Abstract
Purpose
Intravenous thrombolysis and mechanical thrombectomy (MT) are standard of care in patients with acute ischemic stroke due to large vessel occlusion. Data on MT in patients with intracranial hemorrhage prior to intervention is limited to anecdotal reports, as these patients were excluded from thrombectomy trials.
Methods
We analyzed patients from an observational multicenter cohort with acute ischemic stroke and endovascular treatment, the German Stroke Registry—Endovascular Treatment trial, with intracranial hemorrhage before MT. Baseline characteristics, procedural parameters and functional outcome at 90 days were analyzed and compared to a propensity score matched cohort.
Results
Out of 6635 patients, we identified 32 patients (0.5%) with acute ischemic stroke due to large vessel occlusion and preinterventional intracranial hemorrhage who underwent MT. Risk factors of intracranial hemorrhage were head trauma, oral anticoagulation and intravenous thrombolysis. Overall mortality was high (50%) but among patients with a premorbid modified Rankin scale (mRS) of 0–2 (n = 15), good clinical outcome (mRS 0–2) at 90 days was achieved in 40% of patients. Periprocedural and outcome results did not differ between patients with and without preinterventional intracranial hemorrhage.
Conclusion
Preinterventional intracranial hemorrhage in acute ischemic stroke patients with large vessel occlusion is rare. The use of MT is technically feasible and a substantial number of patients achieve good clinical outcome, indicating that MT should not be withheld in patients with preinterventional intracranial hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment for patients with acute ischemic stroke (AIS) due to intracranial large vessel occlusion (LVO) is mechanical thrombectomy (MT), either alone or in combination with intravenous thrombolysis (IVT) [1, 2]. While the added value of IVT in this setting is currently being investigated (SWIFT-DIRECT, MR CLEAN NO IV, DIRECT MT) [3,4,5], the benefit of MT has been firmly established by randomized controlled trials [6].
Intracranial hemorrhage encompasses four broad types of hemorrhage: intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), subdural hemorrhage (SDH) and epidural hemorrhage (EDH). These types of hemorrhage differ in etiology, clinical symptoms and prognosis, and outcomes are highly variable. The ICH, SAH and SDH might occur in patients with LVO prior to intervention due to a variety of causes: firstly, LVO patients are frequently on oral anticoagulation due to atrial fibrillation. Oral anticoagulation is a risk factor for cerebral hemorrhage with annual cerebral bleeding rates of 0.23–0.5% for direct oral anticoagulants and 0.7–0.85% for vitamin K antagonists (VKA) [7,8,9,10]. Cerebral bleeding, on the other hand, necessitates pausing of oral anticoagulants, which in turn dramatically increase the risk of thromboembolic stroke [11]. Furthermore, intracranial hemorrhage and AIS share nonmodifiable risk factors such as age, sex, ethnicity or previous stroke, and modifiable risk factors including arterial hypertension, hyperlipidemia, smoking, obesity and alcohol consumption [12]. Secondly, in acute LVO, sudden-onset paresis or unconsciousness might cause the patient to fall and lead to traumatic intracranial hemorrhage. Thirdly, intracranial hemorrhage is also a complication of IVT, affecting 8.8% of stroke patients in the ECASS II trial [13]. In patients treated with the drip-and-ship paradigm, IVT-associated intracranial hemorrhage can be detected before MT [14, 15].
With increasing MT numbers over the last years reaching, e.g. 16,135 cases (7.2% of hospitalized AIS patients) in Germany in 2019 alone, one would expect to see patients with LVO and preinterventional intracranial hemorrhage now and then, at least in larger stroke centers [16]. While IVT is obviously contraindicated in this setting, the optimal treatment of these patients currently remains unknown as randomized controlled MT trials excluded patients with intracranial hemorrhage [17,18,19,20,21]. Preliminary insight into MT in patients with preinterventional intracranial hemorrhage is based on published anecdotal data, including six patients with ICH after IVT [14, 15], two patients with spontaneous ICH [22, 23], three patients with SDH [24] and two with SAH [15, 25]. Therefore, the goal of this study was to describe a larger cohort of patients undergoing MT with AIS due to LVO and preinterventional intracranial hemorrhage.
Methods
The German Stroke Registry—Endovascular Treatment (GSR-ET; NCT03356392) is an ongoing, prospective, open-label, multicenter registry of patients with AIS due to LVO treated by MT. Patients were prospectively recruited between July 2015 and December 2019 from 25 German hospitals. A detailed description of the trial has been published previously [26]. Medical information was reviewed of all patients with intracranial hemorrhage as reported by the database or if given as a reason for omitting or stopping IVT. Patients were retrospectively included in this analysis if intracranial hemorrhage was detected on neuroimaging before initiation of MT. Patient characteristics were extracted from the registry. Functional independence was defined as a modified Rankin scale (mRS) score of 0–2. A 4:1 propensity score matching (R version 4.0.3, R core team 2020, package MatchIt, R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/) was performed to select a cohort of patients without intracranial hemorrhage matched for age, sex, premorbid mRS and NIHSS with the nearest-neighbour approach. All other analyses were performed with SPSS (version 25, IBM Corp, Armonk, NY, USA). Standard descriptive statistics were provided and parameters were compared between groups by Student’s t‑test, Mann-Whitney U test, and Fisher’s exact test, where appropriate. Significance level was set to α < 0.05 and all tests were two-sided.
Ethics Approval
Data collection was approved by the Ethics Committee of the University Munich, Germany (689-15) and Ethics Committees at the participating centers according to local regulations.
Results
Among 6635 patients in the registry treated with MT, 32 AIS patients with LVO and preinterventional intracranial hemorrhage were identified, resulting in a prevalence of 0.5%. The median age was 79.5 years (interquartile range [IQR] 75–83.75 years) and 50% were female (Table 1). The rate of pretreatment functional independence (mRS ≤ 2) was 56.3%.
The most frequent form of intracranial hemorrhage was SAH in 40.6%, followed by ICH in 37.5% and SDH in 31.1% of cases, with 9.4% of patients having more than one form of intracranial hemorrhage.
Intracranial hemorrhage was ipsilateral (or bilateral) to LVO in 67% and contralateral in 33% of cases. Among ipsilateral intracranial hemorrhage cases, LVO affected the same territory as the hemorrhage in a single case (#3): the patient experienced hemorrhagic transformation after MCA occlusion treated with MT. Reocclusion occurred 7 days later and was successfully treated by MT again. The ICH remained stable on follow-up CT but decompressive hemicraniectomy was necessary due to malignant media infarction.
Arterial hypertension was the most frequent cardiovascular risk factor (CVRF) present in 87.5% of cases, followed by atrial fibrillation (65.6%), dyslipidemia (37.5%) and diabetes (25.0%). Oral anticoagulation was taken by 37.5% of patients, of whom 4 patients were on low molecular weight heparin at the time of AIS and 28.1% of patients received antiplatelet medication.
The most frequently reported cause of bleeding was blunt trauma in 34.4% of cases, and bleeding was assumed to result from the presence of risk factors for hemorrhage, namely anticoagulation in 25% and IVT in 18.8%. Of the patients two had parenchymal hemorrhagic transformation of cerebral ischemia, one patient experienced multiple ICH due to cerebral cavernoma and one patient suffered from aneurysmatic SAH. The cause of bleeding was unknown in three patients. Among patients with a known onset of intracranial hemorrhage, it was on the same day as LVO in 46.4% (13/28 cases) and among these cases mostly attributable to IVT or trauma (46.2% and 30.8%, respectively). In the remaining 15 cases, the hemorrhage occurred at a median of 8 days (IQR, 6.5–10 days) before AIS, and LVO was attributable to the pausation of oral anticoagulation due to hemorrhage in 10/15 patients. LVO occurred before bleeding in 34%, afterwards in 56% and with indeterminable sequence on the same day in 9% of cases.
The most frequent stroke etiology according to the TOAST classification was cardioembolism in 75% of cases. The median NIHSS was 15 (IQR, 5–21), and among patients with anterior circulation LVO, cerebral imaging revealed a median ASPECT score of 9 (IQR 8-10). The occluded vessel was located in the anterior circulation in 81.3% and in the posterior circulation in 18.8% of cases. The median number of thrombectomy maneuvers was 2 (IQR, 1‑3), and successful reperfusion, defined as a modified Thrombolysis in Cerebral Infarction scale (mTICI) score of ≥ 2b, was achieved in 81.3%. Details on preprocedural and periprocedural times as well as clinical characteristics are given in Table 1 and the supplemental material.
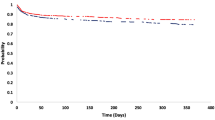
In-hospital mortality was 25%, and 90-day mortality, which was available in 28 patients, was 50%. Functional independence at 90-day follow-up was achieved in 21.4% (6/28) of all patients and in 40% (6/15) of patients with a baseline mRS of 0–2. None of the patients with unsuccessful reperfusion (mTICI ≤ 2a) reached functional independence and 66.7% of them died (4/6).
We performed propensity score matching to generate a control group of patients from the GSR-ET without intracranial hemorrhage matched for age, sex, NIHSS and premorbid mRS. Group comparison revealed no significant difference between patients with and without preinterventional intracranial hemorrhage regarding baseline characteristics, procedural and clinical outcomes (Table 2, supplemental table 3).
Discussion
Preinterventional intracranial hemorrhage is rare in patients with AIS and LVO, affecting 0.5% of cases in our registry. This setting presents a therapeutic dilemma. While IVT is obviously contraindicated in the presence of intracranial hemorrhage (or preceded ICH in the case of IVT-associated hemorrhage), it is at the treating physician’s discretion whether the benefit of MT outweighs the risk of hemorrhage progression or to accept the known detrimental outcome of conservatively treated LVO [6].
We have here reported the largest cohort of patients undergoing MT due to LVO with preinterventional intracranial hemorrhage. Importantly, we found that MT is technically feasible in this setting, with recanalization rates similar to those reported for LVO patients without hemorrhage [6]. Secondly, 40% of successfully treated patients remained functionally independent, whereas morbidity and mortality were high in patients with unsuccessful recanalization. Of note, the pretreatment functional status was often poor as more than half of the patients were functionally dependent before LVO, which is reflected by the high mortality of 50%. Compared to a matched control cohort of patients without intracranial hemorrhage, periprocedural and clinical results were similar. Hence, MT is technically feasible and potentially beneficial in LVO with pre-existing intracranial hemorrhage.
As mentioned above, the four main types of intracranial hemorrhage differ in etiology, management and prognosis and need separate evaluation. An EDH is caused by meningeal artery or less frequently venous bleeding into the epidural space, occurring classically after blunt head trauma. On theoretical grounds, MT seems safe in patients with AIS due to LVO and preinterventional EDH due to the differing vascular territory, but no patient with EDH was included in our cohort.
An SDH occurs in general due to bleeding from the cortical bridging veins into the arachnoid space, most commonly after blunt head trauma, but also spontaneously. As MT theoretically affects only the arterial pressure in the affected territory, MT appears safe in patients with LVO and preinterventional SDH; however, among the 10 cases reported here, most patients (80%) were already functionally dependent before, and morbidity and mortality were high.
We report one case of aneurysmatic SAH referred for aneurysm coiling, where concomitant LVO of the basilar artery was detected and treated during the same intervention but the detrimental outcome precludes any further conclusions. Non-aneurysmatic SAH conveys a better prognosis and is most commonly caused by head trauma, but may also occur in AIS, especially with LVO [25, 27,28,29]. Among 12 cases reported here, functional independence was achieved in 3 cases.
An ICH can occur due to a variety of reasons, including ischemic stroke with hemorrhagic transformation or trauma, further risk factors include cerebral amyloid angiopathy, anticoagulation, or IVT [30]. This heterogeneous etiology is reflected in our cohort. Previous reports focused on IVT-associated ICH, which are more frequently detected in patients treated under the drip-and-ship paradigm [14, 15]. While a detrimental outcome has been reported with a mortality of 4/6 cases, mortality was markedly lower in our cohort of IVT-associated ICH, despite a comparable distribution of age and gender [14, 15]. Among patients with ICH due to oral anticoagulation, mortality was higher.
In the setting of LVO of the supplying artery of the ICH-affected territory, MT might lead to hematoma expansion, compromising neighboring non-ischemic parenchyma or ultimately leading to brain herniation, negatively affecting the patient’s prognosis. In this scenario, established predictors of ICH expansion such as the spot sign are not evaluable, further complicating decision making [31]. We identified one patient with ICH in the LVO-affected territory: hemorrhagic transformation due to LVO was followed by reocclusion of the middle cerebral artery 7 days later, treated with successful reperfusion without hematoma enlargement on follow-up imaging. This case suggests that MT might be feasible in same territory ICH and LVO, but further data are needed to guide decision-making in this setting.
The most important limitation of our study is the lack of a control group without MT, leaving the impact of withholding or performing MT in patients with concomitant bleeding unknown. Another limitation is the nature of a retrospective analysis of the prospectively collected registry. The incidence of LVO with concomitant intracranial hemorrhage might be underestimated as only patients receiving MT were included in the registry. Furthermore, some data (3.3% of values) were missing and details about further clinical management, including the timing of oral anticoagulation resumption in patients with atrial fibrillation, are unknown.
Conclusion
The use of MT in acute stroke patients with LVO and concomitant intracranial hemorrhage is rare but appears to be feasible and potentially beneficial. Further data are needed to guide decision-making in the setting of same territory ICH and LVO.
References
Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, Fiehler J. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4:6-12.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-110. Erratum in: Stroke. 2018;49:e138. Erratum in: Stroke 2018;49:e233-4.
Fischer U, Kaesmacher J, S Plattner P, Bütikofer L, Mordasini P, Deppeler S, Cognard C, Pereira VM, Siddiqui AH, Froehler MT, Furlan AJ, Chapot R, Strbian D, Wiesmann M, Bressan J, Lerch S, Liebeskind DS, Saver JL, Gralla J. SWIFT DIRECT: Solitaire™ With the Intention For Thrombectomy Plus Intravenous t-PA Versus DIRECT Solitaire™ Stent-retriever Thrombectomy in Acute Anterior Circulation Stroke: Methodology of a randomized, controlled, multicentre study. Int J Stroke. 2021; doi: 10.1177/17474930211048768. Epub ahead of print.
Treurniet KM, LeCouffe NE, Kappelhof M, Emmer BJ, van Es ACGM, Boiten J, Lycklama GJ, Keizer K, Yo LSF, Lingsma HF, van Zwam WH, de Ridder I, van Oostenbrugge RJ, van der Lugt A, Dippel DWJ, Coutinho JM, Roos YBWEM, Majoie CBLM; MR CLEAN-NO IV Investigators. MR CLEAN-NO IV: intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion-study protocol for a randomized clinical trial. Trials. 2021;22:141.
Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, Peng Y, Han H, Wang J, Wang S, Yin C, Liu S, Wang P, Fang Q, Shi H, Yang J, Wen C, Li C, Jiang C, Sun J, Yue X, Lou M, Zhang M, Shu H, Sun D, Liang H, Li T, Guo F, Ke K, Yuan H, Wang G, Yang W, Shi H, Li T, Li Z, Xing P, Zhang P, Zhou Y, Wang H, Xu Y, Huang Q, Wu T, Zhao R, Li Q, Fang Y, Wang L, Lu J, Li Y, Fu J, Zhong X, Wang Y, Wang L, Goyal M, Dippel DWJ, Hong B, Deng B, Roos YBWEM, Majoie CBLM, Liu J; DIRECT-MT Investigators. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N Engl J Med. 2020;382:1981-93.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723-31.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-51. Erratum in: N Engl J Med. 2010;363:1877.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-104.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-92.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-91.
Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, Flechsenhar J, Neugebauer H, Jüttler E, Grau A, Palm F, Röther J, Michels P, Hamann GF, Hüwel J, Hagemann G, Barber B, Terborg C, Trostdorf F, Bäzner H, Roth A, Wöhrle J, Keller M, Schwarz M, Reimann G, Volkmann J, Müllges W, Kraft P, Classen J, Hobohm C, Horn M, Milewski A, Reichmann H, Schneider H, Schimmel E, Fink GR, Dohmen C, Stetefeld H, Witte O, Günther A, Neumann-Haefelin T, Racs AE, Nueckel M, Erbguth F, Kloska SP, Dörfler A, Köhrmann M, Schwab S, Huttner HB. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824-36.
Boehme AK, Esenwa C, Elkind MSV. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–95.
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245-51.
Weller JM, Hattingen E, Petzold GC, Bode FJ. Successful mechanical thrombectomy in stroke with thrombolysis-associated intracerebral hemorrhage-a case report. J Stroke Cerebrovasc Dis. 2019;28:285-7.
Styczen H, Gawlitza M, Abdullayev N, Brehm A, Serna-Candel C, Fischer S, Gerber J, Kabbasch C, Psychogios MN, Forsting M, Henkes H, Maus V. Mechanical thrombectomy in acute ischaemic stroke patients with pre-interventional intracranial haemorrhage following intravenous thrombolysis. Neuroradiol J. 2021;34:456-61.
Richter D, Weber R, Eyding J, Bartig D, Misselwitz B, Grau A, Hacke W, Krogias C. Acute ischemic stroke care in Germany - further progress from 2016 to 2019. Neurol Res Pract. 2021;3:14.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-306.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285-95.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20. Erratum in: N Engl J Med. 2015;372:394.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-18.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-30.
Forlivesi S, Bovi P, Cappellari M. Mechanical Thrombectomy for Acute Ischemic Stroke in a Patient with Concomitant Spontaneous Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2017;26:e150-2.
Quintas S, Villacieros-Álvarez J, Bárcena-Ruiz E, Dotor García-Soto J, Vivancos J. Ipsilateral acute ischemic stroke in a patient with concomitant intracerebral hemorrhage successfully treated with mechanical thrombectomy. Neurol Sci. 2019;40:2659-60.
Kim YW, Kang DH, Hwang YH, Kim YS, Park SP. Safe implementation of mechanical thrombectomy in acute stroke patients with major arterial occlusion and concomitant subdural hematoma. Neurointervention. 2013;8:115-9.
Zivelonghi C, Emiliani A, Augelli R, Plebani M, Micheletti N, Tomelleri G, Bonetti B, Cappellari M. Thrombectomy for ischemic stroke with large vessel occlusion and concomitant subarachnoid hemorrhage. J Thromb Thrombolysis. 2021;52:1212-14.
Alegiani AC, Dorn F, Herzberg M, Wollenweber FA, Kellert L, Siebert E, Nolte CH, von Rennenberg R, Hattingen E, Petzold GC, Bode FJ, Pfeilschifter W, Schäfer JH, Wagner M, Röther J, Eckert B, Kraft P, Pham M, Boeckh-Behrens T, Wunderlich S, Bernkopf K, Reich A, Wiesmann M, Mpotsaris A, Psychogios M, Liman J, Maier I, Berrouschot J, Bormann A, Limmroth V, Spreer J, Petersen M, Krause L, Lowens S, Kraemer C, Zweynert S, Lange KS, Thonke S, Kastrup A, Papanagiotou P, Alber B, Braun M, Fiehler J, Gerloff C, Dichgans M, Thomalla G. Systematic evaluation of stroke thrombectomy in clinical practice: The German Stroke Registry Endovascular Treatment. Int J Stroke. 2019;14:372-380. Erratum in: Int J Stroke. 2019;14:NP10-2. Erratum in: Eur J Prev Cardiol. 2020;27:NP16.
Cuvinciuc V, Viguier A, Calviere L, Raposo N, Larrue V, Cognard C, Bonneville F. Isolated acute nontraumatic cortical subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010;31:1355-62.
Nakajima M, Inatomi Y, Yonehara T, Hirano T, Ando Y. Nontraumatic convexal subarachnoid hemorrhage concomitant with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:1564-70.
Introna A, Mezzapesa DM, Petruzzellis M, Savarese M, Chiumarulo L, Zimatore DS, Dicuonzo F, Simone IL. Convexal subarachnoid hemorrhage and acute ischemic stroke: a border zone matter? Neurol Sci. 2019;40:1419-24.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632-44.
Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, Symons SP. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257-62.
Members of GSR-ET Investigators
A. Reich, O. Nikoubashman, J. Röther, B. Eckert, M. Braun, G.F. Hamann, E. Siebert, C.H. Nolte, G. Bohner, R.M. Eckert, J. Borggrefe, P. Schellinger, J. Berrouschot, A. Bormann, C. Kraemer, H. Leischner, M. Petersen, F. Stögbauer, T. Boeck-Behrens, S. Wunderlich, A. Ludolph, K.H. Henn, C. Gerloff, J. Fiehler, G. Thomalla, A. Alegiani, J.H. Schäfer, F. Keil, S. Tiedt, L. Kellert, C. Trumm, U. Ernemann, S. Poli, J. Liman, M. Ernst, K. Gröschel, T. Uphaus
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
F. Dorn received research funding from Cerenovus, speakers fees from Cerenovus and Acandis and serves as a proctor and consultant for Cerus Endovascular, Balt and Cerenovus. J.M. Weller, J.N. Meissner, S. Stösser, G.C. Petzold and F.J. Bode declare that they have no competing interests.
Additional information
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
62_2021_1128_MOESM1_ESM.docx
Supplementary tables 1-3: further baseline and periprocedural characteristics; characteristics of patients with intracerebral, subarachnoid and subdural hemorrhage; periprocedural adverse events for patients with intracranial hemorrhage and matched controls
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weller, J.M., Meissner, J.N., Stösser, S. et al. Mechanical Thrombectomy in Patients with Acute Ischemic Stroke and Concomitant Intracranial Hemorrhage. Clin Neuroradiol 32, 809–816 (2022). https://doi.org/10.1007/s00062-021-01128-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-021-01128-9