Abstract
Introduction
This study was carried out to investigate the global and regional morphometric and iron changes in grey matter (GM) of multiple sclerosis (MS) patients and link them to the white matter (WM) lesions in a multimodal magnetic resonance imaging approach.
Material and Methods
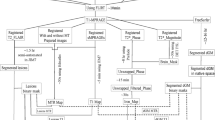
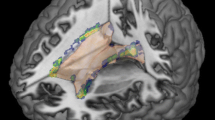
The study involved 30 relapsing-remitting MS (RRMS) patients along with 30 age-matched healthy controls (HC) who were scanned on a 3T Siemens Trio system. The scanning protocol included a 3D, high resolution T1, T2, and T2*-weighted sequences. The T1-w images were used in FreeSurfer for cortical reconstruction and volumetric segmentation, while T2-w images were used to extract the WM T2 lesions; however, iron and magnetic susceptibility were calculated from the phase data of the T2*-w sequence. Surface-based analyses were performed in FreeSurfer to investigate the regional cortical morphometric changes and their correlations with the expanded disability status scale (EDSS), WM T2 lesions load, cortical iron deposition and magnetic susceptibility.
Results
Significant differences were detected between the RRMS patients and HC for all cortical and subcortical morphometric changes. The EDSS and T2 lesion load showed weak to moderate correlation with the reduced cortical morphometric measurements, increased cortical magnetic susceptibility and iron concentration. All deep grey matter (dGM) volumes showed a significant strong positive correlation with the cortical surface area and volume in RRMS patients and HC.
Conclusions
Grey matter is very much involved in the RRMS and cortical morphometric changes occur in a non-uniform pattern and are very likely to be associated with cortical iron deposition and magnetic susceptibility, dGM atrophy, WM T2 lesion load, and disability.





Similar content being viewed by others
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17.
Neema M, Stankiewicz J, Arora A, Dandamudi VS, Batt CE, Guss ZD, Al-Sabbagh A, Bakshi R. T1- and T2-based MRI measures of diffuse gray matter and white matter damage in patients with multiple sclerosis. J Neuroimaging. 2007;17:16S–21S.
Rovaris M, Rocca MA, Filippi M. Magnetic resonance-based techniques for the study and management of multiple sclerosis. Br Med Bull. 2003;65:133–44.
Bö L, Geurts JJ, van der Valk P, Polman C, Barkhof F. Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol. 2007;64:76–80.
Klaver R, De Vries HE, Schenk GJ, Geurts JJ. Grey matter damage in multiple sclerosis. Prion. 2013;7:66–75.
Schutzer SE, Angel TE, Liu T, Schepmoes AA, Xie F, Bergquist J, Vécsei L, Zadori D, Camp DG 2nd, Holland BK, Smith RD, Coyle PK. Gray matter is targeted in first-attack multiple sclerosis. PLoS ONE. 2013;8:e66117.
Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–42.
Dawson JW. The histology of multiple sclerosis. Philos Trans R Soc. 1916;50:517–740.
Kutzelnigg A1, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12.
Geurts JJ, Bö L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol. 2005;26(3):572–7.
Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26.
Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400.
Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–7.
Dutta R, Chang A, Doud MK, Kidd GJ, Ribaudo MV, Young EA, Fox RJ, Staugaitis SM, Trapp BD. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69:445–54.
Bakshi R, Dandamudi VSR, Neema M, De C, Bermel RA. Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):30S-45S.
Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–65.
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–73.
Sadrzadeh SM, Saffari Y. Iron and brain disorders. Am J Clin Pathol. 2004;121 Suppl:S64–70.
LeVine SM. Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res. 1997;760:298–303.
Al-Radaideh AM, Wharton SJ, Lim SY, Tench CR, Morgan PS, Bowtell RW, Constantinescu CS, Gowland PA. Increased iron accumulation occurs in the earliest stages of demyelinating disease: an ultra-high field susceptibility mapping study in Clinically Isolated Syndrome. Mult Scler. 2013;19:896–903.
Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51.
Hebbrecht G, Maenhaut W, De Reuck J. Brain trace elements and aging. Nucl Instrum Methods Phys Res B. 1999;150:208–13.
Ramos P, Santos A, Pinto NR, Mendes R, Magalhães T, Almeida A. Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J Trace Elem Med Biol. 2014;28:13–7.
Yao B, Hametner S, van Gelderen P, Merkle H, Chen C, Lassmann H, Duyn JH, Bagnato F. 7 Tesla magnetic resonance imaging to detect cortical pathology in multiple sclerosis. PLoS ONE. 2014;9:e108863.
Buijs M, Doan NT, van Rooden S, Versluis MJ, van Lew B, Milles J, van der Grond J, van Buchem MA. In vivo assessment of iron content of the cerebral cortex in healthy aging using 7‑Tesla T2*-weighted phase imaging. Neurobiol Aging. 2017;53:20–6.
Moodie J, Healy BC, Buckle GJ, Gauthier SA, Glanz BI, Arora A, Ceccarelli A, Tauhid S, Han XM, Venkataraman A, Chitnis T, Khoury SJ, Guttmann CR, Weiner HL, Neema M, Bakshi R. Magnetic resonance disease severity scale (MRDSS) for patients with multiple sclerosis: a longitudinal study. J Neurol Sci. 2012;315:49–54.
Raz E, Branson B, Jensen JH, Bester M, Babb JS, Herbert J, Grossman RI, Inglese M. Relationship between iron accumulation and white matter injury in multiple sclerosis: a case-control study. J Neurol. 2015;262:402–9.
Wharton S, Schäfer A, Bowtell R. Susceptibility mapping in the human brain using threshold-based k‑space division. Magn Reson Med. 2010;63:1292–304.
Haacke EM, Ayaz M, Khan A, Manova ES, Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F, Kirsch W. Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging. 2007;26:256–64.
Susceptibility weighted imaging in MRI: basic concepts and clinical applications. Eds. EM Haacke, JR Reichenbach, John Wiley & Sons, 2014
Udupa JK, Samarasekera S. Fuzzy connectedness and object definition: theory, algorithms, and applications in image segmentation. Graph Models Image Process. 1996;58:246–61.
Liu M, Habib C, Miao Y, Haacke EM. Measuring iron content with phase. Susceptibility weighted imaging in MRI: basic concepts and clinical applications. Eds. EM Haacke, JR Reichenbach, pp. 369–401, John Wiley & Sons 2011.
Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012;33:2062–71.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94.
Sailer M, Fischl B, Salat D, Tempelmann C, Schönfeld MA, Busa E, Bodammer N, Heinze HJ, Dale A. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–44.
Narayana PA, Govindarajan KA, Goel P, Datta S, Lincoln JA, Cofield SS, Cutter GR, Lublin FD, Wolinsky JS; MRI Analysis Center at Houston; The CombiRx Investigators Group. Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. Neuroimage Clin. 2013;2:120–31.
Geisseler O, Pflugshaupt T, Bezzola L, Reuter K, Weller D, Schuknecht B, Brugger P, Linnebank M. Cortical thinning in the anterior cingulate cortex predicts multiple sclerosis patients’ fluency performance in a lateralised manner. Neuroimage Clin. 2016;10:89–95.
Nygaard GO, Walhovd KB, Sowa P, Chepkoech JL, Bjørnerud A, Due-Tønnessen P, Landrø NI, Damangir S, Spulber G, Storsve AB, Beyer MK, Fjell AM, Celius EG, Harbo HF. Cortical thickness and surface area relate to specific symptoms in early relapsing-remitting multiple sclerosis. Mult Scler. 2015;21:402–14.
Hier DB, Wang J. Reduced cortical surface area in multiple sclerosis. Neurol Res. 2007;29:231–2.
Charil A, Dagher A, Lerch JP, Zijdenbos AP, Worsley KJ, Evans AC. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. Neuroimage. 2007;34:509–17.
Datta S, Staewen TD, Cofield SS, Cutter GR, Lublin FD, Wolinsky JS, Narayana PA; MRI Analysis Center at Houston; CombiRx Investigators Group. Regional gray matter atrophy in relapsing remitting multiple sclerosis: baseline analysis of multi-center data. Mult Scler Relat Disord. 2015;4:124–36.
Filippi M, Rocca MA, Horsfield MA, Hametner S, Geurts JJ, Comi G, Lassmann H. Imaging cortical damage and dysfunction in multiple sclerosis. JAMA Neurol. 2013;70:556-64.
Filippi M, Preziosa P, Copetti M, Riccitelli G, Horsfield MA, Martinelli V, Comi G, Rocca MA. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology. 2013;81:1759–67.
Jacobsen C, Hagemeier J, Myhr KM, Nyland H, Lode K, Bergsland N, Ramasamy DP, Dalaker TO, Larsen JP, Farbu E, Zivadinov R. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatr. 2014;85:1109–15.
Hammond KE, Metcalf M, Carvajal L, Okuda DT, Srinivasan R, Vigneron D, Nelson SJ, Pelletier D. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64:707–13.
Chen X, Zeng C, Luo T, Ouyang Y, Lv F, Rumzan R, Wang Z, Li Q, Wang J, Hou H, Huang F, Li Y. Iron deposition of the deep grey matter in patients with multiple sclerosis and neuromyelitis optica: a control quantitative study by 3D-enhanced susceptibility-weighted angiography (ESWAN). Eur J Radiol. 2012;81:e633–9.
Du S, Sah SK, Zeng C, Wang J, Liu Y, Xiong H, Li Y. Iron deposition in the gray matter in patients with relapse-remitting multiple sclerosis: a longitudinal study using three-dimensional (3D)-enhanced T2*-weighted angiography (ESWAN). Eur J Radiol. 2015;84:1325–32.
Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC, Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci U S A. 2010;107:5130-5.
Prinster A, Quarantelli M, Orefice G, Lanzillo R, Brunetti A, Mollica C, Salvatore E, Morra VB, Coppola G, Vacca G, Alfano B, Salvatore M. Grey matter loss in relapsing–remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage. 2006;29:859–67.
Preziosa P, Pagani E, Mesaros S, Riccitelli GC, Dackovic J, Drulovic J, Filippi M, Rocca MA. Progression of regional atrophy in the left hemisphere contributes to clinical and cognitive deterioration in multiple sclerosis: a 5‑year study. Hum Brain Mapp. 2017;38:5648–65.
Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26:594–601.
Acknowledgements
We would like to thank the Royal Medical Services for their great help and support and for allowing us to use their 3T MRI to scan our patients and healthy controls.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Al-Radaideh, I. Athamneh, H. Alabadi and M. Hbahbih declare that they have no competing interests.
Ethical standards
The present study was approved by the Royal Medical Service ethics committee and carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). All participants were asked to sign a consent form and fill out the MRI safety questionnaire before participating in the study.
Caption Electronic Supplementary Material
Fig _S1
Regions of statistically significant correlations of magnetic susceptibility; Fig _S2 Regions of statistically significant correlations of iron
Rights and permissions
About this article
Cite this article
Al-Radaideh, A., Athamneh, I., Alabadi, H. et al. Cortical and Subcortical Morphometric and Iron Changes in Relapsing-Remitting Multiple Sclerosis and Their Association with White Matter T2 Lesion Load. Clin Neuroradiol 29, 51–64 (2019). https://doi.org/10.1007/s00062-017-0654-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-017-0654-0