Abstract
Neurodegenerative disorders are caused by the progressive death of neuronal cells in specific regions of the brain and spinal cord. The most common neurodegenerative disorders are Alzheimer’s disease and Parkinson’s disease. The inhibition of enzymes that metabolise neurotransmitter amines is an important approach in the treatment of these disorders and monoamine oxidase (MAO) B inhibitors have thus been used for the treatment of Parkinson’s disease. Inhibitors of the MAO-A isoform, in turn, are used clinically for the treatment of affective (e.g., major depression) and anxiety disorders. Recent studies have shown that benzothiazole derivatives act as potent MAO inhibitors. Based on these findings, the present study group synthesised thirteen 2-methylbenzo[d]thiazole derivatives and evaluated their in vitro MAO inhibition properties. The results showed that the benzothiazole derivatives were potent and selective inhibitors of human MAO-B, with all compounds exhibiting IC50 values < 0.017 µM. The most potent MAO-B inhibitor (4d) had an IC50 value of 0.0046 µM, while the most potent MAO-A inhibitor (5e) had an IC50 value of 0.132 µM. It may be concluded that active benzothiazole derivatives may serve as potential leads for the development of MAO inhibitors for the treatment of neuropsychiatric and neurodegenerative disorders.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative disorders occur when the neuronal cells in the central nervous system deteriorate over time, lose their function and die off gradually [1]. These disorders become more prevalent as people age with Alzheimer’s disease and Parkinson’s disease being two of the most common neurodegenerative disorders [2, 3]. The ultimate goal of the treatment of these disorders is to slow or stop the degenerative process and neuronal death, however current treatment only provides symptomatic alleviation. The discovery of disease-modifying drugs for the treatment of neurodegenerative disorders is an important field of research [4, 5]. In this respect, the monoamine oxidase (MAO) enzymes have been linked to mechanisms that underlies neurodegeneration [6]. MAO exists as two isoforms, MAO-A and MAO-B. Both isoforms are present in the brain where their primary function is to metabolise neurotransmitter amines. These flavoenzymes are found on the outer membranes of mitochondria and are responsible for catalysing the oxidative deamination of biogenic amines and other neurotransmitters, a process which produces hydrogen peroxide (H2O2), ammonia (NH3) and aldehydes as products [7, 8]. Some of these products (e.g., H2O2) may lead to oxidative stress and neuronal damage [9]. By reducing H2O2 formation inhibitors of MAO may attenuate the neurodegenerative process.
Currently, MAO inhibitors are used for the symptomatic treatment of depression and Parkinson’s disease [10, 11]. As a symptomatic treatment for Parkinson’s disease, MAO-B inhibitors elevate central dopamine levels and are commonly used in combination with levodopa, the amino acid metabolic precursor of dopamine. MAO inhibitors were among the first pharmacological treatments for depression. Inhibitors that are selective for the MAO-A isoform have been used for decades as antidepressant agents and are also effective in the treatment of other neuropsychiatric disorders. These agents act by enhancing central levels of serotonin and noradrenaline [12, 13]. The mechanism by which MAO inhibitors bind to the enzyme, as well as their specificity of inhibition differ. Phenelzine, tranylcypromine, selegiline and rasagiline are examples of irreversibly acting MAO inhibitors, while moclobemide and safinamide bind reversibly to the enzyme [13]. Certain inhibitors are non-specific MAO inhibitors (e.g., phenelzine, tranylcypromine). Examples of specific MAO-A inhibitors are moclobemide and clorgyline, whereas selegiline, rasagiline and safinamide are specific MAO-B inhibitors [13].
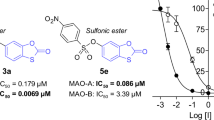
Benzothiazole (1) derivatives have been reported to inhibit the MAO enzymes (Fig. 1) [14,15,16,17,18]. For example, benzothiazole derivative 2 inhibits MAO-B with an IC50 value of 0.028 µM. Interestingly, the benzothiazole derivative, pramipexole (3), has been approved by the Food and Drug Administration for the treatment of patients suffering from restless legs syndrome, which forms part of the clinical profile of advanced Parkinson’s disease [19]. Numerous benzothiazole derivatives have been developed and are now available on the market, each of which treats a different condition [20]. These include the agents riluzole, ethoxzolamide and zopolrestat. For the current study, thirteen benzothiazole derivatives were synthesised in response to an academic interest in the development of novel MAO inhibitors, and as a method of assessing the potential of this class of compounds as in vitro inhibitors of human MAO-A and MAO-B [21].
For this study, thirteen 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g) were synthesised and their in vitro MAO-A and MAO-B inhibition properties were evaluated. Substitution with the methyl on C2 was included due to the commercial availability of the key reagents, 2-methylbenzo[d]thiazol-6-ol (6) or 2-methylbenzo[d]thiazol-5-ol (7). These reagents were readily reacted with an appropriate benzyl bromide to yield derivatives substituted on either C5 or C6 with benzyloxy substituents. The benzyloxy substituent is present in numerous MAO inhibitors (e.g., safinamide) and significantly contributes to inhibitor stabilisation in MAO-B by interacting with the entrance cavity of the enzyme [22]. The MAO-B active site consists of two cavities, an entrance cavity which leads to a larger substrate cavity where catalysis takes place. Cavity-spanning compounds that bind to both MAO-B cavities often display potent inhibition. This is largely due to the productive van der Waals interactions between an appropriate substituent (e.g., benzyloxy moiety) and the lipophilic environment of the entrance cavity. The part of the inhibitor that binds to the substrate cavity often contains hydrogen bonding groups for interaction with conserved waters and polar residues. In this respect the benzothiazole moiety might be suitable. Figure 2 illustrates how the study compounds might interact with the active site of MAO-B, and thus act as inhibitors of this enzyme.
Results and discussion
Chemistry
The 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g) were synthesised according to the literature method [23]. 2-Methylbenzo[d]thiazol-6-ol (6) or 2-methylbenzo[d]thiazol-5-ol (7) was dissolved in DMF after which the appropriate benzyl bromide derivative and K2CO3 were added (Scheme 1). The reaction mixture was stirred at room temperature for 24 h. After completion, ethanol was added to the reaction mixture and the solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate and allowed to recrystallise. NMR and MS were used to characterise the 2-methylbenzo[d]thiazole derivatives, and HPLC was used to determine the purity of the compounds (see supplementary material) [23]. The 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g) were synthesised with yields ranging from 9–82%.
Potencies of monoamine oxidase inhibition
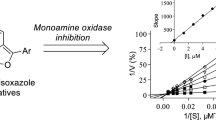
The MAO inhibition potencies of the 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g) were investigated by measuring the IC50 values for the in vitro inhibition of recombinant human MAO-A and MAO-B. Kynuramine was used as a substrate for both MAO isoforms, and fluorescence spectrophotometry was used to quantify the MAO-generated product of kynuramine oxidation, 4-hydroxyquinoline (Supplementary) [24, 25]. The IC50 values are presented in Table 1 and the results showed that 2-methylbenzo[d]thiazole derivatives were inhibitors of both MAO-A and MAO-B. Certain 2-methylbenzo[d]thiazole derivatives (e.g., 4f, 5d) were highly specific for the MAO-B isoform and showed very weak inhibition of MAO-A at the maximal concentration tested (100 µM). For MAO-A, the most potent inhibition was observed for 4d (IC50 = 0.218 μM) and 5e (IC50 = 0.132 μM). Compounds 4d and 5e were substituted with the nitro and chloro groups, respectively, with the most potent inhibitor (5e) being substituted on C5 while 4d was substituted on the C6 position. Other potent MAO-A inhibitors identified in this study were 4e, 5b, 5c and 5g with IC50 values < 1 μM. No clear trends were observed regarding the position of the benzyloxy moiety (C5 vs. C6) and substituent on the benzyloxy ring (e.g., Br, CN, NO2, Cl, CH3). It was however noticeable that both CH3 substituted compounds (4f, 5f) were weak MAO-A inhibitors with 4f showing minimal inhibition at 100 µM. Furthermore, the unsubstituted homologues (4a, 5a) were in most instances weaker MAO-A inhibitors compared to compounds with substituents on the benzyloxy ring (4a vs. 4b–e; 5a vs. 5b,c,e,g). This suggests that substitution on the benzyloxy ring is in most cases beneficial for MAO-A inhibition.
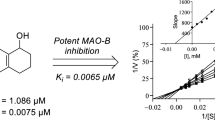
For the inhibition of MAO-B, compounds 4d (IC50 = 0.0046 μM), 5c (IC50 = 0.0056 μM), 5d (IC50 = 0.0052 μM) and 5e (IC50 = 0.0054 μM) displayed the most potent inhibition. It was interesting to observe that compounds 4d and 5d were substituted with the 4-nitrobenzyl moiety on the C6 and C5 positions, respectively. The 4-nitrobenzyl moiety is thus a suitable substituent to obtain potent inhibition of MAO-B irrespective of the position (C5 vs. C6) of the benzyloxy substitution. However, all of the 2-methylbenzo[d]thiazole derivatives proved to be good potency MAO-B inhibitors with IC50 < 0.017 µM. The 2-methylbenzo[d]thiazole derivatives were thus specific inhibitors of MAO-B over the MAO-A isoform in each instance. The weakest MAO-B inhibition was displayed by compound 4b and 5g with IC50 values of 0.017 µM. As for MAO-A, no clear trends were observed regarding the position of the benzyloxy moiety (C5 vs. C6) and substituent on the benzyloxy ring (e.g., Br, CN, NO2, Cl, CH3). Compound 5e may be highlighted as a good potency inhibitor of both MAO isoforms although it was approximately 24-fold more potent for MAO-B. Compounds 4f and 5d may be highlighted as specific MAO-B inhibitors since they displayed IC50 ≤ 0.0076 µM for the inhibition of MAO-B while exhibiting minimal MAO-A inhibition at 100 µM.
Molecular docking studies
Molecular docking simulations were used to investigate the binding orientations and interactions of the 2-methylbenzo[d]thiazole derivatives within the MAO-A and MAO-B active sites. For this purpose, compound 5e was investigated since it acted as a good potency inhibitor of both MAO-A and MAO-B. The life sciences molecular modelling suite, Discovery Studio 3.1 was used for all simulations, and the X-ray crystal structures of human MAO-A and MAO-B complexed to harmine and safinamide, respectively, were used [22, 26].
The binding orientations of 5e to MAO-A and MAO-B are presented in Fig. 3. Compound 5e binds to MAO-A with the benzothiazole moiety in proximity to the FAD and the tyrosine residues of the ‘aromatic sandwich’ (Tyr407 and Tyr444). The benzyloxy side chain extends towards the entrance of the MAO-A active site. The inhibitor is not within hydrogen bond distance to any active site waters or polar residues and is stabilised by van der Waals interactions to mainly Phe208, Gln215, Ile335, Phe352, Tyr407 and Tyr444. The interaction with Gln215 is predicted to be particularly favourable.
Compound 5e binds to the active site of MAO-B also with the benzothiazole moiety in proximity to the FAD and the tyrosine residues of the ‘aromatic sandwich’ (Tyr398 and Tyr435). The benzyloxy side chain extends into the entrance cavity. The orientation of 5e is virtually superimposable on that of safinamide, and 5e is thus also a cavity-spanning inhibitor. Such compounds are known to bind with high affinity to the MAO-B active site and would explain the potent inhibition observed for 5e and the other compounds of this study [27]. Although the inhibitor does not form hydrogen bond interactions with the MAO-B active site, it is stabilised by pi interactions between Ile199 and the benzyloxy ring, and between Tyr398 and the thiazole ring. Van der Waals interactions between the inhibitor and residues Cys172, Ile199, Gln206, Ile316, Tyr326, Tyr398 and Tyr435 occur.
Conclusion
For this study, a series of 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g) were synthesised and their human MAO-A and MAO-B inhibition properties were investigated. With the exception of two compounds (4f, 5d), all compounds inhibited the MAO enzymes. The most potent MAO-A inhibitors, 4d and 5e, exhibited IC50 values of 0.218 µM and 0.132 µM, respectively. The most potent MAO-B inhibitors were 4d (IC50 = 0.0046 μM), 5c (IC50 = 0.0056 μM), 5d (IC50 = 0.0052 μM) and 5e (IC50 = 0.0054 μM). In fact, all compounds were found to be submicromolar MAO-B inhibitors. Compounds 4f and 5d could be highlighted as specific MAO-B inhibitors since they displayed minimal MAO-A inhibition at 100 µM. Compound 5e could be highlighted as a good potency inhibitor of both MAO isoforms. Based on this study, it may be concluded that 2-methylbenzo[d]thiazole derivatives are a class of potent MAO inhibitors that may be used in the future treatment of conditions such as Parkinson’s disease, Alzheimer’s disease and depression.
Experimental section
Materials and instrumentation
The reagents required for the chemical synthesis were purchased from Sigma-Aldrich while 2-methylbenzo[d]thiazol-6-ol (6) and 2-methylbenzo[d]thiazol-5-ol (7) were from Ambeed. Merck provided deuterated dimethyl sulfoxide (DMSO-d6) for use in nuclear magnetic resonance (NMR) spectroscopy. 1H and 13C NMR spectra were recorded on a Bruker Avance III 600 spectrometer at frequencies of 600 MHz and 151 MHz, respectively. Each sample was analysed using dimethyl sulfoxide (DMSO-d6) as solvent. Chemical shifts (δ) are reported in parts per million (ppm) and were referenced to the solvent signal at 2.5 and 39.5 ppm for 1H and 13C NMR, respectively. Multiplicities are abbreviated as follows: s (singlet), d (doublet), dd (doublet of doublets), t (triplet) and m (multiplet). The coupling constant (J) is given in hertz (Hz). A Bruker micrOTOF-Q II mass spectrometer, performing in the atmospheric-pressure chemical ionisation (APCI) mode, was used to record high resolution mass spectra (HRMS). A Büchi melting point B-545 instrument was used to measure melting points. TLC (silica gel 60 F254; Merck) was used to monitor the progress and completion of the chemical reactions. The mobile phase consisted of a mixture of n-hexane and ethyl acetate (3:2). HPLC analyses were carried out to determine the purities of the synthesised compounds according to the published procedure [28].
All the reagents used for the biochemical analyses, including the enzymes and substrates, were obtained from Sigma-Aldrich. A Varian Cary Eclipse fluorescence spectrophotometer (Agilent Technologies) and a SpectraMax iD3 multi-mode microplate reader (Molecular Devices) were used to measure fluorescence intensities.
The synthesis of 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g)
The protocol for the synthesis of 2-methylbenzo[d]thiazole derivatives (4a–f, 5a–g) has been reported [23]. 2-Methylbenzo[d]thiazol-6-ol (6) or 2-methylbenzo[d]thiazol-5-ol (7) (3.026 mmol) was dissolved in N,N-dimethylformamide (DMF; 10 mL) and the appropriate benzyl bromide derivative (4.539 mmol) was added. Anhydrous potassium carbonate (K2CO3; 6.05 mmol) was added and the reaction was stirred for 24 h at room temperature. Ethanol was finally added to the reaction mixture (±50 mL) and the solvent was removed under reduced pressure. Ethyl acetate was added to the residue and the solution was allowed to recrystallise. The crystals were collected by filtration, washed with n-hexane, and air-dried.
6-(Benzyloxy)-2-methylbenzo[d]thiazole (4a)
The title compound (dark grey crystal shards) was produced from 2-methylbenzo[d]thiazol-6-ol and benzyl bromide. Yield: 62%, mp: 70–74 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.80 (d, J = 8.7 Hz, 1H), 7.72–7.65 (m, 1H), 7.48 (d, J = 7.5 Hz, 2H), 7.44–7.37 (m, 2H), 7.37–7.30 (m, 1H), 7.14 (dd, J = 8.8, 2.7 Hz, 1H), 5.16 (s, 2H), 2.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 164.70, 156.38, 147.95, 137.32, 136.92, 128.91, 128.37, 128.27, 122.86, 115.99, 106.43, 70.28, 20.03. Purity: 97%. APCI-HRMS m/z Calc. for C15H14NOS: 256.0791, found 256.0780 (MH+).
6-((4-Bromobenzyl)oxy)-2-methylbenzo[d]thiazole (4b)
The title compound (light purple powder-like crystals) was produced from 2-methylbenzo[d]thiazol-6-ol and 4-bromobenzyl bromide. Yield: 61%, mp: 113–115 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.80 (d, J = 8.9 Hz, 1H), 7.68 (d, J = 2.6 Hz, 1H), 7.60 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 8.1 Hz, 2H), 7.14 (dd, J = 8.9, 2.4 Hz, 1H), 5.15 (s, 2H), 2.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 164.81, 156.16, 148.04, 136.92, 136.81, 131.84, 130.35, 122.89, 121.48, 115.97, 106.52, 69.46, 20.04. Purity: 95%. APCI-HRMS m/z Calc. for C15H13BrNOS: 333.9896, found 333.9885 (MH+).
4-(((2-Methylbenzo[d]thiazol-6-yl)oxy)methyl)benzonitrile (4c)
The title compound (dark purple crystal shards) was produced from 2-methylbenzo[d]thiazol-6-ol and 4-(bromomethyl)benzonitrile. Yield: 80%, mp: 134–136 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.88 (d, J = 7.8 Hz, 2H), 7.81 (d, J = 8.9 Hz, 1H), 7.70 (d, J = 2.3 Hz, 1H), 7.67 (d, J = 7.9 Hz, 2H), 7.17 (dd, J = 8.9, 2.3 Hz, 1H), 5.29 (s, 2H), 2.75 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 164.95, 155.98, 148.14, 143.15, 136.93, 132.89, 128.62, 122.95, 119.20, 115.93, 111.01, 106.58, 69.31, 20.04. Purity: 100%. APCI-HRMS m/z Calc. for C16H13N2OS: 281.0743, found 281.0729 (MH+).
2-Methyl-6-((4-nitrobenzyl)oxy)benzo[d]thiazole (4d)
The title compound (yellowish crystal shards) was produced from 2-methylbenzo[d]thiazol-6-ol and 4-nitrobenzyl bromide. Yield: 32%, mp: 150–152 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.28 (d, J = 7.8 Hz, 2H), 7.82 (d, J = 8.7 Hz, 1H), 7.75 (d, J = 8.1 Hz, 2H), 7.71 (d, J = 2.6 Hz, 1H), 7.19 (dd, J = 8.8, 2.7 Hz, 1H), 5.35 (s, 2H), 2.75 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.00, 155.94, 148.19, 147.51, 145.27, 136.94, 128.78, 124.09, 122.97, 115.94, 106.62, 69.07, 20.04. Purity: 100%. APCI-HRMS m/z Calc. for C15H13N2O3S: 301.0641, found 301.0644 (MH+).
6-((4-Chlorobenzyl)oxy)-2-methylbenzo[d]thiazole (4e)
The title compound (light purple powder-like crystals) was produced from 2-methylbenzo[d]thiazol-6-ol and 4-chlorobenzyl bromide. Yield: 65%, mp: 101–103 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.80 (d, J = 8.8 Hz, 1H), 7.69 (d, J = 2.6 Hz, 1H), 7.51 (d, J = 8.7 Hz, 2H), 7.47 (d, J = 8.7 Hz, 2H), 7.14 (dd, J = 8.9, 2.5 Hz, 1H), 5.17 (s, 2H), 2.74 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 164.80, 156.18, 148.03, 136.92, 136.39, 132.95, 130.06, 128.92, 122.89, 115.97, 106.51, 69.42, 20.04. Purity: 96%. APCI-HRMS m/z Calc. for C15H13ClNOS: 290.0401, found 290.0409 (MH+).
2-Methyl-6-((4-methylbenzyl)oxy)benzo[d]thiazole (4f)
The title compound (sparkling purple crystals) was produced from 2-methylbenzo[d]thiazol-6-ol and 4-methylbenzyl bromide. Yield: 77%, mp: 123–125 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.79 (d, J = 8.8 Hz, 1H), 7.68 (d, J = 2.6 Hz, 1H), 7.36 (d, J = 7.7 Hz, 2H), 7.21 (d, J = 7.6 Hz, 2H), 7.12 (dd, J = 8.8, 2.5 Hz, 1H), 5.11 (s, 2H), 2.74 (s, 3H), 2.31 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 164.64, 156.40, 147.90, 137.62, 136.90, 134.27, 129.45, 128.37, 122.83, 116.01, 106.42, 70.18, 21.24, 20.03. Purity: 97%. APCI-HRMS m/z Calc. for C16H16NOS: 270.0947, found 270.0958 (MH+).
5-(Benzyloxy)-2-methylbenzo[d]thiazole (5a)
The title compound (beige powder-like crystals) was produced from 2-methylbenzo[d]thiazol-5-ol and benzyl bromide. Yield: 78%, mp: 76–78 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.89 (d, J = 8.7 Hz, 1H), 7.54 (d, J = 2.5 Hz, 1H), 7.51–7.47 (m, 2H), 7.43–7.37 (m, 2H), 7.36–7.31 (m, 1H), 7.11 (dd, J = 8.8, 2.5 Hz, 1H), 5.20 (s, 2H), 2.76 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.56, 157.83, 154.67, 137.43, 128.90, 128.30, 128.19, 127.53, 122.72, 115.22, 106.83, 70.06, 20.26. Purity: 88%. APCI-HRMS m/z Calc. for C15H14NOS: 256.0791, found 256.0784 (MH+).
5-((4-Bromobenzyl)oxy)-2-methylbenzo[d]thiazole (5b)
The title compound (small yellow/beige crystals) was produced from 2-methylbenzo[d]thiazol-5-ol and 4-bromobenzyl bromide. Yield: 78%, mp: 121–123 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.89 (d, J = 8.7 Hz, 1H), 7.60 (d, J = 8.3 Hz, 2H), 7.53 (d, J = 2.4 Hz, 1H), 7.45 (d, J = 8.3 Hz, 2H), 7.10 (dd, J = 8.8, 2.5 Hz, 1H), 5.18 (s, 2H), 2.77 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.65, 157.60, 154.64, 136.93, 131.83, 130.29, 127.68, 122.77, 121.40, 115.20, 106.89, 69.22, 20.27. Purity: 85%. APCI-HRMS m/z Calc. for C15H13BrNOS: 333.9896, found 333.9899 (MH+).
4-(((2-Methylbenzo[d]thiazol-5-yl)oxy)methyl)benzonitrile (5c)
The title compound (small, thin off-white crystal shards) was produced from 2-methylbenzo[d]thiazol-5-ol and 4-(bromomethyl)benzonitrile. Yield: 26%, mp: 162–164 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.91 (d, J = 8.8 Hz, 1H), 7.87 (d, J = 8.2 Hz, 2H), 7.68 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 2.5 Hz, 1H), 7.13 (dd, J = 8.8, 2.5 Hz, 1H), 5.32 (s, 2H), 2.77 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.74, 157.46, 154.65, 143.30, 132.87, 128.55, 127.89, 122.84, 119.18, 115.14, 110.97, 106.95, 69.13, 20.27. Purity: 92%. APCI-HRMS m/z Calc. for C16H13N2OS: 281.0743, found 281.0739 (MH+).
2-Methyl-5-((4-nitrobenzyl)oxy)benzo[d]thiazole (5d)
The title compound (small yellow crystal shards) was produced from 2-methylbenzo[d]thiazol-5-ol and 4-nitrobenzyl bromide. Yield: 9%, mp: 178–180 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.27 (d, J = 7.1 Hz, 2H), 7.92 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.55 (s, 1H), 7.15 (d, J = 8.7 Hz, 1H), 5.38 (s, 2H), 2.77 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.80, 157.39, 154.63, 147.47, 145.42, 128.73, 127.92, 124.08, 122.89, 115.14, 106.92, 68.86, 20.28. Purity: 98%. APCI-HRMS m/z Calc. for C15H13N2O3S: 301.0641, found 301.0627 (MH+).
5-((4-Chlorobenzyl)oxy)-2-methylbenzo[d]thiazole (5e)
The title compound (large yellow/white crystal) was produced from 2-methylbenzo[d]thiazol-5-ol and 4-chlorobenzyl bromide. Yield: 81%, mp: 102–106 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.90 (d, J = 8.8 Hz, 1H), 7.53 (d, J = 2.5 Hz, 1H), 7.51 (d, J = 8.5 Hz, 2H), 7.49–7.44 (m, 2H), 7.10 (dd, J = 8.8, 2.5 Hz, 1H), 5.20 (s, 2H), 2.76 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.65, 157.61, 154.63, 136.49, 132.87, 130.00, 128.91, 127.65, 122.78, 115.19, 106.84, 69.16, 20.27. Purity: 90%. APCI-HRMS m/z Calc. for C15H13ClNOS: 290.0401, found 290.0412 (MH+).
2-Methyl-5-((4-methylbenzyl)oxy)benzo[d]thiazole (5f)
The title compound (white crystal shards) was produced from 2-methylbenzo[d]thiazol-5-ol and 4-methylbenzyl bromide. Yield: 31%, mp: 91–93 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.88 (d, J = 8.9 Hz, 1H), 7.52 (d, J = 2.6 Hz, 1H), 7.37 (d, J = 7.5 Hz, 2H), 7.20 (d, J = 7.6 Hz, 2H), 7.08 (dd, J = 8.8, 2.7 Hz, 1H), 5.14 (s, 2H), 2.77 (s, 3H), 2.31 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.53, 157.83, 154.65, 137.54, 134.37, 129.45, 128.30, 127.43, 122.69, 115.25, 106.81, 69.93, 21.24, 20.26. Purity: 90%. APCI-HRMS m/z Calc. for C16H16NOS: 270.0947, found 270.0933 (MH+).
3-(((2-Methylbenzo[d]thiazol-5-yl)oxy)methyl)benzonitrile (5g)
The title compound (grey powder-like granules) was produced from 2-methylbenzo[d]thiazol-5-ol and 3-(bromomethyl)benzonitrile. Yield: 82%, mp: 105–107 °C. 1H NMR (600 MHz, DMSO-d6) δ 7.96 (s, 1H), 7.91 (d, J = 8.8 Hz, 1H), 7.85–7.80 (m, 2H), 7.63 (t, J = 7.8 Hz, 1H), 7.55 (d, J = 2.5 Hz, 1H), 7.13 (dd, J = 8.7, 2.5 Hz, 1H), 5.27 (s, 2H), 2.77 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.73, 157.49, 154.65, 139.22, 132.88, 132.10, 131.49, 130.20, 127.88, 122.82, 119.11, 115.18, 111.94, 106.95, 68.91, 20.27. Purity: 93%. APCI-HRMS m/z Calc. for C16H13N2OS: 281.0743, found 281.0756 (MH+).
MAO activity measurements
MAO activity measurements and inhibition studies were carried out according to the method described in literature [24, 25]. For this purpose, the commercially available recombinant human MAO-A and MAO-B enzymes were used.
Protocol for molecular docking
Molecular docking was carried out according to the protocol previously described [24]. The Discovery Studio 3.1 suite (Accelrys) was used for the simulations and X-ray crystal structures of MAO-A (PDB code: 2Z5X) and MAO-B (PDB code: 2V5Z) bound to harmine and safinamide, respectively, were selected as the protein models [22, 26]. Illustrations were created with the PyMOL molecular graphics system [29].
Abbreviations
- APCI:
-
atmospheric-pressure chemical ionisation
- DMF:
-
N,N-dimethylformamide
- DMSO:
-
dimethyl sulfoxide
- HPLC:
-
high performance liquid chromatography
- HRMS:
-
high resolution mass spectrometry
- MAO:
-
monoamine oxidase
- NMR:
-
nuclear magnetic resonance
- PDB:
-
protein data bank
- SD:
-
standard deviation
- SI:
-
selectivity index
- TLC:
-
thin layer chromatography
References
Hussain R, Zubair H, Pursell S, Shahab M. Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018;8:177. https://doi.org/10.3390/brainsci8090177.
Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020;25:5789. https://doi.org/10.3390/molecules25245789.
Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. https://doi.org/10.1016/s0896-6273(03)00568-3.
Morato X, Pytel V, Jofresa S, Ruiz A, Boada M. Symptomatic and disease-modifying therapy pipeline for Alzheimer’s disease: towards a personalized polypharmacology patient-centered approach. Int J Mol Sci. 2022;23:9305. https://doi.org/10.3390/ijms23169305.
Poewe W, Seppi K, Marini K, Mahlknecht P. New hopes for disease modification in Parkinson’s Disease. Neuropharmacology. 2020;171:108085. https://doi.org/10.1016/j.neuropharm.2020.108085.
Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol. 2006;147:S287–96. https://doi.org/10.1038/sj.bjp.0706464.
Ramsay RR. Monoamine Oxidases: The biochemistry of the proteins as targets in medicinal chemistry and drug discovery. Curr Top Med Chem. 2012;12:2189–209.
Ramsay RR. Inhibitor design for monoamine oxidases. Curr Pharm Des. 2013;19:2529–39. https://doi.org/10.2174/1381612811319140004.
Behl T, Kaur D, Sehgal A, Singh S, Sharma N, Zengin G, et al. Role of monoamine oxidase activity in Alzheimer’s disease: an insight into the therapeutic potential of inhibitors. Molecules. 2021;26:3724. https://doi.org/10.3390/molecules26123724.
Henchcliffe C, Schumacher HC, Burgut FT. Recent advances in Parkinson’s disease therapy: use of monoamine oxidase inhibitors. Expert Rev Neurother. 2005;5:811–21. https://doi.org/10.1586/14737175.5.6.811.
Naoi M, Maruyama W, Shamoto-Nagai M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis. J Neural Transm. 2018;125:53–66. https://doi.org/10.1007/s00702-017-1709-8.
Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35:703–16. https://doi.org/10.1007/s40263-021-00832-x.
Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. https://doi.org/10.1038/nrn1883.
Nam MH, Park M, Park H, Kim Y, Yoon S, Sawant VS, et al. Indole-substituted benzothiazoles and benzoxazoles as selective and reversible MAO-B inhibitors for treatment of Parkinson’s disease. ACS Chem Neurosci. 2017;8:1519–29. https://doi.org/10.1021/acschemneuro.7b00050.
Çevik UA, Osmaniye D, Saglik BN, Levent S, Çavusoglu BK, Karaduman AB, et al. Synthesis of new benzothiazole derivatives bearing thiadiazole as monoamine oxidase inhibitors. J Heterocyclic Chem. 2020;57:2225–33. https://doi.org/10.1002/jhet.3942.
Cao ZC, Wang XY, Zhang TL, Fu XW, Zhang F, Zhu J. Discovery of novel 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives as multifunctional MAO-B inhibitors for the treatment of Parkinson’s disease. J Enzym Inhib Med Ch. 2023;38:2159957. https://doi.org/10.1080/14756366.2022.2159957.
Ilgin S, Osmaniye D, Levent S, Saglik BN, Acar Cevik U, Cavusoglu BK, et al. Design and Synthesis of New Benzothiazole Compounds as Selective hMAO-B Inhibitors. Molecules. 2017;22:2187. https://doi.org/10.3390/molecules22122187.
Turan G, Osmaniye D, Saglik BN, Çevik UA, Levent S, Çavusoglu BK, et al. Synthesis and monoamine oxidase A/B inhibitory evaluation of new benzothiazole-thiazolylhydrazine derivatives. Phosphorus Sulfur. 2020;195:491–7. https://doi.org/10.1080/10426507.2020.1722667.
Benbir G, Guilleminault C. Pramipexole: new use for an old drug - the potential use of pramipexole in the treatment of restless legs syndrome. Neuropsychiatr Dis Treat. 2006;2:393–405. https://doi.org/10.2147/nedt.2006.2.4.393.
Tariq S, Kamboj P, Amir M. Therapeutic advancement of benzothiazole derivatives in the last decennial period. Arch Pharm. 2019;352:e1800170. https://doi.org/10.1002/ardp.201800170.
Guglielmi P, Carradori S, D’Agostino I, Campestre C, Petzer JP. An updated patent review on monoamine oxidase (MAO) inhibitors. Expert Opin Ther Pat. 2022;32:849–83. https://doi.org/10.1080/13543776.2022.2083501.
Binda C, Wang J, Pisani L, Caccia C, Carotti A, Salvati P, et al. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J Med Chem. 2007;50:5848–52. https://doi.org/10.1021/jm070677y.
Spadaro A, Frotscher M, Hartmann RW. Optimization of hydroxybenzothiazoles as novel potent and selective inhibitors of 17beta-HSD1. J Med Chem. 2012;55:2469–73. https://doi.org/10.1021/jm201711b.
Mostert S, Petzer A, Petzer JP. Indanones as high-potency reversible inhibitors of monoamine oxidase. ChemMedChem. 2015;10:862–73. https://doi.org/10.1002/cmdc.201500059.
Weissbach H, Smith TE, Daly JW, Witkop B, Udenfriend S. A rapid spectrophotometric assay of mono-amine oxidase based on the rate of disappearance of kynuramine. J Biol Chem. 1960;235:1160–3.
Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci USA. 2008;105:5739–44. https://doi.org/10.1073/pnas.0710626105.
Hubalek F, Binda C, Khalil A, Li M, Mattevi A, Castagnoli N, et al. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. J Biol Chem. 2005;280:15761–6. https://doi.org/10.1074/jbc.M500949200.
Prinsloo IF, Petzer A, Cloete TT, Petzer JP. Investigation of the monoamine oxidase inhibition properties of benzoxathiolone derivatives. Med Chem Res. 2023;32:827–40. https://doi.org/10.1007/s00044-023-03042-w.
DeLano WL. The PyMOL molecular graphics system. San Carlos, USA: DeLano Scientific; 2002.
Acknowledgements
The NMR and MS spectra were recorded by Danie Otto and Johan Jordaan of the SASOL Centre for Chemistry, North-West University. This work was financially supported by the National Research Foundation of South Africa [Grant specific unique reference numbers (UID) 137997 and PMDS23041894613]. The Grantholders acknowledge that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research are that of the authors, and that the NRF accepts no liability whatsoever in this regard.
Author contributions
Conceptualization: Anél Petzer, Jacobus P Petzer; Methodology: Maryké Shaw, Anél Petzer; Formal analysis and investigation: Anél Petzer, Maryké Shaw; Writing - original draft preparation: Maryké Shaw, Jacobus P Petzer; Writing - review and editing: Anél Petzer, Theunis T Cloete; Funding acquisition: Jacobus P Petzer; Resources: Theunis T Cloete; Supervision: Anél Petzer.
Funding
Open access funding provided by North-West University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaw, M., Petzer, J.P., Cloete, T.T. et al. Synthesis and evaluation of 2-methylbenzothiazole derivatives as monoamine oxidase inhibitors. Med Chem Res (2024). https://doi.org/10.1007/s00044-024-03283-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00044-024-03283-3