Abstract
Spondyliaspis cf. plicatuloides and Glycaspis brimblecombei (Hemiptera: Aphalaridae) are invasive insect pests of Eucalyptus, native to Australia. The insects feed on eucalypt sap, and both psyllid species exhibit clear preferences for different species and hybrids of Eucalyptus. The objective of this study was to identify the constitutive morphological and phytochemical characteristics underlying these host preferences. Four preferred and eight non-preferred eucalypt hosts were selected for evaluation. Thirteen leaf morphological features of the 12 eucalypts were analysed. The non-polar and polar metabolites in and on the surface of leaves of each eucalypt species were extracted, and their chemical composition was analysed using gas chromatography coupled with mass spectrometry. The leaf volatile profiles of hosts and non-hosts of S. cf. plicatuloides and G. brimblecombei did not differ sufficiently to explain the host choices of the two eucalypt psyllids. The leaf polar metabolite profiles of the susceptible hosts of the two psyllids differed significantly but did not explain the host preferences of the two psyllid species. However, preferred hosts of S. cf. plicatuloides and G. brimblecombei had some leaf morphological features and wax metabolites in common. Our results show that particular combinations of leaf morphological features and wax metabolites might influence the host choice of eucalypt-feeding lerp psyllids, but no traits explaining the differences in host-selection behaviour between S. cf. plicatuloides and G. brimblecombei were identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalypt trees, from the genera Angophora, Corymbia and Eucalyptus (family Myrtaceae) are native to Australia, Papua New Guinea (including New Britain), Timor-Leste, Indonesia and the Philippines (Ladiges et al. 2003; Nicolle 2022). These hardy, diverse evergreen trees are planted globally as a source of hardwood timber, paper, pulp, bioenergy, essential oils (Eldridge et al. 1993; Turnbull 1999), phenolic resins (Lourençon et al 2020) and nanocomposites (Matos et al 2020). Angophora, Corymbia and Eucalyptus contain 12, 95 and 730 species, respectively (Grattapaglia et al. 2012; Nicolle 2022). Amongst these genera, Eucalyptus is highly diverse and is comprised of three main subgenera, Eudesmia, Eucalyptus (formally Monocalyptus), Symphyomyrtus as well as seven smaller subgenera, Acerosae, Cuboidea, Alveolata, Cruciformes, Idiogenes, Minutifructus and Primitiva (Brooker 2000; Nicolle 2022). At present, the majority of plantation forestry species grown globally are in the subgenus Symphyomyrtus (Beadle and Turnbull 1992).
Symphyomyrtus species are highly susceptible to pathogens (Wingfield et al. 2008) and suffer greater herbivore damage than those from the subgenus Eucalyptus (Burdon and Chilvers 1974; Specht and Brouwer 1975; Lowman and Heatwole 1987; Noble 1989). Extensive surveys conducted across southern Australia also revealed that Symphyomyrtus species carried significantly more psyllid lerps and other invertebrates than Eucalyptus species (Woinarski and Cullen 1984). As Symphyomyrtus species are planted globally, native Australian pathogens and insect herbivores of these trees have also colonised these new ecosystems (Paine et al. 2011).
The number of emerging invasive insect pests of plantation forests is rapidly increasing globally (Wingfield et al. 2015; Hurley et al. 2016). South Africa, which has a well-established eucalypt plantation forest industry, is particularly affected by introductions of invasive pests. For example, two sap-sucking and lerp-forming Eucalyptus-feeding psyllids (Hemiptera: Aphalaridae), Glycaspis brimblecombei Moore (red gum lerp psyllid) (Fig. 1A) and Spondyliaspis cf. plicatuloides (Froggatt) (shell lerp psyllid) (Fig. 1D) have been reported in South Africa in 2012 and 2014, respectively (Bush et al. 2016).
Glycaspis brimblecombei is a serious pest (de Queiroz et al. 2013) and has spread globally (Makunde et al. 2020; Dittrich-Schröder et al. 2021; Ouvrard 2023). The insects feed on red gums from the subgenus Symphyomyrtus. Adult females lay orange/yellow eggs on young to mature leaf surfaces and after hatching, the nymphal instars (Fig. 1B) produce individual white conical lerps (Fig. 1C) on the leaf surface soon after feeding, by weaving anal exudates. The five nymphal instars feed and develop underneath the lerp until they become adults and the size of the lerp is enlarged by each instar (Hollis 2004). Nymphal instars of G. brimblecombei secrete large amounts of honeydew and eucalypt damage is primarily because of sooty mould on leaf surfaces. Sap sucking gradually causes leaf discolouration, leaf drop, twig dieback and, in severe infestations, death of the entire tree (Paine et al. 2000; Bella and Rapisarda 2013). The host range of G. brimblecombei is well known (Brennan et al. 2001; Ferreira et al. 2009; Huerta et al. 2010; Gonçalves et al. 2013; Bush et al. 2020) and the leaf chemistry and morphological features of preferred and non-preferred hosts have been studied previously (da Silveira et al. 2021; de Oliveira Del et al. 2022; Lucia et al. 2016).
Spondyliaspis cf. plicatuloides was only reported once outside of its native range (Australia) in South Africa (Bush et al. 2016; Makunde et al. 2020). Adult females lay brown eggs in clusters on mature leaf surfaces and the nymphal instars (Fig. 1E) produce individual architecturally hard-ribbed brown scalloped lerps (Fig. 1F) soon after feeding, by weaving anal exudates (Makunde et al. 2023). The five nymphal instars of S. cf. plicatuloides feed and develop underneath the lerps until adulthood (Hollis 2004; Makunde et al. 2023). When susceptible eucalypt species are infested, sap sucking by immature stages result in chlorosis, followed by leaf necrosis and defoliation (Makunde et al. 2023). The host range of this species is not well known, because the insect is uncommon in Australia, and only Eucalyptus rostrata Cav. (= Eucalyptus robusta sm.) and Eucalyptus spp. were identified as hosts (Hollis 2004). In South Africa, our observation was that the psyllid initially infested ornamental and street eucalypt trees and later spread to a wide variety of Symphyomyrtus species and hybrids in commercial plantation forests.
Host preference is comprised of two critical behavioural phases namely host-finding and host selection (Döring 2014). Several studies have found that psyllids use visual cues (White 1970; Farnier et al. 2014, 2015, 2018; Farnier and Steinbauer 2016; Paris et al. 2017) and host volatiles (Mayer et al. 2008a, b, 2011) to find their hosts. However, Farnier et al. (2018) reported that the eucalypt psyllids, Ctenarytaina eucalypti, C. bipartita, Anoeconeossa bundoorensis and G. brimblecombei, did not show positive chemotactic responses to host plant volatiles, despite neurophysiological responses of C. eucalypti to host volatiles (Yuvaraj et al. 2013). In wind tunnel assays, downwind movement of psyllids suggests that long-distance migrations are primarily wind-assisted (Martini et al. 2018). Dispersed individuals can thus land multiple times before reaching a suitable plant, which they evaluate using short-distance physical and chemical cues (Farnier et al. 2018).
Host selection of psyllids that use evergreen eucalypt hosts was shown to be affected by the presence and abundance of non-structural epicuticular waxes which greatly influenced psyllid adhesion to leaf surfaces (Brennan and Weinbaum 2001a), probing (Brennan and Weinbaum 2001b, c), orientation behaviour (Brennan and Weinbaum 2001d) as well as oviposition (Brennan et al. 2001). In addition, the anatomy of eucalypt leaves was shown to be important in the host-selection behaviour of psyllids, particularly the number of stomata on both leaf surfaces and the distribution of oil glands on the leaf area (de Oliviera del Piero et al. 2022). However, a more detailed dissection of the different factors is needed to make general predictions on the host-selection behaviour of poorly studied eucalypt psyllids, such as S. cf. plicatuloides.
Consequently, in this study, we sought to study the morphological and phytochemical characteristics of eucalypts that potentially underpin the host preference of S. cf. plicatuloides. We defined host preference as the ability of psyllids to select, feed and reproduce on a particular eucalypt host species. We addressed the study's goal in three steps. First, we examined levels of susceptibility of 21 eucalypt species planted in a common garden towards S. cf. plicatuloides and G. brimblecombei. Second, we examined the morphological and biochemical characteristics of four highly susceptible and eight moderately to highly resistant eucalypts, and finally, we used multivariate statistics to study the relationship between host preference and leaf characteristics.
Materials and methods
Assessment of eucalypt preferences by lerp psyllids
The host preferences of S. cf. plicatuloides and G. brimblecombei were evaluated in the ‘Zoo Plot’ plantation of the National Zoological Gardens, Rietondale, Pretoria, Gauteng (25° 44′ 8.578″ S, 28° 14′ 24.882″ E). The plantation consists of over 4000 eucalypt trees of 21 species and is naturally infested with both S. cf. plicatuloides and G. brimblecombei. The trees on the study site were planted in 14 blocks, each block has 29 rows and, in each row, each eucalypt species is represented from block 1 to block 14 (132 trees per row). From each block, the first and last trees were not considered and thus, eight trees per species were assessed per block from block 3 to block 6 (Supplementary Figure 1). The host preference of the two lerp psyllids was scored for 19 Eucalyptus species, determining their level of infestation using the following subjective scale: 0 (no lerps in the foliage); 1 (low infestation with lerps in 0.1–10% of the foliage); 2 (moderate infestation with lerps in 11–25% of the foliage); 3 (high infestation with lerps in 26–50% of the foliage); 4 (severe infestation with lerps on > 50% of the foliage). The infestation score took ‘fresh’ lerps and ‘older’ lerps on the entire tree into account. Thirty trees of each eucalypt species in the garden were assessed.
Twelve eucalypt species were selected for further analysis and their characteristics are presented in Supplementary Table 1. Six of the species were infested to varying degrees by S. cf. plicatuloides, namely Eucalyptus botryoides Smith, Eucalyptus camaldulensis Dehnh, Eucalyptus microcorys Muell, Eucalyptus paniculata Smith, Eucalyptus sideroxylon Cunn. Ex Woolls and Eucalyptus tereticornis Smith and amongst these eucalypts, E. tereticornis, E. paniculata and E. camaldulensis were also infested by G. brimblecombei. The other six eucalypt species were non-host species and were selected to compare traits between hosts and non-hosts. These were Corymbia citriodora (Hook.) K.D.Hill & L.A.S.Johnson, Eucalyptus ovata Labill, Eucalyptus pilularis Smith, Eucalyptus propinqua Deane and Maiden, Eucalyptus punctata DC and Eucalyptus viminalis Labill. Nine Eucalyptus species are members of the subgenus Symphyomyrtus (E. botryoides in the series Transversae; E. camaldulensis and E. tereticornis both in the series Exsertae; E. ovata in the series Foveolatae; E. paniculata in the series Rhodoxyla; E. propinqua in the series Connexentes; E. punctata in the series Lepidotae-Fimbriata; E. sideroxylon in the series Melliodorae and E. viminalis in the series Viminales); E. pilularis belongs to the subgenus Eucalyptus (Monocalyptus) in the series Pseudostringybarks; E. microcorys belongs to the subgenus Alveolata in the series Alveolatae and lastly C. citriodora belongs to the subgenus Corymbia in the Maculatae series.
Foliage chemical analysis
Sample collection and processing for analysis of volatile compounds
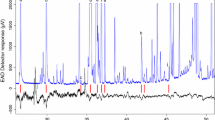
Fresh young leaves (4th and 5th leaf from the bud), collected at a height of approximately 2 m from 5 different trees of each of the 12 eucalypt species from the study site (Zoo Plot, Pretoria), were placed separately in individual aluminium foil packets, zip-locked in plastic bags and placed on ice in a cooler box. The fresh leaf material was stored at − 20 °C until sample preparation. Fresh leaf material (three or four leaves) from each sample were frozen in liquid nitrogen and finely ground to powder using a mortar and pestle and stored at − 80 °C. To extract non-polar compounds, 40 mg (mg) of each sample was weighed, and 1 ml of 95% hexane was added, and the mixture was agitated for 1 h at room temperature. Following a 20 min centrifugation at 14,000 rpm, 700 μl of the supernatant was transferred to a 1.5 ml glass chromatography vial for gas chromatography-mass spectrometry (GC–MS) analysis.
Gas chromatography–mass spectrometry analysis of non-polar compounds
The non-polar components of eucalypts were analysed using an Agilent Technologies gas chromatography system (Agilent 7890B) equipped with an HP-5MS UI GC column (30 m × 250 μm i.d. × 0.25 μm film thickness, 19091S-433UI; Agilent™) interfaced with a mass spectrometer (5977B MSD, Agilent, Santa Clara, USA). Using a 10 μl syringe on an Agilent automatic liquid sampler G4567A (7650A ALS), an aliquot of 1 μl of the hexane extracts of each eucalypt species under study was automatically injected into the GC–MS inlet in split mode (10:1). The chromatographic separation was performed using > 99.9% helium (baseline 5.0, Afrox SA) as a carrier gas set at a flow rate of 1.2 ml/min. The two wash solvents used were 99.9% Sigma-Aldrich dichloromethane and double-distilled hexane. The mass spectrometer was set to 230 °C in electron impact (EI) mode (70 eV), the MS quadrupole heated to 150 °C, and the MSD Transfer Line (Aux 2-Temperature) set to 280 °C. The scan range for the mass spectra was set to analyse from 40 to 450 amu, with a threshold of zero. The temperature programme for the GC was set as follows: temperature started at 40 °C and was kept at that level for 2 min. The ramp temperature was set at 50 °C to 200 °C (4 °C/min) with a final hold time of 2 min. The inlet temperature was set to 250 °C, and the column’s inlet pressure was set to 9.1473 psi (linear velocity: 39.72 cm/s). The purge septum flow rate on the GC was set at 3 ml/min, whilst the total flow rate was set at 16.2 ml/min. The gas saver was set to 20 ml/min after 5 min and the GC run lasted for 44 min.
Analysis of polar metabolites and cuticular waxes
Sample preparation and derivatisation
For the extraction of polar metabolites, a subsample of the homogenised leaf material used for the volatile analysis was freeze-dried at 13 kPa for 24 h using a VirTis AdVantage Pro Freeze Dryer (SP scientific, USA). Forty milligrams (40 mg) of the freeze-dried leaf material were weighed in 2 ml Eppendorf tubes to which 1.8 ml of absolute methanol (purity > 99.8%) was added. The mixture was incubated with occasional shaking for 4 h at room temperature and centrifuged for 20 min at 14,000 rpm, after which 1.2 ml of the supernatant was transferred to separate glass vials.
For cuticular wax analysis, leaf samples of the 12 eucalypt species were collected as described previously from three replicate trees. The three leaves of each eucalypt species were placed separately in 30 ml glass vials with 15 ml chloroform and vortexed for 20 s before the leaves were removed.
The extracts in methanol (polar metabolites) and chloroform (cuticular waxes) were dried separately at 37 °C in an incubator placed in a fume hood and, once dry, the extracts were resuspended in 100 μL pyridine containing 20 mg ml−1 methoxamine HCl followed by incubation at 30 °C for 90 min and centrifugation at 12,000 rpm for 20 min. Subsequently, 30 μl of the supernatant was transferred from each sample to a glass vial insert and silylated with 30 μl of N-methyl-N-(trimethylsilyl)trifluoroacetamide (MS-TFA) (Sigma-Aldrich), followed by incubation at 37 °C for 30 min. Samples were stored in the freezer at − 20 °C prior to the GC/MS analysis.
Gas chromatography–mass spectrometry for polar metabolites and cuticular waxes
The GC–MS was used to separate and quantify the derivatised polar compounds and cuticular waxes using the same instrument as described for the essential oils, with some adjustments to the operation settings. One microliter of the derivatised methanol extracts for each sample was automatically injected in split mode (100:1) and 1 μl of the derivatised chloroform extracts for each sample was automatically injected in split mode (10:1). Each sample was injected into a glass split 4-mm-inner diameter straight inlet liner, Ultra Inert packed with deactivated glass wool (SUPELCO 2–6375: 200 μl) and the inlet temperature was set at 250 °C, with a split flow of 120 ml/min and the column inlet pressure was set at 11.052 psi. Chromatographic separation was performed on an HP-5MS UI GC column (30 m × 250 μm i.d. × 0.25 μm film thickness, 19091S-433UI: Agilent™) with He carrier gas set at a flow rate of 1.2 ml/min. The mass spectrometer was programmed to run using electron impact (EI) ionisation at 70 eV operated at 230 °C, and the MS quadrupole was heated to 150 °C and the MSD Transfer Line was kept at 300 °C. The scan range for the mass spectra was set to range from 40 to 650 amu and the threshold was set to zero. The GC temperature programme: initial temperature at 70 °C and held for 2 min with a solvent delay of 3.5 min. The ramp temperature was set at 5 °C/min to 300 °C and held for 12 min, with a total run time of 60 min. The GC had a septum purge flow rate of 3 ml/min, whilst the total flow rate was set at 124.2 ml/min. The gas saver was set at 20 ml/min after 5 min. Cuticular waxes were analysed using the following adjustments: a split flow of 12 ml/min and the column inlet pressure was set at 11.052 psi. The septum purge flow rate on the GC was set to 3 ml/min, whilst the total flow rate was set to 16.2 ml/min.
Analysis of foliage physical features
The leaf materials used in this study were collected from five individual plants of each taxon at a height of approximately 2 m. A total of 12 undamaged leaves of similar leaf age were collected from each eucalypt species preferred by the 2 psyllids (6 with lerps and 6 without lerps), and only 6 leaves of similar leaf age were collected from non-preferred species. Epidermal impressions were made using clear nail varnish to produce a replica of leaf surfaces in the middle section of the adaxial and abaxial leaf surfaces to measure the stomatal density (number/mm–2) of the leaf lamina and the stomatal size. Using a clear adhesive tape, the clear nail varnish was peeled off the leaf lamina after drying, attached to a glass slide, and viewed under a compound light microscope (Zeiss Axioskop 2 Plus). Using a digital camera (AxioCam 105 colour, Zeiss) mounted on a compound light microscope, images were captured at 20×. Three images per leaf surface were captured to measure stomatal sizes and to calculate stomatal density for each image area (mm2). In addition, measurements were made for leaf lamina thickness (midway between the margin and the midrib at the widest part of the leaf), cuticle thickness, palisade parenchyma thickness, stomatal length (length of sunken guard cells measured on the longitudinal stoma axis) and width (measured as the largest distance found between the outer walls of the guard cells perpendicular to the longitudinal stoma axis). To measure these parameters, one square centimetre of leaf lamina was cut from five representative leaves of each eucalypt species used in this study between the mid vein and the leaf margin at the mid-leaf length. The leaf pieces were separately embedded in tissue freezing medium (Leica) and cross-sectioned at 12 μm thickness into six sections using a cryo-microtome (Leica CM1520) at − 20 °C. The mean measurement of six measurements along the leaf segment was used as a measure of leaf thickness, palisade thickness and cuticle thickness.
Data acquisition and analyses
Data from host preference assessment were analysed using GenStat 17th edition (2014, VSN International). Analysis of variance (ANOVA) was done to test for significant differences in host preference of the two lerp-forming psyllids, S. cf. plicatuloides and G. brimblecombei. The host susceptibility scores were further presented graphically as proportions per eucalypt species. Furthermore, data for each physical trait evaluated were tested for normality and analysed using one-way ANOVA, and the mean values and standard deviation (SD) were calculated using GenStat 17th edition (2014, VSN International). Tukey’s multiple range tests (p < 0.05) were used to compare means.
Tentative identification of eucalypt metabolites was achieved by comparing their mass spectra and retention index values with known spectra in the National Institute of Standard and Technology 17 reference library (NIST17.L). The peak area of the compounds found in three or more genotypes were considered for statistical analysis. Normality of data was tested prior analysis with Shapiro–Wilk tests in R 4.1.3 (R Core Team 2022). In addition, the percentage of the total area of the chromatogram was calculated for each peak by integrating the total peak area of the spectrograms. Data for the essential oil composition, waxes and polar compounds of the eucalypt species were log transformed and subjected to a one-way ANOVA using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) to determine which metabolites were significantly different between the species. A heat map showing the metabolites that were statistically significantly different (p < 0.05) between the different species was generated using the original data (non-normalised). A PCA was performed for each of the metabolite classes as well as for the leaf morphological traits, using the default settings in Metaboanalyst. For the cuticular waxes and the leaf morphological traits, the data were separated into two clusters, based on the PCA, and random forest analysis was conducted to determine which traits contributed most significantly to the separation of the two clusters, using the default settings in Metaboanalyst.
Results
Evaluation of Spondyliaspis cf. plicatuloides and Glycaspis brimblecombei infestation levels on eucalypt species planted in a common garden
The levels of infestation of S. cf. plicatuloides and G. brimblecombei on different eucalypt species in four subgenera (Alveolata, Corymbia, Eucalyptus and Symphyomyrtus) varied considerably (p < 0.001; Supplementary Table 2) and were ranked (for S. cf. plicatuloides) from the most preferred (susceptible), least preferred (less susceptible) to non-preferred (resistant) (Fig. 2). Eight of the 19 assessed eucalypt species were infested with S. cf. plicatuloides. The preference order based on the degree of infestation was E. microcorys > E. sideroxylon > E. camaldulensis > E. paniculata > E. botryoides > E. tereticornis > E. saligna > E. robusta. Amongst these, only E. microcorys, E. sideroxylon and E. camaldulensis had high levels of infestation, whilst lerps were only occasionally observed on the other five moderately resistant eucalypt species.
Damage severity of Spondyliaspis cf. plicatuloides and Glycaspis brimblecombei on 19 Eucalyptus species using a subjective scale (0–4): 0 (no lerps); 1 (low infestation with lerps on 0.1–10% of the foliage); 2 (moderate infestation with lerps on 11–25% of the foliage); 3 (high infestation with lerps on 26–50% of the foliage); 4 (severe infestation with lerps on > 50% of the foliage). The data are presented as a host preference proportion for each eucalypt species
Six eucalypt species were infested with G. brimblecombei and the order of preference was E. tereticornis > E. camaldulensis > E. punctata > Corymbia maculata > E. robusta > E. scoparia. Corymbia citriodora, C. maculata, E. dorrigoensis, E. goniocalyx, E. nicholii, E. ovata, E. punctata, E. propinqua, E. scoparia and E. viminalis and E. pilularis were not infested by either S. cf. plicatuloides or G. brimblecombei, except E. scoparia, with very low infestations of G. brimblecombei. Amongst these, only E. tereticornis had high levels of infestation, whilst lerps were only occasionally observed on the other five moderately resistant eucalypts.
Composition of volatile compounds in host and non-host species
GC–MS analysis identified more than 30 foliar volatile compounds across 12 eucalypt species selected for further analysis. Most of the compounds were mono- and sesquiterpenes. The dominant terpenes were eucalyptol, α-pinene, β-elimene and E-β-caryophyllene (Fig. 3). PCA revealed only minor differences between the volatile profiles of individual species (22.4% and 19.5% in the x- and y-dimensions, respectively). The volatile profile of E. sideroxylon, which is highly susceptible to S. cf. plicatuloides, did not differ significantly from the non-host, E. ovata. In addition, the volatile profiles of E. microcorys and E. camaldulensis (hosts of S. cf. plicatuloides) as well as E. tereticornis (highly susceptible to G. brimblecombei) did not significantly differ from the resistant species, E. propinqua and E. punctata (Fig. 3). The heat map illustrates the relative abundances of the most significantly different volatile metabolites (p < 0.05) between the different eucalypt species. Eucalyptus punctata and E. propinqua had the highest levels of eucalyptol, whilst C. citriodora and E. botryoides had the lowest levels. A very high concentration of α-pinene was observed in leaves of E. microcorys, whereas E. ovata had significantly higher concentrations of sesquiterpenes than the other species. Taken together, these data show that the profile of non-polar compounds of hosts and non-hosts of S. cf. plicatuloides and G. brimblecombei did not differ sufficiently to explain the host choices of eucalypt lerp psyllids.
Volatile metabolites of 12 eucalypt species. Two-dimensional PCA scores plot of the volatiles in eucalypt leaves based on the relative proportions of compounds in the hexane extract. Confidence intervals are presented by lighter coloured circles. The heat map shows the relative average abundance of compounds for each eucalypt species. Only compounds that differed significantly (p < 0.05) between the species based on a one-way ANOVA are shown. The data were log10-transformed for the PCA and ANOVA, but untransformed data are shown in the heat map. Five replicates per species were included. Metabolites were analysed using GC–MS. The images were generated by metaboanalyst and manually enhanced. S Eucalypt species that were highly susceptible to Spondyliaspis cf. plicatuloides, G Eucalypt species that was highly susceptible to Glycaspis brimblecombei
Composition of polar compounds in host and non-host species
GC–MS analysis tentatively identified over 50 polar compounds across all eucalypts used in this study. PCA of the relative concentrations of these compounds revealed significant differences between the different species, with unique metabolite profiles for E. sideroxylon, E. microcorys and E. camaldulensis, which are highly susceptible to S. cf. plicatuloides (Fig. 4). However, the species also differed significantly amongst each other (20.5% and 18.1% in the x- and y-dimensions, respectively). Eucalyptus tereticornis, the most susceptible species to G. brimblecombei, clustered together with the non-host, E. botryoides. The relative abundances of the most significantly different metabolites (p < 0.05) between the eucalypt species included high concentrations of 4,6-dioxohept-2-enoic acid in E. sideroxylon, as well as an unknown triterpene derivative and cyclohexine-1-carboxylate which were both relatively abundant in most species included in this study. Taken together, these data show that susceptible hosts of S. cf. plicatuloides and G. brimblecombei differed significantly from each other in their polar metabolite profiles. These eucalypt traits may, therefore, not play an important role in the host preference of these psyllids.
Polar metabolites of 12 eucalypt species. Two-dimensional PCA scores plot of the metabolites in eucalypt leaves based on the relative proportions of derivatised compounds in the methanol extract. Confidence intervals are presented by lighter coloured circles. The heat map shows the relative average abundance of compounds for each eucalypt species. Only compounds that differed significantly (p < 0.05) between the species based on a one-way ANOVA are shown. The data were log10-transformed for the PCA and ANOVA, but untransformed data are shown in the heat map. Four to five replicates per species were included. Metabolites were analysed using GC–MS. The images were generated by metaboanalyst and manually enhanced. S Eucalypt species that were highly susceptible to Spondyliaspis cf. plicatuloides, G Eucalypt species that was highly susceptible to Glycaspis brimblecombei
Composition of cuticular waxes in host and non-host species
Overall, 28 compounds associated with the surface of eucalypt leaves were detected across all preferred and non-preferred species included in this study. PCA of surface-associated metabolites separated the eucalypt species into two distinct clusters explaining 39.1% of the variation in the x-dimension (Fig. 5). The most susceptible host species (E. microcorys, E. sideroxylon, E. camaldulensis and E. tereticornis) clustered together with E. botryoides and E. paniculata, which were occasionally infested by S. cf. plicatuloides (Fig. 2). The non-host species clustered in a separate cluster. The relative abundances of the most significantly different metabolites between the different species included in this study (p < 0.05) are shown in the heat map. An unknown phenolic, an unknown triterpene and heptacosanol were present in high concentrations and differed most significantly between the different eucalypt species (Fig. 5). Random forest analysis revealed that there were seven metabolites (docosene, octacosane, benzoic acid, undecyl ester, hexadecanoic acid, octadecyl ester, an unknown phenolic, an unknown triterpene and 2-methyl 1,6-methylheptadecanoate) that each explained more than 1% of the differences between the two clusters. Their relative concentrations are shown as a percentage of all the metabolites in the spectrogram of each leaf (Table 1). Docosene was the most significant with rf = 0.12, showing that the metabolite explained 12% of the differences between the two clusters in the PCA (Fig. 5). Taken together, these data show that cuticular wax metabolites were similar in susceptible hosts of S. cf. plicatuloides and G. brimblecombei and might influence the host-selection behaviour of these psyllids.
Cuticular wax metabolites of 12 eucalypt species. Two-dimensional PCA scores plot of the metabolites in eucalypt leaves based on the relative proportions of derivatised compounds in the chloroform extract. Confidence intervals are presented by lighter coloured circles. The heat map shows the relative average abundance of compounds for each eucalypt species. Only compounds that differed significantly (p < 0.05) between the species based on a one-way ANOVA are shown. The data were log10-transformed for the PCA and ANOVA, but untransformed data are shown in the heat map. Three replicates per species were included. Metabolites were analysed using GC–MS. The images were generated by metaboanalyst and manually enhanced. S Eucalypt species that were highly susceptible to Spondyliaspis cf. plicatuloides, G Eucalypt species that was highly susceptible to Glycaspis brimblecombei
Morphological leaf characteristics in host and non-host species
The morphological leaf characters, excluding the adaxial stomatal features (due to their absence in some species without stomata on the adaxial leaf surface), were analysed using PCA. The PCA scores plot of the leaf morphological characters of the 12 eucalypt species separated the species into two distinct clusters explaining 72.1% of the variation in the x-dimension (Fig. 6). The most susceptible host species (E. microcorys, E. sideroxylon, E. camaldulensis and E. tereticornis) clustered in a separate cluster from most of the non-host species. However, three non-host species (C. citriodora, E. ovata, and E. viminalis) clustered together with the highly susceptible species. The morphological features that were most significantly different between the two clusters were the abaxial guard cell area, as well as the adaxial and abaxial stomatal density. Random forest analysis also showed that these three morphological features contributed significantly to the separation of the clusters, with adaxial stomatal density explaining 40% of the variation between the two clusters in the PCA (Table 2). Taken together, these data show that susceptible hosts of S. cf. plicatuloides and G. brimblecombei have some common morphological features that could play an important role in the host-selection behaviour of the two lerp psyllid species.
Morphological leaf traits of 12 eucalypt species. Two-dimensional PCA scores plot of the combined dataset excluding adaxial stomatal traits. Confidence intervals are presented by lighter coloured circles. The heat map shows the relative average differences between traits for each eucalypt species. Traits that differed significantly (p < 0.05) between the species based on a one-way ANOVA are shown. The data were log10-transformed for the PCA and ANOVA, but untransformed data are shown in the heat map. Five replicates per species were included. The images were generated by metaboanalyst and manually enhanced. S Eucalypt species that were highly susceptible to Spondyliaspis cf. plicatuloides, G Eucalypt species that was highly susceptible to Glycaspis brimblecombei
Discussion
The aim of this study was to identify morphological and chemical characteristics of eucalypt leaves associated with the host preference of the shell lerp psyllid, S. cf. plicatuloides, in relation to the red gum psyllid, G. brimblecombei. To achieve this goal, preferred and non-preferred eucalypt host species were identified based on natural infestations of the lerp psyllids in a common garden. Various morphological and chemical characteristics of hosts and non-hosts were evaluated to identify traits in eucalypt leaves that contribute to the host-selection behaviour of these lerp psyllids. The findings of this study revealed that host leaf surface traits, such as leaf wax metabolites and stomatal density, influence the host preference of both psyllid species.
In this study, E. tereticornis was most preferred by G. brimblecombei and less preferred by S. cf. plicatuloides. Eucalyptus tereticornis was also reported elsewhere as a highly preferred eucalypt host of G. brimblecombei, whilst E. viminalis and E. sideroxylon were not infested by this psyllid (Lucia et al. 2016). Susceptibility of eucalypts to S. cf. plicatuloides has not been studied in detail before. This study revealed that E. camaldulensis and two non-hosts of G. brimblecombei, E. microcorys and E. sideroxylon, were the most preferred hosts of S. cf. plicatuloides.
Volatile compounds did not associate with psyllid host-selection behaviour in evergreen eucalypt hosts
It was previously demonstrated that psyllids utilise visual cues such as leaf shape, size and colour, as well as light polarisation to find hosts (White 1970; Farnier et al. 2014, 2015, 2018; Farnier and Steinbauer 2016; Paris et al. 2017). However, not all psyllids can use visual cues, as some have poor visual acuity (Land 1997; Farnier et al. 2015) and must rely on olfactory cues instead. For example, a neurophysiological study of the carrot psyllid (Trioza apicalis), a host-alternating psyllid, revealed that it has several types of olfactory receptor neurons that are extremely selective to a few important substances found both in conifers and carrots (winter and summer hosts, respectively) (Kristoffersen et al. 2008). Bergamotene (a sesquiterpene), a carrot-specific chemical, is assumed to influence the preference of T. apicalis for their summer host (Valterová et al. 1997). Similar findings have been reported in another host-alternating psyllid, the apple psyllid (Cacopsylla picta). This psyllid alternates between deciduous summer hosts (e.g. apples) and coniferous winter hosts (e.g. pine and spruce), which share important volatiles (Gross and Mekonen 2015). In these examples, it has been proposed that the specificity of psyllid olfactory systems is the result of adaptive trade-offs that result in lower detection thresholds for a few essential volatiles that promote seasonal migration (Farnier et al. 2018). However, increased emissions of volatiles in fruit trees infected by Phytoplasmas have also been shown to significantly increase their attractiveness to psyllids (Mayer et al. 2008a, b, 2011; Mann et al. 2012; Aksenov et al. 2014; Patt et al. 2018), suggesting that these psyllids might detect a broader range of compounds.
In contrast, our results showed no strong associations between the volatile profiles of individual eucalypt species and psyllid host preference. These findings agree with previous studies where psyllids that utilise evergreen eucalypt hosts are reported to show no positive response to host plant volatiles. For example, Farnier et al. (2018) reported that Ctenarytaina eucalypti, C. bipartita, Anoeconeossa bundoorensis and G. brimblecombei did not show positive chemotactic responses to HPVs, despite previous physiological data that showed neuronal responses of C. eucalypti to HPVs (Yuvaraj et al. 2013). It was also shown that psyllids move passively downwind and that long-distance migrations are primarily wind-assisted (Martini et al. 2018) without any olfactory orientation. Dispersed individuals thus land multiple times before reaching a eucalypt that is identified as a suitable host by short-range cues such as leaf morphology or surface wax composition.
Surface traits likely influence eucalypt psyllid host choice
After randomly landing on a potential host, eucalypt psyllids need to evaluate its suitability for feeding and oviposition. Psyllids feed on leaves by inserting their mouthparts into the tissues through the stomata (Woodburn and Lewis 1973). Our results showed that eucalypts susceptible to S. cf. plicatuloides and G. brimblecombei had stomata on both sides of the leaves. The other common leaf morphological features shared amongst the main hosts of S. cf. plicatuloides and G. brimblecombei were a slightly larger abaxial guard cell width and a slightly lower abaxial stomatal density. De Oliveira Del et al. (2022) reported that E. camaldulensis and E. grandis, the most preferred species of G. brimblecombei have stomata on both sides of the leaves whereas two other resistant species did not have stomata on their adaxial surface. A similar pattern was observed in the current study except that, E. paniculata, a species with low infestations by both G. brimblecombei and S. cf. plicatuloides had no stomata on the adaxial leaf surface. Furthermore, other morphological features examined in the current study showed no differences between main hosts and non-hosts and this agrees with de Oliveira Del et al. (2022) who showed that epidermis, spongy and palisade parenchyma thickness are not associated with resistance or susceptibility to G. brimblecombei.
The ecological significance of plant cuticular waxes for plant–insect interactions has been extensively studied. Epicuticular wax mostly consists of alkanes and long-chain alcohols (Jetter et al. 2000) and these compounds play a key role in plant–insect interactions (Schoonhoven et al. 2005; Sarkar and Barik 2014). Analysis of surface-associated metabolites separated the eucalypt species into two distinct clusters and the most susceptible host species (E. microcorys, E. sideroxylon, E. camaldulensis and E. tereticornis) clustered together with E. botryoides and E. paniculata, which were occasionally infested by S. cf. plicatuloides. Docosene was the most significant with rf = 0.12, indicating that the metabolite accounts for 12% of the differences between the preferred and non-preferred host clusters in the PCA. Earlier studies have shown that the presence and abundance of non-structural epicuticular waxes greatly influenced psyllid adhesion to leaf surfaces (Brennan and Weinbaum 2001a), probing (Brennan and Weinbaum 2001b, c), orientation behaviour (Brennan and Weinbaum 2001d) as well as oviposition preferences (Brennan et al. 2001). As a result, it is likely that surface wax structure and composition as well as stomatal abundance may play an important role in the host location of both G. brimblecombei and S. cf. plicatuloides. However, these traits did not explain the differences in the preferred hosts of the two psyllid species, and it is still not known why S. cf. plicatuloides prefers E. camaldulensis, E. microcorys and E. sideroxylon above E. tereticornis, the preferred host of G. brimblecombei.
Polar internal leaf metabolites did not associate with psyllid host-selection behaviour
Once a psyllid has landed on a susceptible host plant and has started probing the stomata, host acceptance may be influenced by its nutritional quality and the abundance of defence chemicals (Dethier 1982; Visser 1986). In our study, the most susceptible hosts of S. cf. plicatuloides and G. brimblecombei differed significantly in their polar leaf metabolite profiles, but no clear associations were observed between these traits and psyllid host preference.
Whilst probing the leaf for the phloem tissue, psyllids could come into contact with noxious plant defence metabolites. Plant secondary metabolites are abundant in Eucalyptus foliage, with phenolics, particularly tannins, being the most abundant (Fox and Macauley 1977; Hillis 1966; Marsh et al. 2017). These compounds might be deterrent to the psyllids (Steinbauer and Tanha 2023). We detected phenolic acids, condensed tannin monomers and flavonoids, but their abundances did not correlate with host preferences of S. cf. plicatuloides and G. brimblecombei.
As phloem feeders, S. cf. plicatuloides and G. brimblecombei may require high levels of sugars and amino acids in the phloem of the host. However, levels of the most abundant sugars, including sucrose, glucose, galactose and lactose, were not positively associated with the host preferences of the two psyllid species. Sugar contents in host eucalypt species were generally lower or similar to that of the non-host species. Nevertheless, it is known that phloem sap contains more sugars than needed by the insects and is excreted in the form of honeydew, whilst amino acid concentrations, which we did not detect in our analysis, are limited (Sandström and Moran 1999; Douglas 2006). Sugary honeydew allows for the growth of sooty moulds of the genera Capnodium, Fumago, and Scorias, which might negatively affect the psyllids, by reducing photosynthesis in host leaves. Lower levels of sugars might, therefore, reduce sooty mould growth and be beneficial in increasing host leaf longevity.
Conclusion
Elucidating the mechanisms underpinning host preference in psyllids is a crucial step towards identifying resistant eucalypt varieties that can be planted to manage these pests. Phytochemical examination of volatile and polar metabolites in leaves of the tested eucalypt species did not clearly separate the preferred hosts from non-hosts of the two lerp psyllids, S. cf. plicatuloides and G. brimblecombei and thus could not sufficiently explain the host choices of the two lerp-forming psyllids. However, morphological analysis resulted in identification of abaxial guard cell area, the adaxial and abaxial stomatal density as main features shared amongst the preferred hosts of the two psyllids. Furthermore, cuticular wax composition revealed seven metabolites shared by the preferred hosts and these metabolites separated them from the non-hosts. Specific combinations of some morphological features and wax metabolites could potentially explain different levels of infestation and the choices of females during oviposition in both psyllid species. However, although this study revealed common features positively influencing host selection of both psyllid species, we did not identify any traits explaining the differences in host-selection behaviour between S. cf. plicatuloides and G. brimblecombei. Future studies will address this question by including a larger variety of susceptible species and hybrids as well as using additional methods, including amino acid analyses and electron microscopy of leaf surfaces and stomatal apertures.
Data availability
Data can be obtained from the corresponding author.
References
Aksenov AA, Martini X, Zhao W, Stelinski LL, Davis CE (2014) Synthetic blends of volatile, phytopathogen-induced odorants can be used to manipulate vector behavior. Front Ecol Evol 2:78. https://doi.org/10.3389/fevo.2014.00078
Beadle CL, Turnbull CRA (1992) Comparative growth rates of Eucalyptus in native forest and in plantation monoculture. In: Calder IR, Hall RC, Adlard PG (eds) Growth and water use of forest plantations. Wiley, Chichester, pp 318–331
Bella S, Rapisarda C (2013) First record from Greece of the invasive red gum lerp psyllid Glycaspis brimblecombei Moore (Hemiptera: Psyllidae) and its associated parasitoid Psyllaephagus bliteus Riek (Hymenoptera: Encyrtidae). Redia 96:33–35
Brennan EB, Weinbaum SA (2001a) Effect of epicuticular wax on adhesion of psyllids to glaucous juvenile and glossy adult leaves of Eucalyptus globulus labillardière. Aust J Entomol 40:270–277. https://doi.org/10.1046/j.1440-6055.2001.00229.x
Brennan EB, Weinbaum SA (2001b) Stylet penetration and survival of three psyllid species on adult leaves and “waxy” and “de-waxed” juvenile leaves of Eucalyptus globulus. Entomol Exp Appl 100:355–363. https://doi.org/10.1046/j.1570-7458.2001.00883.x
Brennan EB, Weinbaum SA (2001c) Performance of adult psyllids in no-choice experiments on juvenile and adult leaves of Eucalyptus globulus. Entomol Exp Appl 100:179–185. https://doi.org/10.1046/j.1570-7458.2001.00862.x
Brennan EB, Weinbaum SA (2001d) Psyllid responses to colored sticky traps and the colors of juvenile and adult leaves of the heteroblastic host plant Eucalyptus globulus. Environ Entomol 30:365–370. https://doi.org/10.1603/0046-225X-30.2.365-30.2.365
Brennan EB, Hrusa GF, Weinbaum SA, Levison JW (2001) Resistance of Eucalyptus species to Glycaspis brimblecombei (Homoptera: Psyllidae) in the San Francisco Bay area. Pan-Pac Entomol 77:249–253
Brooker MIH (2000) A new classification of the genus Eucalyptus L’Her. (Myrtaceae). Aust Syst Bot 13:79–148. https://doi.org/10.1071/SB98008
Burdon JJ, Chilvers GA (1974) Fungal and insect parasites contributing to niche differentiation in mixed species stands of eucalypt saplings. Aust J Bot 22:103–114. https://doi.org/10.1071/BT9740103
Bush SJ, Slippers B, Nesser S, Harney M, Dittrich-Schröder G, Hurley BP (2016) Six recently recorded Australian insects associated with Eucalyptus in South Africa. Afr Entomol 24:539–544. https://doi.org/10.4001/003.024.0539
Bush SJ, Slippers B, Brett P, Hurley BP (2020) Eucalypt susceptibility towards the invasive Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae) in South Africa. South for 82:243–252. https://doi.org/10.2989/20702620.2020.1824556
da Silveira AC, de Andrade de Siqueira GL, Mayer FM, da Silveira Lazzarotto SR, Miguel OG, Zini CA, de Queiroz DL, Lazzarotto M (2021) Thermal tool to evaluate essential oil composition of different Eucalyptus genotypes in relation to Glycaspis brimblecombei susceptibility (Hemiptera: Aphalaridae). J Therm Anal Calorim 147:7363–7371. https://doi.org/10.1007/s10973-021-11027-3
de Oliveira Del FH, Wilcken CF, Domingues MM, Favoreto AL, Rodella RA, Pereira AI, Silva WM, Serrão JE, Zanuncio JC (2022) Anatomical indicators of Eucalyptus spp. resistance to Glycaspis brimblecombei (Hemiptera: Aphalaridae). PeerJ 10:e13346. https://doi.org/10.7717/peerj.13346
de Queiroz DL, Majer J, Burckhardt D, Zanetti R, Fernandez JIR, Queiroz EC, Garrastazu M, Fernandes BV, Anjos N (2013) Predicting the geographical distribution of Glycaspis brimblecombei (Hemiptera: Psylloidea) in Brazil. Austr J Entomol 52:20– 30. https://doi.org/10.1111/aen.12001
Dethier VG (1982) Mechanisms of host-plant recognition. Entomol Exp Appl 31:49–56. https://doi.org/10.1111/j.1570-7458.1982.tb03118.x
Dittrich-Schröder G, Garnas JR, Arriagada-Cares D, Ahumada R, Hurley BP, Lawson SA, Slippers B (2021) Diversity and introduction history of Glycaspis brimblecombei reflects a history of bridgeheads and distinct invasions. Front for Glob Change 4:783603. https://doi.org/10.3389/ffgc.2021.783603
Döring TF (2014) How aphids find their host plants, and how they don’t. Ann Appl Biol 165:3–26. https://doi.org/10.1111/aab.12142
Douglas A (2006) Phloem-sap feeding by animals: problems and solutions. J Exp Bot 57:747–754. https://doi.org/10.1093/jxb/erj067
Eldridge K, Davidson J, Harwood C, van Wyk G (1993) Eucalypt domestication and breeding. Clarendon Press, Oxford, p 288
Farnier K, Steinbauer MJ (2016) Elevated anthocyanins protect young Eucalyptus leaves from high irradiance but also indicate foliar nutritional quality to visually attuned psyllids. Ecol Entomol 41:168–181. https://doi.org/10.1111/een.12286
Farnier K, Dyer AG, Steinbauer MJ (2014) Related but not alike: Not all Hemiptera are attracted to yellow. Front Ecol Evol 2:67. https://doi.org/10.3389/fevo.2014.00067
Farnier K, Dyer AG, Taylor GS, Peters RA, Steinbauer MJ (2015) Visual acuity trade-offs and microhabitat-driven adaptation of searching behaviour in psyllids (Hemiptera: Psylloidea: Aphalaridae). J Exp Biol 218:1564–1571. https://doi.org/10.1242/jeb.120808
Farnier K, Davies NW, Steinbauer MJ (2018) Not led by the nose: volatiles from undamaged Eucalyptus hosts do not influence psyllid orientation. InSects 9:166–179. https://doi.org/10.3390/insects904016
Ferreira RDA, Blaziza AAB, Anzolin MG, Firmino-Winckler DC (2009) Flutuação populacional do psilídeo-de-concha Glycaspis brimblecombei Moore (Hemiptera: Psyllidae) EM Eucalyptus spp. No Munícipio De Garça. SP Rev Cient Eletrônica Eng Florest 8:29–46
Fox LR, Macauley BJ (1977) Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 29:145–162
Gonçalves JLM, Alvares CA, Higa AR, Silva LD, Alfenas AC, Stahl J, Ferraz SFB, Lima WP, Brancalion PHS, Hubner A, Bouillet JD, Laclau J, Nouvellon Y, Epron D (2013) Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For Ecol Manag 301:6–27. https://doi.org/10.1016/j.foreco.2012.12.030
Grattapaglia D, Vaillancourt RE, Shepherd M, Thumma BR, Foley W, Külheim C, Potts BM, Myburg AA (2012) Progress in Myrtaceae genetics and genomics: Eucalyptus as the pivotal genus. Tree Genet Genom 8:463–508. https://doi.org/10.1007/s11295-012-0491-x
Gross J, Mekonen N (2015) Plant odours influence the host finding behaviour of apple psyllids (Cacopsylla picta; C. melanoneura). IOBC Wprs Bull 28:351–355
Hillis WE (1966) Variation in polyphenol composition within species of Eucalyptus L’Herit. Phytochemistry 5:541–556. https://doi.org/10.1016/S0031-9422(00)83632-8
Hollis D (2004) Australian Psylloidea: jumping plant lice and lerp insects. Australian Biological Resources Study, Canberra
Huerta A, Faúndez M, Araya JE (2010) Susceptibility of Eucalyptus spp. to an introduced infestation of red gum lerp psyllid Glycaspis brimblecombei Moore (Hemiptera: Psyllidae) in Santiago. Chile Cienc Investig Agrar 37:27–33
Hurley BP, Garnas J, Wingfield MJ, Branco M, Richardson DM, Slippers B (2016) Increasing numbers and intercontinental spread of invasive insects on eucalypts. Biol Invasions 18:921–933. https://doi.org/10.1007/s10530-016-1081-x
Jetter R, Schaffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23:619–628. https://doi.org/10.1046/j.1365-3040.2000.00581.x
Kristoffersen L, Larsson MC, Anderbrant O (2008) Functional characteristics of a tiny but specialized olfactory system: olfactory receptor neurons of carrot psyllids (Homoptera: Triozidae). Chem Senses 33:759–769. https://doi.org/10.1093/chemse/bjn034
Ladiges PY, Udovicic F, Nelson G (2003) Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae. J Biogeogr 30:989–998. https://doi.org/10.1046/j.1365-2699.2003.00881.x
Land MF (1997) Visual acuity in insects. Annu Rev Entomol 42:147–177. https://doi.org/10.1146/annurev.ento.42.1.147
Lourençon TV, Alakurtti S, Virtanen T, Jääskeläinen A, Liitiä T, Hughes M, Magalhães WLE, Muniz GIB, Tamminen T (2020) Phenol-formaldehyde resins with suitable bonding strength synthesized from “less-reactive” hardwood lignin fractions. Holzforschung 74:175–183. https://doi.org/10.1515/hf-2018-0203
Lowman MD, Heatwole H (1987) The impact of defoliating insects on the growth of eucalypt saplings. Aust J Ecol 12:175–181. https://doi.org/10.1111/j.1442-9993.1987.tb00938.x
Lucia A, Naspi C, Zerba E, Masuh H (2016) Infestation of Glycaspis brimblecombei Moore on thirteen Eucalyptus species and their relationship with the chemical composition of essential oils. J inSects. https://doi.org/10.1155/2016/6340579
Makunde PT, Slippers B, Burkhardt D, de Queiroz DL, Lawson SA, Hurley BP (2020) Current and potential threat of psyllids (Hemiptera: Psylloidea) on eucalypts. South for 82:233–242. https://doi.org/10.2989/20702620.2020.1813650
Makunde PT, Slippers B, Bush SJ, Hurley BP (2023) Biology of the invasive shell lerp psyllid, Spondyliaspis cf. plicatuloides (Froggatt) (Hemiptera: Aphalaridae). Afr Entomol 31:e13747. https://doi.org/10.17159/2254-8854/2023/a13747
Mann RS, Ali JG, Hermann SL, Tiwari S, Pelz-Stelinski KS, Alborn HT, Stelinski LL (2012) Induced release of a plant-defense volatile “deceptively” attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog 8:e1002610. https://doi.org/10.1371/journal.ppat.1002610
Marsh KJ, Kulheim C, Blomberg SP, Thornhill AH, Miller JT, Wallis IR, Nicolle D, Salminen JP, Foley WJ (2017) Genus-wide variation in foliar polyphenolics in eucalypts. J Phytochem 144:197–207. https://doi.org/10.1016/j.phytochem.2017.09.014
Martini X, Rivera M, Hoyte A, Sétamou M, Stelinski L (2018) Effects of wind, temperature, and barometric pressure on Asian citrus psyllid (Hemiptera: Liviidae) flight behavior. J Econ Entomol 111:2570–2577. https://doi.org/10.1093/jee/toy241
Matos M, Mattos BD, Cademartori PHG, Lourencon TV, Hansel F, Zanoni PRS, Yamamoto CI, Magalhaes WLE (2020) Pilot-scaled fast-pyrolysis conversion of Eucalyptus wood fines into products: Discussion toward possible applications in biofuels, materials and precursors. Bioenerg Res 13:411–412. https://doi.org/10.1007/s12155-020-10094-y
Mayer CJ, Vilcinskas A, Gross J (2008a) Phytopathogen lures its insect vector by altering host plant odor. J Chem Ecol 34:1045–1049. https://doi.org/10.1007/s10886-008-9516-1
Mayer CJ, Vilcinskas A, Gross J (2008b) Pathogen-induced release of plant allomone manipulates vector insect behavior. J Chem Ecol 34:1518–1522. https://doi.org/10.1007/s10886-008-9564-6
Mayer CJ, Vilcinskas A, Gross J (2011) Chemically mediated multitrophic interactions in a plant-insect vector-phytoplasma system compared with a partially non vector species. Agric for Entomol 13:25–35. https://doi.org/10.1111/j.1461-9563.2010.00495.x
Nicolle D (2022) Classification of the Eucalypts (Angophora, Corymbia and Eucalyptus) Version 6. http://www.dn.com.au/Classification-Of-The-Eucalypts.pdf. Accessed 22 Apr 2022
Noble IR (1989) Ecological traits of the Eucalyptus L’Herit subgenera Monocalyptus and Symphyomyrtus. Aust J Bot 37:207–224. https://doi.org/10.1071/BT9890207
Ouvrard D (2023) Psyl’list—the world Psylloidea database. http://www.hemiptera-databases.com/psyllist. Accessed 23 Apr 2023. https://doi.org/10.5519/0029634
Paine TD, Dahlsten DL, Millar JG, Hoddle MS, Hanks LM (2000) UC scientists apply IPM techniques to new Eucalyptus pests. Calif Agric 54:8–13. https://doi.org/10.3733/ca.v054n06p8
Paine TD, Steinbauer MJ, Lawson SA (2011) Native and exotic pests of Eucalyptus: a worldwide perspective. Annu Rev Entomol 56:181–201. https://doi.org/10.1146/annurev-ento-120709-144817
Paris TM, Allan SA, Udell BJ, Stansly PA (2017) Evidence of behavior-based utilization by the Asian citrus psyllid of a combination of UV and green or yellow wavelengths. PLoS ONE 12:e0189228. https://doi.org/10.1371/journal.pone.0189228
Patt JM, Robbins PS, Niedz R, McCollum G, Alessandro R (2018) Exogenous application of the plant signalers methyl jasmonate and salicylic acid induces changes in volatile emissions from citrus foliage and influences the aggregation behavior of Asian citrus psyllid (Diaphorina citri), vector of Huanglongbing. PLoS ONE 13:e0193724. https://doi.org/10.1371/journal.pone.0193724
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: http://www.R-project.org/
Sandström J, Moran N (1999) How nutritionally imbalanced is phloem sap for aphids? In: Proceedings of the 10th international symposium on insect-plant relationships. Springer, Dordrecht. pp 203–210
Sarkar N, Barik A (2014) Alkanes from bitter gourd as allelochemicals in olfactory responses of Epilachna dodecastigma (Wied.). Allelopathy J 33:43–51
Schoonhoven LM, Van Loon JJ, Dicke M (2005) Insect-plant biology. Oxford University Press on Demand, New York
Specht RL, Brouwer YM (1975) Seasonal shoot growth of Eucalyptus species in the Brisbane area of Queensland (with notes on shoot growth and litter fall in other areas of Australia). Aust J Bot 23:459–474. https://doi.org/10.1071/BT9750459
Steinbauer MJ, Tanha R (2023) Abundance of white lace lerp psyllids on understorey and canopy river red gums and relationships with foliar sugars and tannins. Agric for Entomol 25:20–37. https://doi.org/10.1111/afe.12528
Turnbull JW (1999) Eucalypt plantations. New for 17:37–52
Valterová I, Nehlin G, Borg-Karlson AK (1997) Host plant chemistry and preferences in egg-laying Trioza apicalis (Homoptera, Psylloidea). Biochem Syst Ecol 25:477–491. https://doi.org/10.1016/S0305-1978(97)00028-8
Visser JH (1986) Host odor perception in phytophagous insects. Annu Rev Entomol 31:121–144. https://doi.org/10.1146/annurev.en.31.010186.001005
White TCR (1970) The nymphal stage of Cardiaspina densitexta (Homoptera: Psyllidae) on leaves of Eucalyptus fasciculosa. Aust J Zool 18:273–293. https://doi.org/10.1071/ZO9700273
Wingfield MJ, Slippers B, Hurley BP, Coutinho TA, Wingfield BD, Roux J (2008) Eucalypt pests and diseases: growing threats to plantation productivity. South for 70:139–144
Wingfield MJ, Brockerhoff EG, Wingfield BD, Slippers B (2015) Planted forest health: The need for a global strategy. Science 349:832–836. https://doi.org/10.1126/science.aac6674
Woinarski JCZ, Cullen JM (1984) Distribution of invertebrates on foliage in forests of south-eastern Australia. Austr J Ecol 9:207–232. https://doi.org/10.1111/j.1442-9993.1984.tb01359.x
Woodburn TL, Lewis EE (1973) A comparative histological study of the effects of feeding by nymphs of four psyllid species on the leaves of eucalypts. J Aust Entomol Soc 12:134–138. https://doi.org/10.1111/j.1440-6055.1973.tb01650.x
Yuvaraj JK, Andersson MN, Steinbauer MJ, Farnier K, Anderbrant O (2013) Specificity and sensitivity of plant odor-detecting olfactory sensory neurons in Ctenarytaina eucalypti (Sternorrhyncha: Psyllidae). J Insect Physiol 59:542–551. https://doi.org/10.1016/j.jinsphys.2013.03.004
Acknowledgements
We thank members of Tree Protection Co-operative Program (TPCP), DSI-NRF Centre of Excellence in Plant Health Biotechnology, University of Pretoria, South Africa for financial support.
Funding
Open access funding provided by University of Pretoria.
Author information
Authors and Affiliations
Contributions
PTM—conceptualisation, material preparation, investigation, data curation, formal analysis, writing—original draft and editing, writing—review and editing. JCJ—material preparation, methodology, data curation, formal analysis, writing—review and editing. BS—funding acquisition, conceptualisation, methodology, supervision, writing—review and editing. BPH—funding acquisition, conceptualisation, methodology, supervision, writing—review and editing. AH—funding acquisition, conceptualisation, methodology, data curation, formal analysis, supervision, writing—review and editing. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have not disclosed any competing interests.
Additional information
Communicated by Günther Raspotnig.
Supplementary Information
Below is the link to the electronic supplementary material.
49_2023_387_MOESM1_ESM.tif
Supplementary file1 Supplementary figure 1: Spatial arrangement of eucalypt species on the study site and the selected eucalypt species for the study are colour coded. Yellow = Eucalypt species that were preferred by S. cf. plicatuloides only, Blue = Eucalypt species that were preferred by G. brimblecombei only, Pink = Eucalypt species that were preferred by both S. cf. plicatuloides and G. brimblecombei, Red = Eucalypt species that were non-preferred by both S. cf. plicatuloides and G. brimblecombei. (TIF 457 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makunde, P.T., Joubert, J.C., Slippers, B. et al. Leaf surface traits may influence host specificity in psyllids of Eucalyptus, Spondyliaspis cf. plicatuloides (Froggatt) and Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae). Chemoecology 33, 83–98 (2023). https://doi.org/10.1007/s00049-023-00387-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-023-00387-x