Abstract
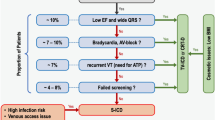
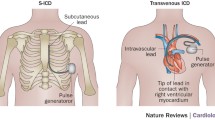
Subcutaneous implantable cardioverter/defibrillators (S-ICDs) have been developed to offer ICD treatment to patients without venous access to the heart and to overcome complications associated with transvenous leads, particularly lead fracture/insulation defects and endocarditis. Several studies and registries have demonstrated the feasibility and safety of S‑ICD in different groups of patients. Further developments in S‑ICD technology involve the combination with devices that can provide anti-bradycardia and anti-tachycardia pacing if needed. The extravascular ICD (EV-ICD) is a new system that similarly offers ICD therapy without a transvenous lead but uses a substernal instead of a subcutaneous lead to facilitate detection of ventricular fibrillation and to provide anti-tachycardia and also temporary anti-bradycardia pacing. The first animal but also clinical data on EV-ICDs have been published. This review discusses the current state, potential advantages and limitations, and future research of both S‑ICD and EV-ICD.
Zusammenfassung
Der subkutane implantierbare Kardioverter/Defibrillator (S-ICD) wurde entwickelt, um eine ICD-Therapie auch Patienten ohne venösen Zugang zum Herzen zu ermöglichen und um Komplikationen, die mit der Implantation transvenöser Elektroden assoziiert sind, zu vermeiden, insbesondere Elektrodenbrüche/Isolierungsdefekte und Endokarditis. Mehrere Studien und Register haben die Machbarkeit und Sicherheit des S‑ICD-Einsatzes bei verschiedenen Patientengruppen gezeigt. Weiterentwicklungen der S‑ICD-Technologie zielen auf eine Kombination mit Geräten zur antibradykarden und antitachykarden Stimulation ab. Der extravaskuläre ICD (EV-ICD) stellt ein neues System dar, das in ähnlicher Weise eine ICD-Therapie ohne transvenöse Elektrode erlaubt und stattdessen eine substernale Elektrode verwendet, um die Detektion von Kammerflimmern zu vereinfachen und eine antitachykarde sowie temporäre antibradykarde Stimulation zu ermöglichen. Erste experimentelle und klinische Ergebnisse zum EV-ICD wurden bereits veröffentlicht. In der vorliegenden Übersicht werden der aktuelle Stand, mögliche Vorteile und Limitationen sowie die weitere Forschung zu beiden Systemen, S‑ICD und EV-ICD, diskutiert.





Similar content being viewed by others
References
Connolly SJ, Hallstrom AP, Cappato R et al (2000) meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs implantable defibrillator study. Cardiac arrest study hamburg. Canadian implantable defibrillator study. Eur Heart J 21(24):2071–2078
Moss AJ, Zareba W, Hall WJ et al (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346(12):877–883
Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al (2015) 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the europe. Eur Heart J 36(41):2793–2867
Al-Khatib SM, Stevenson WG, Ackerman MJ et al (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm 15(10):e73–189
Maisel WH, Moynahan M, Zuckerman BD et al (2006) Pacemaker and ICD generator malfunctions: analysis of food and drug administration annual reports. JAMA 295(16):1901–1906
Habib A, Le KY, Baddour LM et al (2013) Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 111(6):874–879
Bardy GH, Smith WM, Hood MA et al (2010) An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med 363(1):36–44
van Dijk VF, Boersma LV (2020) The subcutaneous implantable cardioverter defibrillator in 2019 and beyond. Trends Cardiovasc Med 30(6):378–384. https://doi.org/10.1016/j.tcm.2019.09.006
McLeod CJ, Boersma L, Okamura H, Friedman PA (2017) The subcutaneous implantable cardioverter defibrillator: state-of-the-art review. Eur Heart J 38(4):247–257
Boersma LV, Barr CS, Burke MC et al (2017) Performance of the subcutaneous implantable cardioverter-defibrillator in patients with a primary prevention indication with and without a reduced ejection fraction versus patients with a secondary prevention indication. Heart Rhythm 14(3):367–375
Weiss R, Knight BP, Gold MR et al (2013) Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 128(9):944–953
Quast ABE, van Dijk VF, Yap SC et al (2018) Six-year follow-up of the initial Dutch subcutaneous implantable cardioverter-defibrillator cohort: long-term complications, replacements, and battery longevity. J Cardiovasc Electrophysiol 29(7):1010–1016. https://doi.org/10.1111/jce.13498
Burke MC, Aasbo JD, El-Chami MF et al (2020) 1‑year prospective evaluation of clinical outcomes and shocks: the subcutaneous ICD post approval study. JACC Clin Electrophysiol 6(12):1537–1550
Gold MR, Lambiase PD, El-Chami MF et al (2021) Primary results from the understanding outcomes with the S‑ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 143(1):7–17
Knops RE, Olde Nordkamp LRA, Delnoy P‑PHM et al (2020) Subcutaneous or transvenous defibrillator therapy. N Engl J Med 383(6):526–536
Brisben AJ, Burke MC, Knight BP et al (2015) A new algorithm to reduce inappropriate therapy in the S‑ICD system. J Cardiovasc Electrophysiol 26(4):417–423
Theuns DAMJ, Brouwer TF, Jones PW et al (2018) Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 15(10):1515–1522
Moss AJ, Schuger C, Beck CA et al (2012) Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 367(24):2275–2283
Wilkoff BL, Fauchier L, Stiles MK et al (2016) 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. J Arrhythmia 32(1):1–28
Quast A‑FBE, Baalman SWE, Brouwer TF et al (2019) A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: the PRAETORIAN score. Heart Rhythm 16(3):403–410
Quast A‑FBE, Baalman SWE, Betts TR et al (2019) Rationale and design of the PRAETORIAN-DFT trial: a prospective randomized CompArative trial of SubcutanEous ImplanTable CardiOverter-DefibrillatoR ImplANtation with and without DeFibrillation testing. Am Heart J 214:167–174
Tjong FVY, Brouwer TF, Kooiman KM et al (2016) Communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol 67:1865–1866
Quast A‑FBE, Tjong FVY, Koop BE et al (2018) Device orientation of a leadless pacemaker and subcutaneous implantable cardioverter-defibrillator in canine and human subjects and the effect on intrabody communication. Europace 20(11):1866–1871
Tjong FVY, Brouwer TF, Koop B et al (2017) Acute and 3‑month performance of a communicating leadless antitachycardia pacemaker and subcutaneous implantable defibrillator. JACC Clin Electrophysiol 3(13):1487–1498
Mondesert B, Dubuc M, Khairy P et al (2015) Combination of a leadless pacemaker and subcutaneous defibrillator: first in-human report. HeartRhythm Case Rep 1(6):469–471
Botto GL, Forleo GB, Capucci A et al (2017) The Italian subcutaneous implantable cardioverter-defibrillator survey: S‑ICD, why not? Europace 19(11):1826–1832
Tung SK, Bennett M, Yeung-Lai-Wah JALH (2007) Minimal invasive extra cardiac placement of high voltage defibrillator leads. Heart Rhythm 4(5):S200
Bhagwandien RE, Kik C, Yap S‑C, Szili-Torok T (2016) Substernal ICD lead implantation in a patient not suitable for subcutaneous ICD implantation without venous access due to superior vena cava syndrome. HeartRhythm Case Rep 3(1):97–99
Sholevar DP, Tung S, Kuriachan V et al (2018) Feasibility of extravascular pacing with a novel substernal electrode configuration: the substernal pacing acute clinical evaluation study. Heart Rhythm 15(4):536–542
Chan JYS, Lelakowski J, Murgatroyd FD et al (2017) Novel extravascular defibrillation configuration with a coil in the substernal space: the ASD clinical study. JACC Clin Electrophysiol 3(8):905–910
Boersma LVA, Merkely B, Neuzil P et al (2019) Therapy from a novel substernal lead: the ASD2 study. JACC Clin Electrophysiol 5(2):186–196
Crozier I, Haqqani H, Kotschet E et al (2020) First-in-human chronic implant experience of the substernal extravascular implantable cardioverter-defibrillator. JACC Clin Electrophysiol 6(12):1525–1536
Boersma L, Barr C, Knops R et al (2017) Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol 70(7):830–841
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.V. Boersma is a consultant for Boston Scientific and Medtronic. V.F. van Dijk is a consultant for Boston scientific.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
van Dijk, V.F., Boersma, L.V.A. Non-transvenous ICD therapy: current status and beyond. Herz 46, 520–525 (2021). https://doi.org/10.1007/s00059-021-05077-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-021-05077-4
Keywords
- Subcutaneous ICD
- Extravascular ICD
- Sudden cardiac death
- Defibrillation
- Inappropriate shocks
- Anti-tachycardia pacing