Abstract
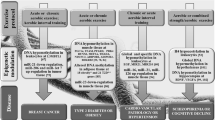
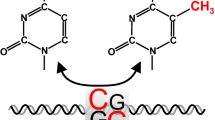
During the last decade, epigenetics became one of the fastest growing research fields in numerous clinical and basic science disciplines. Evidence suggests that chromatin modifications (e.g., histone modifications and DNA methylation) as well as the expression of micro-RNA molecules play a crucial role in the pathogenesis of several cardiovascular diseases. On the one hand, they are involved in the development of general risk factors like chronic inflammation, but on the other hand, epigenetic modifications are conducive to smooth muscle cell, cardiomyocyte, and endothelial progenitor cell proliferation/differentiation as well as to extracellular matrix processing and endothelial function (e.g., endothelial nitric oxide synthase regulation). Therefore, epigenetic medical drugs have gained increased attention and provided the first promising results in the context of cardiovascular malignancies. Beside other lifestyle factors, physical activity and sports essentially contribute to cardiovascular health and regeneration. In this review we focus on recent research proposing physical activity as a potent epigenetic regulator that has the potential to counteract pathophysiological alterations in almost all the aforementioned cardiovascular cells and tissues. As with epigenetic medical drugs, more knowledge about the molecular mechanisms and dose–response relationships of exercise is needed to optimize the outcome of preventive and rehabilitative exercise programs and recommendations.
Zusammenfassung
Die Epigenetik stellte während der letzten Dekade eines der am schnellsten wachsenden Forschungsfelder in vielen klinischen und grundlagenwissenschaftlichen Disziplinen dar. Mittlerweile ist bekannt, dass epigenetische Modifikationen am Chromatin (z. B. postranslationale Histonmodifikationen und DNA-Methylierungen) sowie die Expression von mikro-RNA-Molekülen eine wichtige Rolle bei der Pathogenese zahlreicher kardiovaskulärer Erkrankungen spielen. Veränderungen epigenetischer Faktoren sind sowohl bei der Ausprägung kardiovaskulärer Risikofaktoren als auch bei chronischen Entzündungsprozessen von Bedeutung. Die erwähnten Mechanismen tragen auch zur Proliferation, Differenzierung und Funktion von glatten Muskelzellen in der Gefäßwand, von Kardiomyozyten sowie von Endothelzellen bei und modulieren die Extrazellularmatrix. Es verwundert deshalb nicht, dass die Epigenetik modifizierende Medikamente auch bei kardiovaskulären Erkrankungen vermehrt getestet werden und erste vielversprechende Ergebnisse zeigen. Neben anderen Lebensstilfaktoren tragen körperliche Aktivität und Sport fundamental zur kardiovaskulären Gesundheit und Regeneration bei. Körperliche Aktivität fungiert als epigenetischer Regulator, der pathophysiologischen Entwicklungen im Herz-Kreislauf-System entgegenwirken kann. Ganz ähnlich wie bei der Erforschung neuer Medikamente wird es zukünftig notwendig sein, das Wissen über die zugrunde liegenden Mechanismen und Dosis-Wirkungs-Beziehungen von körperlicher Aktivität auf epigenetische Modifikationen zu mehren, um präventive und rehabilitative Bewegungsprogramme zu optimieren.


Similar content being viewed by others
References
Lee IM, Shiroma EJ, Lobelo F, Puska P et al (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380(9838):219–229
Schmid D, Leitzmann MF (2014) Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 25(7):1293–1311
Mattson MP (2015) Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. pii:S1568-1637(15)00002-1
Waddington CH (2012) The Epigenotype 1942. Int J Epidemiol 41(1):10–13
Li E, Bird A (2006) DNA methylation in mammals. In: Allis CD, Jenuwein T, Reinberg D (eds) Epigenetics. 1st edn. Cold Spring Harbor Laboratory, New York, pp 341–356
Kouzarides T, Berger SL (2006) Chromatin modifications and their mechanism of action. In: Allis CD, Jenuwein T, Reinberg D (eds) Epigenetics, 1st edn. Cold Spring Harbor Laboratory, New York, pp 191–209
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10(3):155–159
Lee S, Vasudevan S (2013) Post-transcriptional stimulation of gene expression by microRNAs. Adv Exp Med Biol 768:97–126
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1080
Sfanos KS, Hempel HA, De Marzo AM (2014) The role of inflammation in prostate cancer. Adv Exp Med Biol 816:153–181
Singhal G, Jaehne EJ, Corrigan F, Toben C et al (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8:315
Paneni F, Beckman JA, Creager MA, Cosentino F (2013) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 2436–2443
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071
Brasier AR (2010) The nuclear factor-kappaB-interleukin-6 signaling pathway mediating vascular inflammation. Cardiovasc Res 86(2):211–218
Ito K, Hanazawa T, Tomita K, Barnes PJ et al (2004) Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun 315:240–245
Ito K, Barnes PJ, Adcock IM (2000) Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol 20(18):6891–6903
Ito K, Jazrawi E, Cosio B, Barnes PJ et al (2001) p65-activated histone acetyltransferase activity is repressed by glucocorticoids: mifepristone fails to recruit HDAC2 to the p65-HAT complex. J Biol Chem 276:30208–30215
Kotla S, Singh NK, Heckle MR, Tigyi GJ et al (2013) The transcription factor CREB enhances interleukin-17A production and inflammation in a mouse model of atherosclerosis. Sci Signal 6(293):ra83
Kaptoge S, Seshasai SR, Gao P, Freitag DF et al (2014) Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 35(9):578–589
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88(4):1379–1406
Hayashino Y, Jackson JL, Hirata T, Fukumori N et al (2014) Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism 63(3):431–440
Saban KL, Mathews HL, DeVon HA, Janusek LW (2014) Epigenetics and social context: implications for disparity in cardiovascular disease. Aging Dis 5(5):346–355
Bekkering S, Quintin J, Joosten LA, Meer JW van der et al (2014) Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 34(8):1731–1738
Nakajima K, Takeoka M, Mori M, Hashimoto S et al (2010) Exercise effects on methylation of ASC gene. Int J Sports Med 31:671–675
Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75(3):487–517
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK (2006) Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol 26(12):2579–2581
Hoofnagle MH, Wamhoff BR, Owens GK (2004) Lost in transdifferentiation. J Clin Invest 113(9):1249–1251
Kawahara K, Kawabata H, Aratani S, Nakajima T (2003) Hyper nuclear acetylation (HNA) in proliferation, differentiation and apoptosis. Ageing Res Rev 2:287–297
Alexander MR, Owens GK (2012) Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 74:13–40
McDonald OG, Owens GK (2007) Programming smooth muscle plasticity with chromatin dynamics. Circ Res 100:1428–1441
McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK (2006) Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 116:36–48
Archer SL, Marsboom G, Kim GH, Zhang HJ et al (2010) Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a base for excessive cell proliferation and a new therapeutic target. Circulation 121:2661–2671
Kim GH, Ryan JJ, Archer SL (2013) The role of redox signaling in epigenetics and cardiovascular disease. Antioxid Redox Signal 18(15):1920–1936
Cao D, Wang Z, Zhang CL, Oh J et al (2005) Modulation of smoothmuscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol 25(1):364–376
Kim YS, Galis ZS, Rachev A, Han HC et al (2009) Matrix metalloproteinase-2 and -9 are associated with high stresses predicted using a nonlinear heterogeneous model of arteries. J Biomech Eng 131(1):011009
Chen KC, Wang YS, Hu CY, Chang WC et al (2011) OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J 25(5):1718–1728
Bátkai S, Thum T (2012) MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep 14(1):79–87
Maeda S, Tanabe T, Otsuki T, Sugawara J et al (2004). Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res 27(12): 947–953
Nualnim N, Barnes JN, Tarumi T, Renzi CP et al (2011) Comparison of central artery elasticity in swimmers, runners and the sedentary. Am J Cardiol 107(5):783–787
Montero D (2014) The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. Eur J Prev Cardiol. pii:2047487314553780
Vital TM, Stein AM, Melo Coelho FG de et al (2014) Physical exercise and vascular endothelial growth factor (VEGF) in elderly: a systematic review. Arch Gerontol Geriatr 59(2):234–239
Silva JF, Rocha NG, Nóbrega AC (2009) Mobilization of endothelial progenitor cells with exercise in healthy individuals: a systematic review. Arq Bras Cardiol 98(2):182–191
Suhr F, Rosenwick C, Vassiliadis A, Bloch W et al (2010) Regulation of extracellular matrix compounds involved in angiogenic processes in short- and long-track elite runners. Scand J Med Sci Sports 20(3):441–448
Yan J, Tie G, Park B, Yan Y et al (2009) Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg 50(6):1412–1422
Gurjar MV, Sharma RV, Bhalla RC (1999) eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol 19(12):2871–2877
Wu XD, Zeng K, Liu WL, Gao YG et al (2014) Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int J Sports Med 35(4):344–350
Illi B, Nanni S, Scopece A, Farsetti A et al (2003) Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ Res 93(2):155–161
Marin T, Gongol B, Chen Z, Woo B et al (2013) Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic Biol Med 64:61–68
Weber M, Baker MB, Moore JP, Searles CD (2010) MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun 393(4):643–648
Shirodkar AV, St Bernard R, Gavryushova A, Kop A et al (2013) A mechanistic role for DNA methylation in endothelial cell (EC)-enriched gene expression: relationship with DNA replication timing. Blood 121(17):3531–3540
Gielen S, Sandri M, Erbs S, Adams V (2011) Exercise-induced modulation of endothelial nitric oxide production. Curr Pharm Biotechnol 12(9):1375–1384
Fernandes T, Magalhães FC, Roque FR, Phillips MI et al (2012) Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertension 59(2):513–520
Reddy MA, Natarajan R (2011) Epigenetic mechanisms.in diabetic vascular complications. Cardiovasc Res 90(3):421–429
Shiva Shankar TV, Willems L (2014) Epigenetic modulators mitigate angiogenesis through a complex transcriptomic network. Vascul Pharmacol 60(2):57–66
Fraineau S, Pal CG, Allan DS, Brand M (2014) Epigenetic regulation of endothelial cell-mediated vascular repair. FEBS J (Epub ahead of print)
Sun Z, Singh N, Mullican SE et al (2011) Diet-induced lethality due to loss of HDAC3 in heart and skeletal muscle. J Biol Chem 286(38):33301–33309
Montgomery RL, Potthoff MJ, Haberland M et al (2011) Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest 118(11):3588–3597
Zhang LX, DeNicola M, Qin X, Du J et al (2014).Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. Am J Physiol Cell Physiol 307(4):C358–C372
Nguyen MA, Karunakaran D, Rayner KJ (2014) Unlocking the door to new therapies in cardiovascular disease: microRNAs hold the key. Curr Cardiol Rep 16(11):539
McGee SL, Fairlie E, Garnham AP, Hargreaves M (2009) Exercise-induced histone modifications in human skeletal muscle. J Physiol 587(Pt 24):5951–5958
Safdar A, Abadi A, Akhtar M, Hettinga BP et al (2009) miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One 4:(5) e5610
Rönn T, Volkov P, Davegårdh C, Dayeh T et al (2013) A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet 9(6):e1003572
Fernandes T, Soci UP, Oliveira EM (2013) MiRNA-208a targeting Purβ gene Regulates the β-MHC content in cardiac hypertrophy induced by exercise training. Circulation Res 128:A21942
Soci UP, Fernandes T, Rosa KT, Irigoyen MC et al (2013) The role of microRNA-208a in cardiac hypertrophy induced by aerobic physical training. FASEB 27:975.4 (Abstract)
Soci UP, Fernandes T, Hashimoto NY, Mota GF et al (2011) MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rat. Physiol Genomics 43:665–673
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA et al (2011) Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124:1537–1547
Tao H, Yang JJ, Shi KH, Deng ZY et al (2014) DNA methylation in cardiac fibrosis: new advances and perspectives. Toxicology 323:125–129
Kwak HB (2013) Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil 9(3):338–347
Kwak HB, Kim JH, Joshi K, Yeh A et al (2011) Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J 25(3):1106–1117
Denham J, O’Brien BJ, Marques FZ, Charchar FJ (2014) Changes in the leukocyte methylome and its effect on cardiovascular related genes after exercise. J Appl Physiol (1985). Jap 00878.2014
Zimmer P, Baumann FT, Bloch W, Schenk A et al (2014) Impact of exercise on pro inflammatory cytokine levels and epigenetic modulations of tumor-competitive lymphocytes in Non-Hodgkin-Lymphoma patients-randomized controlled trial. Eur J Haematol 93(6):527–532
Zimmer P, Bloch W, Schenk A, Zopf EM et al (2014)Exercise-induced natural killer cell activation is driven by epigenetic modifications. Int J Sports Med. DOI:10.1055/s-0034-1398531
Compliance with ethical guidelines
Conflict of interest. P. Zimmer and W. Bloch state that there are no conflicts of interest. The accompanying manuscript does not include studies on humans or animals.
Acknowledgments
The authors would like to thank Mrs. Christine Koliamitra and Mrs. Saskia Schulz for editorial support. Furthermore, the authors thank Mr. Alexander Schenk for graphical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimmer, P., Bloch, W. Physical exercise and epigenetic adaptations of the cardiovascular system. Herz 40, 353–360 (2015). https://doi.org/10.1007/s00059-015-4213-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-015-4213-7