Abstract
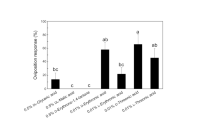
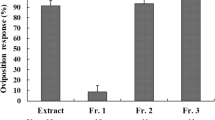
The sulfur butterfly, Colias erate, utilizes various legumes as host plants. We examined the chemical constituents of its primary host plant, Trifolium repens (white clover), to identify phytochemicals inducing oviposition by C. erate females. Since one of the four aqueous subfractions prepared from a methanolic extract of the plant has previously been shown to be the most responsible for the oviposition-stimulatory activity exerted by the plant, chemical analyses were conducted of the fraction concerned. Activity-directed fractionation of the subfraction by ion-exchange chromatography revealed that the key substance(s) resided in the neutral fraction. Preparative TLC of the neutral fraction and subsequent spectral analyses identified d-(+)-pinitol, glycerin, methyl β-d-glucoside, and myo-inositol as characteristic components together with ubiquitous sugars (e.g., sucrose and glucose). Of these, only pinitol singly evoked significant oviposition responses at concentrations over 0.05%. In dual-choice bioassays, however, females laid significantly more eggs on pinitol solutions admixed with glycerin or methyl β-d-glucoside than on pinitol alone. Two cyanoglucosides, linamarin, and lotaustralin, occurring in the other aqueous subfractions, also synergistically increased the oviposition response in combination with pinitol. The results clearly indicated that pinitol is a crucial oviposition stimulant involved in host recognition, while glycerin, methyl β-d-glucoside, linamarin, and lotaustralin function as synergists. We further examined the oviposition responses of C. erate females to aqueous fractions, along with their chemical compositions, that had been prepared from five other host plants and a non-host plant, Aristolochia debilis (Aristolochiaceae), on which oviposition occasionally took place in an outdoor cage during the experiments. The plant species accepted by ovipositing females were all found to contain pinitol in amounts enough to induce egg laying by the butterfly, thus leading to the conclusion that pinitol serves as the essential mediator in recognizing and accepting potential host plants.





Similar content being viewed by others
References
Berenbaum MR, Feeny PP (2008) Chemical mediation of host-plant specialization: the papilionid paradigm. In: Tilmon K (ed) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, California, pp 3–19
Chew FS, Renwick JAA (1995) Host-plant choice in Pieris butterflies. In: Cardé RT, Bell WJ (eds) Chemical ecology of insects 2. Chapman and Hall, New York, pp 214–238
Chew FS, Robbins RK (1984) Egg-laying in butterflies. In: Vane-Wright IR, Ackery PR (eds) The biology of butterflies. Academic Press, London, pp 65–79
Dreyer DL, Binder RG, Chan BG, Waiss AC Jr, Hartwig EE, Beland GL (1979) Pinitol, a larval growth inhibitor for Heliothis zea in soybeans. Experientia 35:1182–1183
Endo S, Nihira I (1990) Larval food of Japanese butterflies. Group Tamamushi, Tokyo (in Japanese)
Feeny P, Sachdev K, Rosenberry L, Cater M (1988) Luteolin 7-O-(6′′-O-malonyl)-β-d-glucoside and trans-chlorogenic acid: Oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439–3448
Ford CW (1984) Accumulation of low molecular weight solutes in water-stressed tropical legumes. Phytochemistry 23:1007–1015
Honda K (1990) Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J Chem Ecol 16:325–337
Honda K (1995) Chemical basis of differential oviposition by lepidopterous insects. Arch Insect Biochem Physiol 30:1–23
Honda K (2005) Larval feeding habit and host selection. In: Honda K, Kato Y (eds) Biology of butterflies. University of Tokyo Press, Tokyo, pp 255–301 (in Japanese)
Honda K, Nishii W, Hayashi N (1997) Oviposition stimulants for sulfur butterfly, Colias erate poliographus: cyanoglucosides as synergists involved in host preference. J Chem Ecol 23:323–331
Honda K, Ômura H, Hayashi N, Abe F, Yamauchi T (2001) Oviposition-stimulatory activity of phenanthroindolizidine alkaloids of host-plant origin to a danaid butterfly, Ideopsis similis. Physiol Entomol 26:6–10
Honda K, Ômura H, Hayashi N, Abe F, Yamauchi T (2004) Conduritols as oviposition stimulants for the danaid butterfly, Parantica sita, identified from a host plant, Marsdenia tomentosa. J Chem Ecol 30:2285–2296
Honda K, Ômura H, Hori M, Kainoh Y (2010) Allelochemicals in plant-insect interactions. In: Mander L, Lui H-W (eds) Comprehensive natural products II. Chemistry and biology. Elsevier, Oxford, pp 563–594
Huang X, Renwick JAA, Sachdev-Gupta K (1994) Oviposition stimulants in Barbarea vulgaris for Pieris rapae and P. napi oleracea: isolation, identification and differential activity. J Chem Ecol 20:423–438
Hudlicky T, Cebulak M (1993) Cyclitols and their derivatives: a handbook of physical, spectral, and synthetic data. VCH Publishers Inc, New York
McManus MT, Bieleski RL, Caradus JR, Barker DJ (2000) Pinitol accumulation in mature leaves of white clover in response to a water deficit. Environ Exp Bot 43:11–18
Miller JS, Feeny PP (1989) Interspecific differences among swallowtail larvae (Lepidoptera: Papilionidae) in susceptibility to aristolochic acids and berberine. Ecol Entomol 14:287–296
Nakayama T, Honda K, Ômura H, Hayashi N (2003) Oviposition stimulants for the tropical swallowtail butterfly, Papilio polytes, feeding on a rutaceous plant, Toddalia asiatica. J Chem Ecol 29:1621–1634
Nishida R, Fukami H (1989) Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alcinous. J Chem Ecol 15:2565–2575
Nishida R, Ohsugi T, Kokubo S, Fumaki H (1987) Oviposition stimulants of a Citrus-feeding swallowtail butterfly, Papilio xuthus L. Experientia 43:342–344
Numata A, Hokimoto K, Shimada A, Yamaguchi H, Takaishi K (1979) Plant constituents biologically active to insects. I. Feeding stimulants for the larvae of the yellow butterfly. Eurema hecabe mandarina. Chem Pharm Bull 27:602–608
Numata A, Yamaguchi H, Hokimoto K, Ohtani M, Takaishi K (1985) Host-plant selection by the yellow butterfly larvae, Eurema hecabe mandarina (Lepidoptera: Pieridae): attractants and arrestants. Appl Entomol Zool 20:314–321
Orthen B, Popp M (2000) Cyclitols as cryoprotectants for spinach and chickpea thylakoids. Environ Exp Bot 44:125–132
Papaj DR, Feeny P, Sachdev-Gupta K, Rosenberry L (1992) D-(+)-pinitol, an oviposition stimulant for the pipevine swallowtail butterfly, Battus philenor. J Chem Ecol 18:799–815
Reese JC, Chan BG, Waiss AC Jr (1982) Effects of cotton condensed tannin, maysin (corn) and pinitol (soybeans) on Heliothis zea growth and development. J Chem Ecol 8:1429–1436
Renwick JAA, Chew FS (1994) Oviposition behavior in Lepidoptera. Annu Rev Entomol 39:377–400
Sachdev-Gupta K, Feeny PP, Carter M (1993) Oviposition stimulants for the pipevine swallowtail butterfly, Battus philenor (Papilionidae), from an Aristolochia host plant: synergism between inositols, aristolochic acids and a monogalactosyl diglyceride. Chemoecology 4:19–28
Stefanescu C, Jubany J, Dantart J (2006) Egg–laying by the butterfly Iphiclides podalirius (Lepidoptera, Papilionidae) on alien plants: a broadening of host range or oviposition mistake? Anim Biodiv Conserv 29:83–90
Traynier RMM, Truscott RJW (1991) Potent natural egg-laying stimulant for cabbage butterfly Pieris rapae. J Chem Ecol 17:1371–1380
van Loon JJA, Blaakmeer A, Griepink FC, van Beek TA, Schoonhoven LM, de Groot A (1992) Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: Pieridae). Chemoecology 3:39–44
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honda, K., Minematsu, H., Muta, K. et al. d-Pinitol as a key oviposition stimulant for sulfur butterfly, Colias erate: chemical basis for female acceptance of host- and non-host plants. Chemoecology 22, 55–63 (2012). https://doi.org/10.1007/s00049-011-0098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-011-0098-y