactions were found for M1 and M6 regions as a two-way subset and M1 + M6 + insula regions as a three-way subset. It can be deduced that for proximal MCA occlusions, that were mainly included in this study, it is not necessarily the specific function of an individual area that is in the focus, but rather the regional extent and thus ultimately the infarct size, stretching from the central insula area to the peripheral M1 and M6 regions in unfavorable circumstances.
Machine-learning methods are becoming increasingly important in medical research and with good reason, as it has been shown that they can be superior to conventional statistical methods in the life sciences, specifically in outcome prediction after ischemic stroke [22]. We therefore decided to analyze our hypothesis not only with traditional logistic regressions, but mainly using a machine-learning approach. The hypothesis here was that the machine-learning models would receive the information from all the affected regions and could internally deduce which region would have a higher importance compared to the others in the overall setting. With respect to this approach on individual ASPECTS regions, the existing literature is also scarce, which makes it difficult to contextualize our findings. Nevertheless, the insula region also showed the highest impact on the clinical outcome by far, followed by the cortical regions in varying order depending on the clinical endpoint. Overall, the results of the machine-learning approach thus corroborate our findings in the logistic regressions, indicating that the overall extent of the early signs of ischemia, especially the combination of central and peripheral ASPECTS regions, is of highest importance. Still, the influence of the insula region on its own should not be overestimated, as the insula as the sole predictor performed significantly worse than the cumulative ASPECTS or the combination of all aspects regions, as indicated by our results.
There are some limitations to this study that we need to acknowledge. Although we analyzed a large dataset, our results only reflect the circumstances of a single institution and would benefit from external or multi-institutional validation. Furthermore, the validity of this study is limited by its retrospective design, although the stroke database was maintained in a prospective manner. Machine-learning models generally outperform conventional statistical methods in medical research for the purpose of outcome prediction. The use of a mixed-effect model could have been reasonable when using multiple variables that may influence each other. While there has been progress in incorporating mixed model effects into existing machine-learning approaches, these are not performed by default and were not part of the final analysis of this manuscript. As only patients with an EVT were included, the effects of selection bias must be considered. Moreover, endovascular treatment of included patients was mostly finished in shorter time windows below 6 h, and findings for patients in a longer onset to treatment time might show different results. Even though ASPECTS is a stable measure in the quantification of early signs of infarction, there is a certain variability in its evaluation. The evaluation by one radiologist in consensus with the e‑ASPECTS evaluation is therefore another limitation of this study.
In summary our results suggest that the functional differences in affected areas are not the main feature of native stroke imaging, but ultimately the extent of early signs of infarction. Consequently, the original ASPECTS assessment in LVO, defined as early as 2001, remains a stable and reliable predictor for clinical outcome and is not inferior to a weighted (location-specific) ASPECTS assessment. Nevertheless, we believe that future research with access to more and sharper data should continue to look for ways to enhance the original ASPECTS to provide an even better simple and reliable assessment of the early signs of infarction and thereby improve the treatment of acute stroke patients.
References
Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–4. Erratum in: Lancet 2000;355:2170.
Ryu CW, Shin HS, Park S, Suh SH, Koh JS, Choi HY. Alberta Stroke Program Early CT Score in the Prognostication after Endovascular Treatment for Ischemic Stroke: A Meta-analysis. Neurointervention. 2017;12:20–30.
Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, Baumgart M, Leischner H, Schön G, Minnerup J, Thomalla G, Fiehler J, Kemmling A. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. 2019;142:1399–407. Erratum in: Brain. 2019;142:e26.
Payabvash S, Souza LC, Kamalian S, Wang Y, Passanese J, Kamalian S, Fung SH, Halpern EF, Schaefer PW, Gonzalez RG, Furie KL, Lev MH. Location-weighted CTP analysis predicts early motor improvement in stroke: a preliminary study. Neurology. 2012;78:1853–9.
Payabvash S, Benson JC, Tyan AE, Taleb S, McKinney AM. Multivariate Prognostic Model of Acute Stroke Combining Admission Infarct Location and Symptom Severity: A Proof-of-Concept Study. J Stroke Cerebrovasc Dis. 2018;27:936–44.
Seyedsaadat SM, Neuhaus AA, Pederson JM, Brinjikji W, Rabinstein AA, Kallmes DF. Location-Specific ASPECTS Paradigm in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2020;41:2020–6.
Fukuda K, Keppetipola K, Davison M, Chen M. O‑005 Utility of aspects region location in predicting stroke thrombectomy outcomes. J NeuroIntervent Surg. 2017;9(Suppl 1):A3–4.
Nagel S, Sinha D, Day D, Reith W, Chapot R, Papanagiotou P, Warburton EA, Guyler P, Tysoe S, Fassbender K, Walter S, Essig M, Heidenrich J, Konstas AA, Harrison M, Papadakis M, Greveson E, Joly O, Gerry S, Maguire H, Roffe C, Hampton-Till J, Buchan AM, Grunwald IQ. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. 2017;12:615–22.
Neuberger U, Nagel S, Pfaff J, Ringleb PA, Herweh C, Bendszus M, Möhlenbruch MA, Kickingereder P. Impact of slice thickness on clinical utility of automated Alberta Stroke Program Early Computed Tomography Scores. Eur Radiol. 2020;30:3137–45.
Gerber JC, Miaux YJ, von Kummer R. Scoring flow restoration in cerebral angiograms after endovascular revascularization in acute ischemic stroke patients. Neuroradiology. 2015;57:227–40.
McTaggart RA, Jovin TG, Lansberg MG, Mlynash M, Jayaraman MV, Choudhri OA, Inoue M, Marks MP, Albers GW; DEFUSE 2 Investigators. Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and MRI: reader agreement, modality agreement, and outcome prediction. Stroke. 2015;46:407–12.
Haranhalli N, Mbabuike N, Grewal SS, Hasan TF, Heckman MG, Freeman WD, Gupta V, Vibhute P, Brown BL, Miller DA, Jahromi BS, Tawk RG. Topographic correlation of infarct area on CT perfusion with functional outcome in acute ischemic stroke. J Neurosurg. 2019;132:33–41.
Rangaraju S, Streib C, Aghaebrahim A, Jadhav A, Frankel M, Jovin TG. Relationship Between Lesion Topology and Clinical Outcome in Anterior Circulation Large Vessel Occlusions. Stroke. 2015;46:1787–92.
Sheth SA, Malhotra K, Liebeskind DS, Liang CW, Yoo AJ, Jahan R, Nogueira RG, Pereira V, Gralla J, Albers G, Goyal M, Saver JL. Regional Contributions to Poststroke Disability in Endovascular Therapy. Interv Neurol. 2018;7:533–43.
Payabvash S, Kamalian S, Fung S, Wang Y, Passanese J, Kamalian S, Souza LC, Kemmling A, Harris GJ, Halpern EF, González RG, Furie KL, Lev MH. Predicting language improvement in acute stroke patients presenting with aphasia: a multivariate logistic model using location-weighted atlas-based analysis of admission CT perfusion scans. AJNR Am J Neuroradiol. 2010;31:1661–8.
Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, Caplan LR. Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Arch Neurol. 2005;62:1081–5.
Arnold M, Slezak A, El-Koussy M, Lüdi R, Findling O, Mono ML, Heldner MR, Fischer U, Mordasini P, Schroth G, Mattle HP, Gralla J, Jung S. Occlusion Location of Middle Cerebral Artery Stroke and Outcome after Endovascular Treatment. Eur Neurol. 2015;74:315–21.
Khan M, Baird GL, Goddeau RP Jr, Silver B, Henninger N. Alberta Stroke Program Early CT Score Infarct Location Predicts Outcome Following M2 Occlusion. Front Neurol. 2017;8:98.
Weyland CS, Mokli Y, Vey JA, Kieser M, Herweh C, Schönenberger S, Bendszus M, Möhlenbruch MA, Ringleb PA, Nagel S. Predictors for Failure of Early Neurological Improvement After Successful Thrombectomy in the Anterior Circulation. Stroke. 2021;52:1291–8. Erratum in: Stroke. 2021;52:e211.
Tan BY, Kong WY, Ngiam JN, Teoh HL, Sharma VK, Yeo LL. The Role of Topographic Collaterals in Predicting Functional Outcome after Thrombolysis in Anterior Circulation Ischemic Stroke. J Neuroimaging. 2017;27:217–20.
Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402.
Nishi H, Oishi N, Ishii A, Ono I, Ogura T, Sunohara T, Chihara H, Fukumitsu R, Okawa M, Yamana N, Imamura H, Sadamasa N, Hatano T, Nakahara I, Sakai N, Miyamoto S. Predicting Clinical Outcomes of Large Vessel Occlusion Before Mechanical Thrombectomy Using Machine Learning. Stroke. 2019;50:2379–88.
Acknowledgements
None
Funding
UN was funded by the Physician-Scientist Program from the University of Heidelberg Medical Faculty.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Nagel and C. Herweh received consulting fees from Brainomix. U. Neuberger, D.F. Vollherbst, C. Ulfert, S. Schönenberger, P.A. Ringleb, M.A. Möhlenbruch, M. Bendszus and P. Vollmuth declare that they have no competing interests.
Ethical standards
The study was conducted in accordance with the Declaration of Helsinki and its later amendments. Because of its retrospective character, additional written informed consent was waived by the local ethics committee (S-784/2018).
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neuberger, U., Vollherbst, D.F., Ulfert, C. et al. Location-specific ASPECTS does not improve Outcome Prediction in Large Vessel Occlusion compared to Cumulative ASPECTS. Clin Neuroradiol 33, 661–668 (2023). https://doi.org/10.1007/s00062-022-01258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-022-01258-8