Abstract
Background
Atrial fibrillation (AF) is the most common arrhythmia worldwide and is associated with increased morbi-mortality. The prevalence of AF in the Western world is increasing; however, reports on the prevalence of AF in the past decade are scarce, and whether the prevalence of AF increased during the last decade in Switzerland remains uncertain. Therefore, using data from a Swiss population-based sample, we aimed to assess the point prevalence of AF from 2014 to 2017 and to investigate determinants of AF.
Methods
A cross-sectional analysis of 4616 Caucasian participants aged 45–86 years (55% women) from a population-based sample was designed to explore the point prevalence and determinants of cardiovascular risk factors in the population of Lausanne, Switzerland. AF was assessed using electrocardiography (ECG) between 2014 and 2017.
Results
Overall, the point prevalence of AF was 0.9% (95% confidence interval [95% CI]: 0.7–1.2%) and the combined AF + atrial flutter (AFL) point prevalence was 1.1% (95% CI: 8.4–1.5%). The point prevalence of AF was higher among men (81% vs. 19% in women) and increased with age, reaching 3.1% in participants aged ≥ 80. In multivariable analysis, male gender (odds ratio and 95% CI: 4.98 [1.01–24.6]) and increasing age (2.86 [1.40–5.87] per decade) were associated with AF.
Conclusion
The point prevalence of AF and of AF + AFL, assessed between 2014 and 2017 in the city of Lausanne (Switzerland), was low but increased with age and in men.
Zusammenfassung
Hintergrund
Vorhofflimmern (VF) stellt weltweit die häufigste Arrhythmie dar und geht mit einer erhöhten Morbidität und Mortalität einher. In der westlichen Welt nimmt die Prävalenz des VF immer weiter zu, jedoch sind Publikationen zur Prävalenz des VF im vergangenen Jahrzehnt rar, und ob die Prävalenz des VF im letzten Jahrzehnt in der Schweiz gestiegen ist, bleibt unklar. Daher war es das Ziel der Autor*innen, anhand der Daten einer Stichprobe aus der Schweizer Bevölkerung die Punktprävalenz des VF von 2014 bis 2017 zu ermitteln und Einflussfaktoren auf das VF zu untersuchen.
Methoden
Eine Querschnittsanalyse von 4616 kaukasischen Teilnehmer*innen im Alter von 45–86 Jahren (55 % Frauen) aus einer bevölkerungsbasierten Stichprobe sollte dazu dienen, die Punktprävalenz und Einflussfaktoren hinsichtlich kardiovaskulärer Risikofaktoren in der Bevölkerung von Lausanne (Schweiz) zu ermitteln. Das Vorliegen eines VF wurde mittels Elektrokardiographie (EKG) zwischen 2014 und 2017 diagnostiziert.
Ergebnisse
Dabei lag die Punktprävalenz für VF bei 0,9 % (95 %-Konfidenzintervall [95 %-KI]: 0,7–1,2 %), und die Punktprävalenz für die Kombination aus VF und Vorhofflattern betrug 1,1 % (95 %-KI: 8,4–1,5 %). Bei Männern war die Punktprävalenz für VF höher als bei Frauen (81 vs. 19 %), sie stieg mit dem Alter und erreichte 3,1 % bei Teilnehmer*innen im Alter von ≥ 80 Jahren. In der multivariablen Analyse waren männliches Geschlecht (Odds Ratio und 95 %-KI: 4,98 [1,01–24,6]) und erhöhtes Alter (2,86 [1,40–5,87] pro Jahrzehnt) mit VF verknüpft.
Schlussfolgerung
Die Punktprävalenz für VF und für die Kombination aus VF und Vorhofflattern wurde zwischen 2014 und 2017 in der Stadt Lausanne (Schweiz) untersucht und stellte sich als niedrig heraus, sie nahm jedoch mit dem Alter zu und war bei Männern erhöht.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atrial fibrillation (AF) is the most common arrhythmia worldwide and is associated with increased mortality [1] and morbidity, particularly stroke [2], myocardial infarction, and heart failure [3].
Several studies performed in Western countries showed variable prevalence of AF, ranging from 0.3% to 7.2%, notably because of heterogeneity in their design and in the age groups studied [4,5,6,7,8]. Indeed, the biggest risk factor for AF is age. The prevalence of AF in adults younger than 55 years is very low and increases to 9% in adults older than 80 years [9]. Due to the aging of the population, prediction models show that more than 18 million AF cases can be expected by 2060 in Europe, which correspond to an increase of 200% in 40 years [9,10,11,12].
In the UK, based on data from NHS general practitioners, the prevalence of AF rose from 1.71% in 2015 to 2.05% in 2019 [13]. In the Framingham Heart Study, age-adjusted prevalence of AF over periods of 50 years doubled in men and increased slightly in women (12.6 during 1958–1967 to 25.7 per 1000 person-years during 1998–2007 in men, trend p = 0.0007; 8.1 to 11.8 per 1000 person-years in women, trend p = 0.009). A population-based survey of individuals aged 35–74 years living in the city of Geneva (Switzerland) at the beginning of 2000 showed an overall prevalence of AF below 1% [8].
However, reports on the prevalence of AF in the past decade are scarce, and whether the prevalence of AF increased during the past decade in Switzerland remains uncertain. Therefore, we aimed to assess, using data from a Swiss population-based sample, aged 45–86 years, and ECG data collected between 2014 and 2017, the point prevalence of AF and to investigate determinants of AF and atrial flutter (AFL).
Methods
Recruitment of participants
A detailed description of the recruitment procedure for the CoLaus|PsyCoLaus study and the follow-up process has been published [14]. Briefly, the CoLaus|PsyCoLaus study is a population-based cohort exploring the prevalence and determinants of cardiovascular risk factors in the population of Lausanne, Switzerland. Between 2003 and 2006, a nonstratified, representative sample was recruited based on the following inclusion criteria: (a) age 35–75 years, (b) willingness to participate, and (c) Caucasian origin. The first follow-up was performed between April 2009 and September 2012, 5.6 years on average after baseline; the second follow-up was performed between May 2014 and July 2017, 10.9 years on average after baseline. All study periods included an interview, a physical examination, and blood analysis. At the second follow-up, 12-lead electrocardiograms (ECG) were performed. Hence, this study was conducted using data from the second follow-up.
Electrocardiographic examination and atrial fibrillation assessment
A resting 12-lead body surface ECG was performed using a portable ECG machine (CARDIOVIT MS-2015, Schiller Reomed® AG, Dietikon, Switzerland) with the patients in supine position, at a paper speed of 25 mm/s. Data on basic rhythm, ventricular rate, P waves, PQ interval, QRS width, and QT interval were collected. Atrial fibrillation was defined as irregular RR intervals and no discernible, distinct P waves. Typical AFL was defined as an atrial activity seen as a “saw tooth” pattern (also called “F waves”), especially in the inferior leads (II, III, aVF), with a variable ventricular rate (ratio of atrial-to-ventricular contraction ranging from 4:1 to 2:1). Atypical AFL was defined as an atrial rate of more than 180/min with no F waves in the inferior leads. All ECGs were interpreted by two physicians (DS, FB). In the case of disagreement, a senior cardiologist (JS) was consulted and his interpretation was retained.
For this study, we considered two main outcomes: “pure” AF (noted as “AF” in this study) and combined AF and typical AFL (noted as „AF + AFL“ in this study). Previous studies [8] grouped AF and typical AFL into the same category, as both arrhythmias share the same risk factors and complications [15].
Covariates
Covariates were collected by self-administered questionnaires, interview, or physical examination. The detailed definitions of the covariates have been described elsewhere [16].
We collected personal and family history of cardiovascular events. Alcohol consumption was self-reported and categorized as alcohol drinkers (yes/no). Smoking status was defined as never, former, and current. Physical activity (PA) was assessed using a validated wrist-worn triaxial accelerometer (GENEActiv, Activinsights Ltd., UK) and classified in accordance with the World Health Organization (WHO) criteria [17].
Overweight was defined as a body mass index (BMI) of ≥ 25 and < 30 kg/m2, and obesity as BMI ≥ 30 kg/m2. Hypertension was defined as a systolic blood pressure (BP) of ≥ 140 mm Hg and/or diastolic BP ≥ 90 mm Hg and/or presence of an antihypertensive drug treatment.
Blood samples after an overnight fast included levels of total cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, glucose and glycated hemoglobin (HbA1c), creatinine, high-sensitive C‑reactive protein (hs-CRP) and pro-brain natriuretic peptide (pro-BNP). Low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald formula. Dyslipidemia was defined as an HDL-cholesterol < 1 mmol/L in men and < 1.29 mmol/L in women and/or LDL-cholesterol ≥ 4.1 mmol/L (≥ 2.6 mmol/L if personal history of cardiovascular disease [CVD] or diabetes) and/or triglyceride ≥ 2.2 mmol/L, and/or presence of a hypolipidemic drug treatment. Diabetes was defined as a fasting plasma glucose level of ≥ 7 mmol/L and/or a HbA1c ≥ 48 mmol/mol 6.5% and/or presence of oral hypoglycemic or insulin treatment.
Obstructive sleep apnea (OSA) is a risk factors for AF or AFL [12]; thus, we performed a subgroup analysis using participants with OSA data (n = 1920), i.e., the Berlin Questionnaire [18]. This instrument was developed to identify people at high risk of OSA in the ambulatory setting. It is composed of three categories, based on different symptoms of sleep or sleepiness. High risk of OSA is defined as ≥ 2 positive categories.
Exclusion criteria
We excluded patients (a) without ECG data and (b) with ≥ 1 missing covariate(s) mentioned above.
Statistical analysis
Statistical analyses were performed using Stata version 15.0 for windows (Stata Corp, College Station, TX, USA). Descriptive results are expressed as number of participants (percentage) for categorical variables and as average ± standard deviation or median and [interquartile range] for continuous variables. Bivariate analyses were performed using chi-square or Fisher’s exact test for qualitative variables and Student’s t test, analysis of variance, or the Kruskal–Wallis test for quantitative variables. Multivariable analysis was performed using logistic regression and the results are expressed as odds ratio (OR) and 95% confidence interval (CI). Statistical significance was assessed for a two-sided test with p < 0.05.
Due to its paroxysmal nature, it was possible that some participants already presented with AF or AFL in the past. As it was unknown who had already presented with these conditions, we hypothesized that participants taking anticoagulants might have AF or AFL. Therefore, we decided to exclude them from the sensitivity analysis.
Ethical statement
The institutional Ethics Committee of the University of Lausanne, which later became the Commission cantonale d’éthique de la recherche sur l’être humain (www.cer-vd.ch) approved the baseline CoLaus|PsyCoLaus study (reference 16/03) and the approval was renewed for the first (reference 33/09) and second (reference 26/14) follow-ups. The study was performed in agreement with the Helsinki Declaration and all participants gave their signed informed consent before joining the study.
Results
Characteristics of the sample
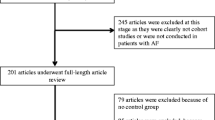
On the initial 4881 participants, 4616 (94.6%) were retained for analysis. The exclusion criteria are indicated in Fig. 1 and the characteristics of the included and excluded participants are summarized in Supplementary Table 1. Included participants were younger, had lower levels of hypertension, diabetes, dyslipidemia, creatinine, hs-CRP, and pro-BNP, and less frequently reported a personal history of CVD. Included participants also reported higher levels of alcohol units consumed per week and were more physically active. Similar findings were obtained when exclusion criteria were extended to participants with at least one missing covariate(s) (Supplementary Table 2), except that included participants were more frequently overweight and had dyslipidemia and less frequently alcohol drinkers. To summarize, excluded patients were older and sicker, which might explain the absence of ECG data as those patients are harder to keep in a prospective study [19, 20].
Point prevalence of AF/AFL
Overall, 42 cases of AF were detected, resulting in an AF point prevalence of 0.9% (95% CI: 0.7–1.2). The point prevalence of AF by sex and age is presented in Fig. 2.
Furthermore, 10 cases of typical AFL were recorded, but no atypical AFL was detected. Therefore, the combined point prevalence of AF + AFL is 1.1% (95% CI: 0.4–1.5%). The point prevalence of combined AF + AFL by sex and age is presented in Fig. 3.
Factors associated with AF/AFL
The bivariate comparisons of the clinical and sociodemographic characteristics between AF and non-AF cases are summarized in Table 1. Participants with AF were older, more often men (81%), presented more often with hypertension, dyslipidemia, or diabetes, had higher levels of BMI, and had more frequently a personal history of CVD. Participants with AF also had higher levels of creatinine, hs-CRP, and pro-BNP, were more often alcohol drinkers, reported higher alcohol consumption, and were less physically active. The AF participants were slightly more at risk of OSA but without reaching a statistically significant difference.
Similar findings were obtained when comparing participants with and without AF + AFL (combined outcome; Table 2) or when restricting the analysis to participants with all covariates, except that no difference was found regarding dyslipidemia (Supplementary Table 3).
Multivariable analysis identified increasing age and male gender as being positively associated with AF and AF + AFL (Table 3). The others risk factors associated with AF and AF + AFL also tended to be positively associated, but were not statistically significant.
In the subgroup of participants with OSA data, multivariate analysis including the Berlin Questionnaire showed overall similar results, except that a high risk of OSA was not statistically significantly associated with AF or AF/AFL (Supplementary Table 4).
Discussion
Our study reported an AF point prevalence of 0.9% (95% CI: 0.7–1.2%). This finding is similar to the population-based survey conducted in Geneva, Switzerland, using data from 2000, where the prevalence of AF among subjects aged ≥ 50 years was 0.9% [8]. Later, in the 2010s, the Swiss AF prevalence was estimated between 0.6% and 0.7% [12]. Therefore, our study shows an overall stability in the AF point prevalence in Lausanne, Switzerland. Whether the Swiss prevalence of AF will steeply increase in the future is uncertain and further studies are needed to explore prevalence of AF overtime.
The point prevalence of AF was higher among men, which is in agreement with previous studies [8, 12]. In the CoLaus|PsyCoLaus cohort, men had a more unfavorable cardiovascular risk factor profile, which can explain why they were at more risk of developing AF [14, 21, 22]. Moreover, according to the BiomarCaRE Consortium study, increased height seems to also be a risk factor in AF [23]. The larger cardiac dimension and higher excitability of the conduction system might also lead to a greater susceptibility to arrhythmia [24], but the exact pathophysiology must be studied further. On the other hand, a cohort study showed that, after adjusting for height and other AF risk factors, male sex was no longer significantly associated with AF [25, 26]. Indeed, the lifetime risk of developing AF is approximately 40% in women and men, but men develop it earlier with an incidence that increases rapidly after 50 years of age [12], whereas in women, the incidence increases a decade later [23, 24]. This phenomenon might be explained by the protector effect of estrogens [27,28,29].
Increasing age is one of the leading risk factors of AF [12]. In participants aged ≥ 80, the point prevalence of AF in our study peaked at 3.1% (95% CI: 2.0–4.2%). This finding confirms previously published results [5, 8, 30, 31]. As the human body ages, the arterial walls stiffen leading to atrial volume overload, which causes AF [1, 32]. Another reason is the presence of other cardiac comorbidities such as coronary artery disease and heart failure, which also increase with age [32].
Hypertension, higher BMI, personal history of CVD, diabetes, dyslipidemia, and physical activity were associated with AF in the bivariate analysis, which was expected as they are known risk factors for AF [1, 12, 32, 33]. Higher creatinine and pro-BNP levels were also associated with AF. However, these associations were no longer significant in the multivariable analysis. Our sample size for the multivariable analysis was smaller than for the bivariate analysis (n = 2421 participants) and therefore could explain the reduced statistical power. The trends of the OR still confirm the results previously published [1, 12, 32, 33]. Furthermore, according to the European Society of Cardiology guidelines, the accumulation of these comorbidities plays a major role in the development of AF [12]. Thus, early intervention and better control of modifiable risk factors should decrease the incidence of AF, but further studies are needed to confirm this.
No association was found between alcohol drinking and AF. An explanation is the relatively low level of alcohol consumption in our study (median of 7.5 units/week among AF cases), as AF is mostly associated with high levels of alcohol consumption (> 21 units/week; [34]).
No association was found between OSA and AF. For a sizable number of participants there were no data for the Berlin Questionnaire and the statistical power was therefore reduced. The link between OSA and AF should be examined better, using a larger number of patients with polysomnography data.
For AF + AFL, the point prevalence was 1.1% (95% CI: 0.4–1.5%) in our study, which is in line with previously published reports [8, 12]. The evolution is also stable along the years and further studies are needed to monitor the prevalence. The point prevalence of AF + AFL was also higher in men than in women. Increased height and larger cardiac dimension may also be responsible [23, 24]. The point prevalence of AF + AFL also peaked at 4.7% (95% CI: 3.4–6.0%) for participants aged ≥ 80, which was expected. Arterial stiffness and atrial volume overload may be also responsible. As with AF, the point prevalence of AF + AFL increased with the presence of modifiable risk factors, such as hypertension, higher BMI, heart failure, diabetes, sedentary lifestyle, and chronic kidney disease. However, the results of multivariable analysis were not significant, but the trends of the OR were similar to those with AF. Alcohol consumption and OSA were not associated AF + AFL.
Study limitations
This study has some limitations. First, our study potentially excludes participants with more comorbidities, since participation in population-based studies are selective and healthy people participate more frequently [20]. Thus, our study might have underestimated the prevalence of AF and AFL. However, this effect could be partly counterbalanced by the fact that our study included only participants of Caucasian origin (inclusion criteria at baseline) and it is known that individuals of non-European ancestry have a lower prevalence of AF [35]. Furthermore, the prevalence of AF and AFL was derived from a single time point ECG, and therefore we could only calculate point prevalence, which means we might have underestimated the true AF prevalence, as paroxysmal AF might have been missed. Furthermore, we did not detect atypical AFL in our study, which is also caused by atrial volume overload and we cannot make a statement about its prevalence. Smartphone-based ambulatory monitoring will probably be an attractive alternative in the future [36]. Finally, the small number of participants with AF reduced the statistical power and precluded the identification of some determinants of AF.
Conclusion
The prevalence of atrial fibrillation and of atrial fibrillation + atrial flutter, assessed between 2014 and 2017 in the city of Lausanne (Switzerland), was low but increased with age and in male participants.
References
Benjamin EJ, Levy D, Vaziri SM et al (1994) Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham heart study. JAMA 271(11):840–844
Currie CJ, Jones M, Goodfellow J et al (2006) Evaluation of survival and ischaemic and thromboembolic event rates in patients with non-valvar atrial fibrillation in the general population when treated and untreated with warfarin. Heart 92(2):196–200
Miyasaka Y, Barnes ME, Gersh BJ et al (2006) Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J 27(8):936–941
Murphy NF, Simpson CR, Jhund PS et al (2007) A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart 93(5):606–612
Heeringa J, van der Kuip DA, Hofman A et al (2006) Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 27(8):949–953
Hobbs FD, Fitzmaurice DA, Mant J et al (2005) A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 9(40):1–74
Mairesse GH, Moran P, Van Gelder IC et al (2017) Screening for atrial fibrillation: a European heart rhythm association (EHRA) consensus document endorsed by the heart rhythm society (HRS), asia pacific heart rhythm society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE). Europace 19(10):1589–1623
Schmutz M, Beer-Borst S, Meiltz A, Urban P, Gaspoz JM, Costanza MC et al (2010) Low prevalence of atrial fibrillation in asymptomatic adults in Geneva, Switzerland. Europace 12(4):475–481
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV et al (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 285(18):2370–2375
Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A et al (2013) Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 34(35):2746–2751
Stefansdottir H, Aspelund T, Gudnason V, Arnar DO (2011) Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace 13(8):1110–1117
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al (2020) 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 42(5):373–498
NHS (2020) Quality and outcomes framework, 2019–20. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2019-20. Accessed 19 Nov 2020
Firmann M, Mayor V, Vidal PM et al (2008) The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord 8:6
Rodriguez Ziccardi M, Goyal A, Maani CV (2019) Atrial flutter. StatPearls, Treasure Island (FL)
Samim D, Mean M, Clair C, Marques-Vidal P (2018) A 10-year observational study on the trends and determinants of smoking status. PLoS ONE 13(7):e200010
World-Health-Organization (2018) WHO physical activity. http://www.webcitation.org/6yltNEnFK. Accessed 22 Mar 2018
Netzer NC, Stoohs RA (1999) Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 131(7):485–491
McMurdo MET, Roberts H, Parker S et al (2011) Improving recruitment of older people to research through good practice. Age Ageing 40(6):659–665
Tolonen H, Ahonen S, Jentoft S et al (2015) Differences in participation rates and lessons learned about recruitment of participants—the European health examination survey pilot project. Scand J Public Health 43(2):212–219
Vollenweider P, Hayoz D, Preisig M et al (2006) Health examination survey of the Lausanne population: first results of the CoLaus study. Rev Med Suisse 2(86):2528–2530
Antiochos P, Marques-Vidal P, Waeber G, Vollenweider P (2015) Five year trends in dyslipidaemia prevalence and management in Switzerland: the CoLaus study. Nutr Metab Cardiovasc Dis 25(11):1007–1015
Magnussen C, Niiranen TJ, Ojeda FM et al (2017) Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (biomarker for cardiovascular risk assessment in europe). Circulation 136(17):1588–1597
Staerk L, Sherer JA, Ko D et al (2017) Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 120(9):1501–1517
Alonso A, Krijthe BP, Aspelund T et al (2013) Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2(2):e102
Ko D, Rahman F, Schnabel RB et al (2016) Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol 13(6):321–332
Merz AA, Cheng S (2016) Sex differences in cardiovascular ageing. Heart 102(11):825–831
Iorga A, Cunningham CM, Moazeni S et al (2017) The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 8(1):33
Knowlton AA, Lee AR (2012) Estrogen and the cardiovascular system. Pharmacol Ther 135(1):54–70
Svennberg E, Engdahl J, Al-Khalili F et al (2015) Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 131(25):2176–2184
Lowres N, Olivier J, Chao T‑F et al (2019) Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med 16(9):e1002903
Chugh SS, Havmoeller R, Narayanan K et al (2014) Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 129(8):837–847
Schnabel RB, Yin X, Gona P et al (2015) 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet 386(9989):154–162
Djousse L, Levy D, Benjamin EJ et al (2004) Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham study. Am J Cardiol 93(6):710–713
Rahman F, Kwan GF, Benjamin EJ (2014) Global epidemiology of atrial fibrillation. Nat Rev Cardiol 11(11):639–654
Perez MV, Mahaffey KW, Hedlin H et al (2019) Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 381(20):1909–1917
Acknowledgements
The authors would like to express their gratitude to the participants in the Lausanne CoLaus|PsyCoLaus study and to the investigators who contributed to the recruitment. We thank Federica Bocchi (FB) and Jürg Schläpfer (JS) for their contribution to the ECG analysis.
Funding
The CoLaus|PsyCoLaus study was and is supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of the University of Lausanne, and the Swiss National Science Foundation (grants 3200B0-105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468 and 33CS30-148401). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
DS collected data, conducted part of the statistical analysis and wrote most of the article; PMV collected data, conducted most of the statistical analysis and wrote part of the article; PV and GW reviewed the manuscript for important intellectual content. DC and MM wrote part of the article and MM reviewed the manuscript for important intellectual content. PMV had full access to the data and is the guarantor of the study.
Corresponding author
Ethics declarations
Conflict of interest
D. Samim, D. Choffat, P. Vollenweider, G. Waeber, P. Marques-Vidal and M. Méan declare that they have no competing interests.
In accordance with the 1964 Helsinki Declaration and its later amendments. All participants signed the informed consent before any data was collected. The CoLaus|PsyCoLaus study was approved by the Ethic committee of the canton of Vaud (reference 16/03, reference 33/09, reference 26/14). This study reused the data collected for CoLaus|PsyCoLaus and its aim is within the initial objectives of the CoLaus|PsyCoLaus study. Therefore, no new approval from the Ethic committee of the canton of Vaud was needed. The principal investigators of the CoLaus|PsyCoLaus study were consulted and agreed to this study.
Supplementary Information
59_2021_5090_MOESM1_ESM.pdf
Supplementary Table 1: characteristics of included and excluded (without ECG) participants at 2nd follow-up, CoLaus|PsyCoLaus study, Lausanne, Switzerland
59_2021_5090_MOESM2_ESM.pdf
Supplementary Table 2: characteristics of included and excluded (without ECG or missing covariate(s) ≥ 1) participants at 2nd follow-up, CoLaus|PsyCoLaus study, Lausanne, Switzerland
59_2021_5090_MOESM3_ESM.pdf
Supplementary Table 3: characteristics of participants with and without AF, CoLaus|PsyCoLaus study, Lausanne, Switzerland. Analysis restricted to participants with all covariates
59_2021_5090_MOESM4_ESM.pdf
Supplementary table 4: multivariable analysis of the factors associated with AF or with AF + AFL at 2nd follow-up, CoLaus|PsyCoLaus study, Lausanne, Switzerland. Analysis including risk of sleep apnea
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samim, D., Choffat, D., Vollenweider, P. et al. Prevalence of atrial fibrillation. Herz 48, 48–54 (2023). https://doi.org/10.1007/s00059-021-05090-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-021-05090-7