Abstract
Aims
In earlier studies, we reported structural remodeling was associated with atrial fibrillation (AF) and showed that the renin–angiotensin–aldosterone system (RAAS) was linked to AF. It is reasonable to hypothesize that there is a cycle, from RAAS to structural remodeling to AF.
Patients and methods
Our study group consisted of 80 patients scheduled for mitral valve replacement surgery. Tissue samples of the left atrial appendages were obtained. Masson’s trichrome staining and immunohistochemical staining were performed to assess the extent of fibrosis. Radioimmunoassay was carried out to investigate the expression levels of local RAAS. RAAS-related genes were analyzed by RT-PCR.
Results
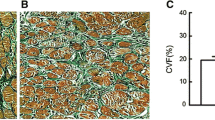
There was a significantly increased degree of fibrosis in AF patients compared with sinus rhythm (SR) patients (p = 0.023). There were significant differences in the expression levels of local angiotensin (Ang) II between the SR and the AF groups (p = 0.002). The expression levels of local Ang II correlated with the duration of AF (r = 0.727851, p = 0.001) and with collagen type I (r = 0.672189, p = 0.032). In the AF group, the mRNA expressions of the AT1R and ACE genes were markedly up-regulated in comparison with the SR group (p = 0.021 and p = 0.037).
Conclusions
On the basis of this study, and in combination with results of our previous studies, we demonstrate for the first time that there is a cycle involving RAAS, structural remodeling, and AF. RAAS, structural remodeling, and AF are the principal aspects in this cycle.
Zusammenfassung
Ziele
In einer vorherigen Studie berichteten die Autoren über strukturelles Remodeling in Zusammenhang mit Vorhofflimmern (VF). In einer anderen Studie zeigten die Autoren, dass das Renin-Angiotensin-Aldosteron-System (RAAS) mit VF in Zusammenhang steht. Es ist begründet, die Hypothese aufzustellen, dass es einen Kreislauf vom RAAS über strukturelles Remodeling bis zum VF gebe.
Methoden
Die Studiengruppe bestand aus 80 Patienten, bei denen eine Operation zum Mitralklappenersatz vorgesehen war. Gewebeproben wurden aus dem linken Herzohr entnommen. Die Masson-Trichromfärbung und eine immunhistochemische Färbung wurden durchgeführt, um das Ausmaß der Fibrose zu ermitteln. Die Bestimmung der Expression des lokalen RAAS erfolgte mit einem Radioimmunoassay. Die RAAS-bezogenen Gene wurden mittels RT-PCR analysiert.
Ergebnisse
Das Ausmaß der Fibrose wies eine signifikante Zunahme bei VF-Patienten gegenüber Patienten mit Sinusrhythmus (SR) auf (p = 0,023). Es bestanden signifikante Unterschiede bei der Expression von lokalem Angiotensin II (Ang II) zwischen der Gruppe mit SR und der Gruppe mit VF (p = 0,002). Die Expression von lokalem Ang II korrelierte mit der Dauer des VF (r = 0,727851; p = 0,001) und mit Kollagen vom Typ I (r = 0,672189; p = 0,032). In der Gruppe mit VF war die mRNA-Expression des AT1R- und ACE-Gens deutlich hochreguliert im Vergleich zu der Gruppe mit SR (p = 0,021 bzw. p = 0,037).
Schlussfolgerungen
Gemäß der vorliegenden Studie und in Kombination mit den vorhergehenden Studien wurde hier erstmals gezeigt, dass es einen Kreislauf zwischen RAAS, strukturellem Remodeling und VF gibt. RAAS, strukturelles Remodeling und VF sind die grundlegenden, wesentlichen Aspekte dieses Kreislaufs.





Similar content being viewed by others
References
Burstein B, Nattel S (2008) Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51:802–809
Zhang S (2009) Atrial fibrillation in mainland China: epidemiology and current management. Heart 95:1052–1055
Li Y, Jian Z, Yang ZY et al (2013) Increased expression of connective tissue growth factor and transforming growth factor-Beta-1 in atrial myocardium of patients with chronic atrial fibrillation. Cardiology 124(4):233–240
Oliveira IM de, Oliveira BD, Scanavacca MI et al (2013) Fibrosis, myocardial crossings, disconnections, abrupt turns, and epicardial reflections: do they play an actual role in human permanent atrial fibrillation? A controlled necropsy study. Cardiovasc Pathol 22(1):65–69
Qian YJ, Meng J, Tang H et al (2010) Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace 12(3):371–377
Qian YJ, Liu Y, Tang H et al (2013) Circulating and local renin-angiotensin-aldosterone system express differently in atrial fibrillation patients with different types of mitral valvular disease. J Renin Angiotensin Aldosterone Syst 14:204–211
Qian YJ, Shao HZ, Luo TX et al (2008) Plasma angiotensin converting enzyme level and permanent atrial fibrillation with mitral valvular disease. Lab Medicine 39(11):674–677
Swartz MF, Fink GW, Sarwar MF et al (2012) Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation. J Am Coll Cardiol 60(18):1799–1806
Nattel S, Burstein B, Dobrev D (2008) Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythmia Electrophysiol 1:62–73
Polyakova V, Miyagawa S, Szalay Z et al (2008) Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med 12:189–208
Thomas L, Mckay T, Byth K (2007) Abnormalities of left function after cardioversion: an atrial strain rate study. Heart 93:89–95
Marchesi C, Rehman A, Rautureau Y et al (2013) Protective role of vascular smooth muscle cell PPARgamma in angiotensin II-induced vascular disease. Cardiovasc Res 97(3):562–570
Singh JP, Kulik A, Levin R et al (2012) Renin-angiotensin-system modulators and the incidence of atrial fibrillation following hospitalization for coronary artery disease. Europace 14(9):1287–1293
Disertori M, Barlera S, Staszewsky L et al (2012) Systematic review and meta-analysis: renin-Angiotensin system inhibitors in the prevention of atrial fibrillation recurrences: an unfulfilled hope. Cardiovasc Drugs Ther 26(1):47–54
Kiryu M, Niwano S, Niwano H et al (2012) Angiotensin II-mediated up-regulation of connective tissue growth factor promotes atrial tissue fibrosis in the canine atrial fibrillation model. Europace 14(8):1206–1214
Dai HL, Guo Y, Guang XF et al (2013) The changes of serum angiotensin-converting enzyme 2 in patients with pulmonary arterial hypertension due to congenital heart disease. Cardiology 124(4):208–212
Kang SJ, You A, Kwak MK (2011) Suppression of Nrf2 signaling by angiotensin II in murine renal epithelial cells. Arch Pharm Res 34(5):829–836
Kaschina E, Grzesiak A, Li J et al (2008) Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 118(24):2523–2532
Yang KC, Jay PY, McMullen JR, Nerbonne JM (2012) Enhanced cardiac PI3Kalpha signalling mitigates arrhythmogenic electrical remodelling in pathological hypertrophy and heart failure. Cardiovasc Res 93(2):252–262
Qian YJ, Shao HZ, Zhou WX et al (2013) Histopathological characteristics and oxidative injury secondary to atrial fibrillation in the left atrial appendages of patients with different forms of mitral valve disease. Cardiovasc Pathol 22(3):211–218
Topal NP, Ozben B, Hancer VS et al (2011) Polymorphisms of the angiotensin-converting enzyme and angiotensinogen gene in patients with atrial fibrillation. J Renin Angiotensin Aldosterone Syst 12(4):549–556
Acknowledgments
We thank all the personnel of the echocardiography department and the laboratory of experimental medicine for their collaboration throughout this study. The authors would like to thank Dr. Adam P. Allen for his editorial and review assistance. This work was supported by grants from the Specialized Research Fund for the Doctoral Program of Higher Education, PCR (Grant No. 20120181120041), and the Science and Technology Bureau of Chengdu, Sichuan Province, PRC (Grant No. 12PPYB192SF-002).
Compliance with ethical guidelines
Conflict of interest. Q. Yongjun, S. Huanzhang, Z. Wenxia, T. Hong, and X. Xijun state that there are no conflicts of interest. All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qian Yongjun and Shao Huanzhang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Yongjun, Q., Huanzhang, S., Wenxia, Z. et al. From changes in local RAAS to structural remodeling of the left atrium. Herz 40, 514–520 (2015). https://doi.org/10.1007/s00059-013-4032-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-013-4032-7