Abstract
Cancers present a significant medical problem despite the development of medical and pharmaceutical sciences leading to a search for further therapeutic approaches. One such approach could involve the use of curcumin or its derivatives. Curcumin reveals interesting antineoplastic effects that could help in the treatment of cancer diseases. However, this natural product possesses some limitations which prevent its application in medicine. Among its limitations, it is characterized by poor water solubility, low stability, and unsatisfactory bioavailability. Aiming to improve the pharmacokinetic properties and enhance the biological effects of curcumin, a series of 30 chemical compounds inspired by its structure was synthesized and characterized. New compounds were subjected to a preliminary MTT viability assessment of 5637 and SCaBER bladder cancer cell lines. Some derivatives revealed the cytotoxic activities already at the concentration of 1 µM. The most active compounds showed no significant acute toxicity in the Microtox test. Intracellular uptake on the basis of the fluorescent properties of the new compounds was analyzed. It was also found that the presence of the morpholine group in the structure improved the biological activity of studied curcumin derivatives. As selected compounds could be considered potential drug candidates, further studies are necessary towards recognition of the exact mechanism of cellular action, the in vivo stability, and toxicity.

A series of 30 derivatives of curcumin was synthesized, characterized and subjected to a MTT viability assessment on 5637 and SCaBER bladder cancer cell lines. Some derivatives revealed the cytotoxic activities at the concentration of 1 µM. Compounds were subjected to acute toxicity study (Microtox test) and intracellular uptake analysis. The presence of the morpholine group in the structure was important for the biological activity of studied derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural products are valuable source of compounds with interesting chemical structures and modes of action, as well as having prospective applications in medicine [1]. However, developing new drugs from such natural products is a demanding process with much work and many resources involved. One of the most common natural products found in foods are curcuminoids. They constitute a group of biologically active compounds built on the 1,7-diarylheptane scaffold. Among them, the most abundant is curcumin, present in the rhizome of turmeric (Curcuma longa L.), along with two other derivatives: demethoxycurcumin and bisdemethoxycurcumin. Despite having already discovered a variety of naturally occurring analogs of curcumin, which have been isolated and identified, the amount of synthetically produced ones continues to rise [2].
The medical applications of curcumin were well-known in Ayurvedic medicine in the Indian subcontinent, where it was used to treat various diseases such as asthma, anorexia, cough, liver disease, diabetes, heart disease, and Alzheimer’s disease, as well as in wound healing. Nowadays, curcumin and its derivatives have been employed in various biological studies assessing their anti-infective, anti-inflammatory, antioxidant, hepatoprotective, thrombosuppressive, cardioprotective, anti-arthritic, chemotherapeutic, antimicrobial, and antidiabetic activities [3, 4]. Moreover, the antineoplastic effects of curcuminoids towards various cancers have been examined, including squamous cell lung, breast, pancreas, head and neck, large intestine, and prostate [5].
Ascribed as a microtubule-targeting agent (MIT) [6], it is believed that in the regions where curcumin constitutes a staple part of the diet, it may actually be responsible for the reduced appearance of tumors in the urinary tract [7, 8]. In the in vivo studies, curcumin effectively inhibited implantation and tumor growth in the intra-bladder tumor implant model and prevented the formation of induced bladder cancer in animal models [7, 9, 10].
Literature data show that the anticancer properties of curcuminoids depend on the presence of OH groups in the phenolic ring (at C4 and C4′ positions) [11, 12]. These groups are well known for their antioxidant activity. The methoxy groups at positions C3 and C3′ of curcumin chemical structure increase the antioxidant properties of curcuminoids, whereas the substitution at the C2 and C2′ positions of phenolic rings increases all activities compared to the unsubstituted analogs. The anti-inflammatory activity increases, especially when additional hydroxyl groups are introduced [13]. Moreover, the cyclization in the central part of the compound and the introduction of heteroatoms (oxygen and nitrogen) lead to the formation of derivatives with enhanced antitumor and anti-angiogenic activities. The cytotoxic activity of curcumin derivatives can also be achieved by metal complexation at the β-diketone moiety [14]. Moreover, the attachment of solubilizing groups to the hydroxyls at 4 and 4′ positions is responsible for the cytotoxicity of curcuminoids. In contrast, elimination of one of the methoxy groups stimulates the activity directed against human immunodeficiency virus type one (HIV-1) integrase [12]. The studies performed by Ferrari et al. showed that substitution of β-keto-enolic moiety with an alkyl group leads to increased stability of such derivatives in the physiological conditions compared to parent curcuminoids [15].
Curcumin aggregation impedes its water-solubility and in this way hampers its therapeutic use in acidic and neutral media, whilst in alkaline conditions curcumin decomposition is induced [16, 17]. The low bioavailability of curcumin is further complicated by its fast metabolism to either sulfates or glucuronides that are easily eliminated from the human body [18, 19], or alternatively to hydrogenated analogs whose biological half-life is shorter than curcumin’s. Therefore, it highlights that there is a great need to search for new derivatives with improved pharmacokinetic and pharmacodynamic parameters.
Herein, we present the synthesis, physicochemical and spectral characterization, and biological activity assessment of the series of 30 curcuminoids and quasicurcuminoids. The in vitro studies concern the evaluation of their cytotoxicity against urinary tract neoplastic cell lines. In addition, acute toxicity studies were performed using a bioluminescence-based assay.
Results and discussion
Chemistry
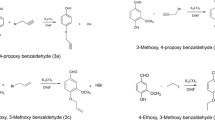
A series of compounds was prepared according to the synthetic routes presented in Fig. 1. In this study, binding the β-diketone moiety within acetylacetone or benzoylacetone with the BF2 group allows to retain the enol form and to avoid the Knoevenagel condensation at the C3 atom [20]. The chemical reaction was performed using a boron trifluoride-diethyl ether complex in dichloromethane (DCM). It is worth noting that the addition of the BF2 group improves the photostability and optical properties of the resulting curcuminoid derivatives [21]. This reaction leads to the BF2-curcumin complexes at a minimum work-up with high yields and purities.
Synthetic pathways and chemical structures of compounds included in the study. Reagents and conditions: (i) BF3·Et2O, CH2Cl2, 40 °C, 24 h; (ii) benzaldehyde derivative, toluene, tributyl borate, N-butylamine, 70 °C, 18–24 h; (iii) methanol, water, sodium oxalate, 140 °C, 8 min, microwave irradiation
Functionalization of aldehydes with morpholine-based building blocks was performed by Williamson ether synthesis in dry N,N-dimethylformamide (DMF) with potassium carbonate acting as a base [22, 23]. The resulting yields of this stage were very good, reaching 91%. Curcuminoids and quasicurcuminoids were synthesized via aldol condensation of modified aldehydes in toluene with tri-n-butyl borate as a dehydrating agent and N-butylamine as a catalyst [24]. Again, the obtained yields were excellent, reaching up to 99% depending on the structure of the compound. The pure product was usually obtained as a colorful precipitate after the condensation reaction. When necessary, non-morpholinated or morpholinated compounds were purified with column chromatography using n-hexane: ethyl acetate or dichloromethane: methanol as mobile phase, respectively. In order to compare the biological activities of BF2 complexes and free diketones, the decomplexation reactions were performed using microwave irradiation. The following conditions were proven to be the most efficient: 140 °C, 8 min, methanol: water 4:1, with the addition of sodium oxalate, giving yields up to 99% [25].
All the synthesized compounds were subjected to experiments such as 1D and 2D Nuclear Magnetic Resonance spectroscopy (NMR): 1H NMR, 13C NMR, Correlated Spectroscopy (COSY), Heteronuclear Single Quantum Coherence (HSQC), Heteronuclear Multiple Bond Correlation (HMBC), High Resolution Mass Spectrometry (HR-MS), Ultraviolet-visible spectroscopy (UV-vis), and melting point (MP) measurement. Detailed 1D and 2D NMR analysis made it possible to assign individual chemical shifts to all atoms and confirm the identity of the obtained compounds. In the HR-MS, the observed signals did not deviate from the expected values by more than 6 ppm. The UV-vis absorption maxima of curcuminoids with the BF2 group were present at about 500 nm, whereas for those curcuminoids without the BF2 group at about 420 nm. Blocked BF2 quasicurcuminoids showed absorption maxima at around 450 nm, while unblocked–around 390 nm. The mentioned differences result from the length of the conjugated system of bonds and the presence of the BF2 group. The purity of compounds was proven to exceed 95% via high-performance liquid chromatography (HPLC) method employing two types of detection (Diode Array Detector–DAD, evaporative light scattering detector–ELSD). 1D NMR spectra and precise descriptions of gradients used in the HPLC technique are available in the Supplementary Information.
Acute toxicity assessment
The compounds were screened for their acute toxicity using the Microtox test. The basis of the Microtox assay is the measurement of the bioluminescence of Aliivibrio fischeri bacteria, which decreases upon contact with a toxic substance. Since the organism involved is a Gram-negative bacteria, Microtox may be considered for an initial assessment of the toxicological properties of tested samples [26, 27]. The results are summarized in Fig. 2 and Table S2 (in the Supplementary Information).
Almost all the tested compounds exerted a decrease in A. fischeri bioluminescence. The negative values obtained in some of the experiments (i.e., 4a-B, 4m-B, 4e-B, 2c-B, 4k-B, 4h-B, 4j-B, 4a, 4g, 4h, 2b) may result from the phenomenon of hormesis and are usually observed for the lower concentrations of the compounds which are insufficient to induce toxic effects [28]. The highest activities in the test were observed for compounds 4b, 4c, 4f, and 4i. For all these derivatives, twenty percent effect concentration (EC20) was lower than 1 µM. An unusual effect was observed for compounds 4b-B, 4c-B, and 2b, for which the effect was lower in the higher concentrations tested. This can presumably be attributed to the aggregation of these non-polar derivatives, as the resulting aggregates can be larger and unable to interact with the cellular targets. The least active compounds were 4b-B, 4h-B, and 4h, which did not reach the arbitrary threshold of 20% bioluminescence decrease, regarded as a limit of toxic concentration [29]. Considering these results, compounds 4b, 4c, 4f, and 4i were selected for further antimicrobial studies.
Cell viability assessment
In preliminary experiments, 30 compounds were studied regarding biological activity toward 5637 and SCaBER cell lines to classify their anticancer potential (Fig. 3). Based on these studies, five compounds with the most potent activity against both cancer cell lines were selected for further experiments to determine half maximal inhibitory concentration (IC50) values.
Screening for the cytotoxic effects of curcumin derivatives in 5637 and SCaBER cell lines. Section A presents data for the 5637 cell line, while section B shows results for the SCaBER cell line. Depending on the cell viability decrease, the tested derivatives were classified as non-active (cell viability ≥71%), moderate (cell viability 51–70%), active (cell viability 11–50%), and very active (cell viability ≤10%)
In addition, the MRC-5 cell line was included in the study to evaluate the selectivity of these compounds. The selected derivatives 2a-B, 2a, 2b-B, 2b, and 2c-B exerted stronger activity than curcumin against cancer cells. In our previous studies, the IC50 values of curcumin calculated for 5637 were 12.65 ± 3.03 µM and 12.86 ± 2.35 µM after incubation lasting 24 and 48 h, respectively [30]. A similar cytotoxic activity of curcumin has also been demonstrated for the SCaBER cell line, for which the IC50 values were 13.14 ± 0.68 µM, and 12.57 ± 0.62 µM after 24 and 48 h treatment, respectively [30]. In contrast, curcumin exhibited lower activity against MRC-5 cells, with IC50 values of 70.35 ± 12.45 µM and 45.33 ± 3.18 µM after 24 and 48 h of incubation, respectively [30]. The IC50 values are presented in Table 1, while Fig. 4 shows the dose-dependent curves.
The dose-response curves plotted for the compounds 2a-B, 2a, 2b-B, 2b, and 2c-B. Cell viability was measured using the MTT assay after 24 and 48 h incubation with tested compounds. The compounds were tested against two human bladder cancer cell lines (5637 and SCaBER) and non-cancerous MRC-5 cells. Data are presented as mean values ± SD calculated from three independent experiments
The most active derivative was 2a-B, with IC50 values of 1.2 ± 0.4 µM and 2.2 ± 0.8 µM for 5637 and SCaBER cells, respectively, after 24 h incubation. However, this compound also decreased the viability of the non-cancerous MRC-5 cells with IC50 values of 8.7 ± 0.9 and 6.7 ± 2.7 µM for incubation lasting 24 and 48 h, respectively. Noteworthy is the fact that a significant difference in cytotoxic activity against 5637 and SCaBER cell lines was not observed.
Additionally, to estimate the specificity towards cancer cells, the selectivity index (SI) was calculated according to the formula available in the literature [31] and presented in Table 1. The SI value for compounds 2a-B and 2b-B exceeded 3 being above the limit at which a compound would be considered worth conducting further research on [32]. Significantly, the SI values for compounds 2a-B and 2b-B exceeded the values calculated for the reference compounds: curcumin and curcumin BF2. This means that the most active compounds, apart from exhibiting higher efficacy than the reference compounds, also demonstrate a better safety profile towards healthy cells and higher specificity towards cancer cells. It should be noted that despite the further improvement of SI, in the case of the tested compounds, SI > 10, which is considered the perfect value, was not achieved. Additional in silico analysis describing the expected toxicity, molecular targets and physicochemical parameters of the selected most active compounds is included in the Supplementary Information. Moreover, the solubility-aggregation assessment of 2a-B and 2b-B was performed and is presented in the Supplementary Information. Interestingly, one of the compounds, namely 2b-B, demonstrates at least 15-33 times better water solubility (20 µg/mL) than the reference curcumin (1.34 µg/mL [33]; 0.6 µg/mL [34]) [35, 36].
Intracellular uptake
Further experiments were designed to assess the intracellular uptake of selected curcumin derivatives 2a-B and 2a. Firstly, absorbance and fluorescence scans were performed to determine the excitation and emission values of compounds 2a-B and 2a (Fig. 5). As previously described for BF2 curcuminoids, the diketo moiety fixed by the BF2 group enhanced stability and resulted in a bathochromic shift in the absorption and emission spectra [30, 37]. The 2a-B shows an absorption maximum in dimethylsulfoxide (DMSO) at around 520 nm, while for compound 2a it appears around 430 nm. Since both compounds are fluorescent, the uptake of both compounds by 5637 cells was studied. A quantitative cellular uptake study revealed that the highest concentration of both compounds occurred after 2 h incubation. The uptake was also confirmed by fluorescent microscopy (Figs. 6 and 7).
The UV-vis absorption and emission spectra of compounds 2a-B and 2a in DMSO (A and B, respectively). The absorbance and fluorescence spectra were measured using the Infinite® M Plex microplate reader (Tecan Trading AG, Männedorf, Switzerland). The standard curves of compounds 2a-B and 2a were prepared in RIPA lysis buffer (C and D, respectively). The fluorescence of compounds 2a-B (excitation and emission wavelength of 470 nm and 605 nm, respectively) and 2a (excitation and emission wavelength of 430 nm and 520 nm, respectively). Data are presented as mean ± SD from five independent experiments. The concentration of compounds 2a-B and 2a in cell lysates: the 5637 cells were incubated with 2a-B (E) and 2a (F) at concentrations of 2 and 8 µM for 0.5, 1, 2, 4, 6, and 8 h. The concentration of compounds was calculated using the standard curves and normalized to µg/mL protein in the sample
Fluorescent images showing cellular uptake of curcumin derivative 2a-B. The 5637 cells were incubated with 2a-B at a concentration of 8 µM. Control refers to cells treated with DMSO. The blue fluorescence corresponds to nuclei stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), and green signals correspond to the tested compounds. The scale bars correspond to 100 µm
Intracellular uptake of curcumin derivative 2a using fluorescence microscopy. The 5637 cells were incubated with 2a at a concentration of 8 µM. Control refers to cells treated with DMSO. The blue fluorescence corresponds to nuclei stained with DAPI, and green signals correspond to the tested compounds. The scale bars correspond to 100 µm
The structure-activity relationship discussion
The research showed that symmetric curcuminoids demonstrate higher anticancer activity than quasi-curcuminoids. The structure-activity relationship analysis of the most active compounds (presented in Table 1) indicates that modification of the β-diketone moiety in the curcumin structure by the BF2 group increases the cytotoxic effect compared to the parental compound - curcumin. However, it was proved only for symmetrical derivatives. Interestingly, asymmetric derivatives 4j-B and 4k-B bearing BF2 moiety and only one morpholine group at the 3- or 4-position of the phenyl ring were inactive. Although morpholine moiety in the phenyl ring increases the cytotoxic activity of symmetrical derivatives slightly, the BF2 incorporation is more critical in decreasing cell viability. This is confirmed by the results obtained for derivative 2c-B. This compound exerted strong activity towards cancer cells with IC50 values of 2.6 ± 0.3 µM, and 2.7 ± 0.4 µM for 5637 and SCaBER cells after 24 h incubation, respectively. It should be highlighted that compound 2c (analog of 2c-B without BF2 moiety) exerted moderate activity against 5637 cells after 24 h, while it was non-active towards SCaBER cells. The incorporation of BF2 as a strategy to increase curcumin cytotoxic effects was also demonstrated by several studies [25, 30].
The next effect that could be verified in our study concerns the presence of the hydroxy and methoxy groups in the phenyl rings. Huang and co-workers reported that depending on cancer cell lineage, the number of methoxy groups on the phenyl ring could modulate cytotoxic activity [38]. The authors found that curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC) exerted similar activity towards osteosarcoma (HOS and U2OS) cancer cells, while curcumin and DMC were more active towards breast (MDA-MB-231) and melanoma (A2080) cells than BDMC [38]. The presence of methoxy groups can modulate the cancer cell death pathways. Curcumin and DMC induced the apoptosis of HOS cells by activating Smad2/3 signaling pathway, while the lack of methoxy groups induced caspase-mediated apoptosis through the Akt signaling pathways. It is well-known that the presence of hydroxy and methoxy substituents in phenyl rings impacts the biological activity of curcumin. On the other hand, Basil et al. showed that bisdehydroxy curcumin (bDHC) is more cytotoxic to human colon carcinoma HCT116 and LOVO cells than curcumin [39]. Moreover, bDHC did not affect the viability of primary fibroblasts. The bDHC induced mitochondria-dependent apoptotic and autophagic cell death pathways [39]. Considering that both the hydroxyl and methoxy groups in the phenyl rings modulate the biological activity of curcumin, a very interesting result is the strong cytotoxicity of the 2c-B (bisdehydroxy- and bisdemethoxy- derivative with BF2 moiety). However, the proposed modification did not secure selectivity towards cancer cells.
Interestingly, the substitution of the hydroxy groups in the phenyl ring with 2‐(morpholin‐4‐yl)ethoxy moieties in the case of derivatives 2a and 2b increased cytotoxic activity against cancer cells compared to the parental compound, while it did not affect the non-cancerous cell line. Furthermore, switching the methoxy and 2‐(morpholin‐4‐yl)ethoxy moieties in positions 3 and 4 of the phenyl ring did not change the activity significantly. Morpholine is a versatile moiety, a privileged pharmacophore, and an outstanding heterocyclic motif widely used in medicinal chemistry [40]. Since 1935, several morpholine-containing drugs have been approved by the FDA, such as Gefitinib (anticancer), Moclobemide (anti-depressant), Doxapram (respiratory stimulant, analeptic agent), Timolol (antiglaucoma, antihypertension), Rivaroxaban (anti-coagulant), and others [41]. The morpholine group provides a balance between lipophilic and hydrophilic properties and improves the pharmacokinetic profile. The presence of oxygen determines the formation of weak hydrogen bonds, while a relatively electron-deficient ring is responsible for hydrophobic interactions [41]. Although morpholine is widely used in medicinal chemistry as a scaffold or substituent, the curcumin modified by morpholine motif is not a very common modification. Roy and co-workers synthesized a stable 1,3-diketone coordinated Ru(II)-p-cymene complex made from curcumin modified at the phenolic -OH by an ethyl morpholine substituent [42]. This functionalization led to selective accumulation in lysosomes and increased stability in physiological conditions [42]. It is known that the morpholine group can increase lysosome specificity [43, 44], as it diffuses into the acidic environment due to the protonation of its amine group [44]. Morpholine is a relatively strong base (pKa 8.7) but weaker than other nitrogen‐containing heterocycles [41]. Moreover, the basicity of the substituted morpholine ring ranges from pKa 6.0 to 7.9. On the other hand, the improved stability might be related to a decrease in the formation of the quinone methide species generated during the degradation of curcumin [42]. Thus, the presence of a morpholine motif in curcumin structure might overcome the well-known curcumin’s limitations, such as low water solubility and instability, and could be a viable strategy for increasing cytotoxicity.
Conclusions
In this work, a series of chemical compounds inspired by the curcumin scaffold were synthesized and subjected to physicochemical and biological studies. The compounds were obtained using known synthetic methods with satisfactory or excellent yields and subjected to standard characterization to confirm the chemical identity. The following methods were used to confirm the structure and assess purity: NMR, ESI-MS, HPLC, UV-VIS, TLC, and melting point.
The acute toxicities of the tested compounds were determined using the Microtox test. Results allowed to conclude that the most active derivatives at anticancer active concentrations do not pose a threat to the natural environment. The MTT test performed on the 5637 and SCaBER cell lines allowed to select compounds presenting the highest in vitro anticancer activity (2a-B, 2a, 2b-B, 2b, 2c-B). The structure-activity relationship analysis of the most active compounds indicates that symmetric curcuminoids demonstrate higher anticancer activity than quasi-curcuminoids. Moreover, it was revealed that modifying the β-diketone part of the parental compound - curcumin with the BF2 group increases the cytotoxic effect. It is worth noting that substituting the hydroxy groups in the phenyl ring of curcumin with 2‐(morpholin‐4‐yl)ethoxy moieties improved the cytotoxic effect against cancer cells, while it did not affect the non-cancerous cell line. Since compounds 2a-B and 2a revealed fluorescent properties, their uptake by 5637 cells was determined, and the highest intracellular concentration was observed after 2 h incubation.
The results provide a solid basis for further studies on these derivatives. It will be necessary to clarify the intracellular mechanism of action of these compounds and to answer whether they also show satisfactory biological activity in the in vivo model.
Materials and methods
The laboratory glassware in which the chemical reactions were carried out was heated in an oven at 140 °C each time before use. The temperature of the water bath in the rotary evaporator did not exceed the temperature of the reaction in which a particular compound was formed with a maximum of 70 °C for reactions carried out in DMF. The reaction temperature was read from the display of a Heidolph MR Hei-Tec magnetic heating stirrer equipped with an external Pt1000 sensor measuring the temperature of the Heat-On heating jacket. Chemical reagents and solvents of high-grade purity were purchased from commercial suppliers (Sigma Aldrich, Fluorochem, Tokyo Chemical Industry Co., Ltd., and Acros Organics) and used without additional processing. Reactions performed under microwave irradiation were carried out using an Anton Paar Monowave 400 microwave reactor (Anton Paar, Graz, Austria). Thin-layer chromatography (TLC) was performed on precoated TLC plates (SiliaPlate TLC, thickness 200 µm, F-254, SiliCycle Inc., Quebec, Canada) and visualized under a UV lamp (λ = 254 and 365 nm). Chromatographic separations were performed on silica gel columns using the flash method–flash column chromatography (FCC), with the use of silica gel as the stationary phase (SiliaFlash P60, particle size 40–63 µm, SiliCycle Inc., Quebec, Canada). Melting points were determined in open glass capillaries using a Stuart SMP10 apparatus (Bibby Sterilin Ltd., Stone, Staffordshire, UK) and are uncorrected. Nuclear magnetic resonance (NMR) spectra (1D: 1H, 13C; 2D: COSY, HSQC, HMBC) were recorded on an Agilent DD2 800 spectrometer (Agilent Technologies, Santa Clara, California, USA) equipped with a 5 mm 1H{13C/15N} probe; data are reported as follows: chemical shift (ppm value), multiplicity (indicated as: br, broad signal; s, singlet; d, doublet; t, triplet; q, quartet; p, quintet; m, multiplet), coupling constants (J) in Hertz (Hz) and integrated intensity. High-resolution mass spectra (HR-MS) were recorded on a Bruker Impact HD apparatus (Bruker Daltonics, Billerica, Massachusetts, USA) operating in electrospray mode (ESI) with positive ionization. Detailed compound data such as NMR spectra, HR-MS spectra and UV-Vis spectra can be found in the Supplementary Information.
Chromatographic purity analysis of compounds was carried out on an Agilent 1260 Infinity II LC System (Agilent Technologies, Bolinem, Germany) equipped with a quaternary pump (model G7111B) and degasser, a vial sampler (model G7129A) set at 20 °C, multicolumn thermostat (model G7116A) set at 35 °C, and detector diode array (DAD) WR, model G7115A. The detection wavelength was adjusted for each tested compound at its absorption maximum. The elution of compounds was achieved on the reverse phase column used as stationary phase (C-18(2) 100 Å Luna, 150 × 4.6 mm ID, 5 µm, Phenomenex®, USA) and a mobile phase with the flow rate 1.0 mL/min based on gradient solvent systems consisting of acetic acid (0.4% in water) (phase A) and acetonitrile (phase B). A sample volume of 10 µL was injected into the column. Gradient conditions for the morpholine-bearing derivatives of curcumin are given in Table S3 (gradient A), and for the other compounds in Table S4 (gradient B) in Supplementary Information.
Reagents used for in vitro experiments, such as Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin-L-glutamine solution, phosphate-buffered saline (PBS), trypsin-EDTA, molecular-grade dimethylsulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), were purchased from Sigma Aldrich (St. Louis, MO, USA). The Roswell Park Memorial Institute 1640 (RPMI) medium and Eagle’s Minimum Essential Medium (EMEM) were purchased from Thermofisher (Waltham, MA, USA). The DMSO for dissolving formazan crystals was obtained from Avantor Performance Materials (Gliwice, Poland).
Synthesis of 2,2-difluoro-6-methyl-4-phenyl-1,3,2-dioxaborinine (3-B) and 2,2‐difluoro‐4,6‐dimethyl‐1,3,2‐dioxaborinine (1-B)
BF2-blocked diketones (acetylacetone and benzoylacetone) were synthesized according to the procedure reported in the literature [24]. For example, benzoylacetone (3, 5.00 g, 30.83 mmol, 1 equiv.) was dissolved in 100 mL of dichloromethane. Then 5.7 mL (6.55 g, 46.25 mmol, 1.5 equiv.) of boron trifluoride diethyl etherate was added and stirred for 24 h at 40 °C under argon. The reaction was cooled to room temperature, and 100 mL of water was added. The organic layer was separated and further extracted with water several times until the pH of the aqueous layer was neutral. Then the organic layer was dried with anhydrous magnesium sulfate and evaporated under reduced pressure to obtain the pure product as a white powder with 85% yield (5.53 g, 26.32 mmol).
Synthesis of morpholine-modified aldehydes
Aldehydes containing a morpholinoethoxy group were synthesized according to the literature and our previous experiments [22, 23]. For example, 3‐methoxy‐4‐[2‐(morpholin‐4‐yl)ethoxy]benzaldehyde (morpholinoethoxyvanillin) was synthesized as follows. First, 750 mg of vanillin (4.93 mmol, 1.0 equiv.), 1.10 g of 4-(2-chloroethyl)morpholine hydrochloride (5.92 mmol, 1.2 equiv.), and 4.08 g of potassium carbonate (29.61 mmol, 6 equiv.) were transferred to a dry round bottom flask. Then, 20 mL of N,N-dimethylformamide (DMF) was added, and the reaction was carried out for 24 h under inert gas at 70 °C with constant stirring. The reaction mixture was cooled to room temperature and filtered to remove potassium carbonate. The filtrate was evaporated to give a yellowish oil which was then purified by column chromatography (DCM: MeOH 20:1), giving a colorless oil with a yield of 91% (1.19 g, 4.49 mmol). The aldehyde was stored in a refrigerator (2−8 °C) for further use.
Synthesis of curcuminoids and quasicurcuminoids (aldol condensation)
The synthesis of quasicurcuminoids and curcuminoids followed the aldol condensation mechanism and was performed according to the procedure described earlier in the literature [24]. For example, the synthesis of 4c-B has been described below. Initially, 300 mg of 3-B (1.43 mmol, 1.0 equiv.) and 217 mg of aldehyde (isovanillin, 1.43 mmol, 1 equiv.) were transferred to a dry round bottom flask. Then 10 mL of toluene, 358 µL of tributyl borate (328 mg, 1.43 mmol, 1.0 equiv.), and 14 µL of N-butylamine (10 mg, 0.14 mmol, 0.1 equiv.) were added consecutively. The reaction was carried out for 24 h under inert gas at 70 °C with constant stirring. The reaction was cooled to room temperature, and the resulting colorful precipitate was filtered. The solid was washed several times with toluene and dried in the air, giving 470 mg (1.37 mmol) of 4c-B with 96% yield. In the case of symmetrically substituted curcuminoids, 2.0 equiv. of aldehyde, 2.0 equiv. tributyl borate and 0.2 equiv. of N-butylamine were used. The synthesis and preliminary physicochemical characterization of 2c-B [24], 4f-B [45] was previously reported in the literature.
Detachment of the BF2 moiety
Curcuminoid decomplexation reaction involving cleavage of the BF2 group and release of the diketone moiety was performed in a microwave reactor according to the literature protocol [25]. Briefly, 5 mL of aqueous methanol (methanol: water 4:1, v/v), 200 mg of compound 4c-B (0.6 mmol, 1.0 equiv.), and 156 mg of sodium oxalate (1.2 mmol, 2 equiv.) were mixed in the microwave reaction vial. Then, the sealed vial was placed in a microwave reactor for 8 min at 140 °C. After cooling to ambient temperature, the solvent was evaporated. Afterward, water was added to precipitate the product. The resulting yellow precipitate of 4c was filtered off, washed several times with water, and dried. The reaction yield was 84% (145 mg). The synthesis and preliminary physicochemical characterization of 2c [46], 4a [47], 4b [48], 4c [49], 4d [50], 4e [49], 4f [48], 4g [47] and 4i [47, 48] was previously reported in the literature.
Characterization of the obtained compounds
4‐(2‐{4‐[(1E)‐2‐{2,2‐difluoro‐6‐[(1E)‐2‐{3‐methoxy‐4‐[2‐(morpholin‐4‐yl)ethoxy]phenyl}ethenyl]‐1,3,2‐dioxaborinin‐4‐yl}ethenyl]‐2‐methoxyphenoxy}ethyl)morpholine (2a-B)
Yield: 77%; ESI MS recorded: 643.3009 m/z [M+H]+, expected for C33H41BF2N2O8: 643.2997 m/z [M+H]+; Rf = 0.45 (DCM:MeOH 10:1); MP = 122 °C; λmax (ACN) nm (logε): 502 (5.04); HPLC purity: DAD 96.64%. 1H NMR (800 MHz, acetone-d6) δ 7.97 (d, J = 15.6 Hz, 2H), 7.47 (d, J = 1.9 Hz, 2H), 7.42 (dd, J = 8.3, 2.0 Hz, 1H), 7.11 (d, J = 8.3 Hz, 2H), 6.98 (d, J = 15.6 Hz, 2H), 6.39 (s, 1H), 4.24 (t, J = 5.8 Hz, 4H), 3.91 (s, 6H), 3.61 (t, J = 4.6 Hz, 8H), 2.80 (s, 4H), 2.55 (s, 8H); 13C NMR (201 MHz, acetone-d6) δ 180.8, 153.4, 151.0, 147.4, 128.5, 125.6, 119.8, 113.9, 112.5, 102.2, 68.0, 67.5, 58.2, 56.4, 55.0.
(1E,4Z,6E)‐5‐hydroxy‐1,7‐bis({3‐methoxy‐4‐[2‐(morpholin‐4‐yl)ethoxy]phenyl})hepta‐1,4,6‐trien‐3‐one (2a)
Yield: 33%; ESI MS recorded: 595.3012 m/z [M+H]+, expected for C33H42N2O8: 595.3014 m/z [M+H]+; Rf = 0.39 (DCM:MeOH 10:1); MP = 100 °C; λmax (ACN) nm (logε): 421 (4.94); HPLC purity: DAD 96.64%. 1H NMR (800 MHz, acetone-d6) δ 7.61 (d, J = 15.8 Hz, 2H), 7.34 (d, J = 2.0 Hz, 2H), 7.23 (dd, J = 8.4, 1.8 Hz, 2H), 7.04 (d, J = 8.3 Hz, 2H), 6.75 (d, J = 15.8 Hz, 2H), 6.00 (s, 1H), 4.19 (t, J = 5.8 Hz, 4H), 3.89 (s, 6H), 3.62–3.59 (m, 8H), 2.77 (t, J = 5.8 Hz, 4H), 2.54 (s, 8H); 13C NMR (201 MHz, acetone-d6) δ 184.5, 151.8, 150.9, 141.2, 129.2, 123.6, 123.0, 114.1, 111.8, 101.8, 67.9, 67.5, 58.3, 56.3, 55.1.
4‐(2‐{5‐[(1E)‐2‐{2,2‐difluoro‐6‐[(1E)‐2‐{4‐methoxy‐3‐[2‐(morpholin‐4‐yl)ethoxy]phenyl}ethenyl]‐1,3,2‐dioxaborinin‐4‐yl}ethenyl]‐2‐methoxyphenoxy}ethyl)morpholine (2b-B)
Yield: 50%; ESI MS recorded: 643.3030 m/z [M+H]+, expected for C33H41BF2N2O8: 643.2997 m/z [M+H]+; Rf = 0.45 (DCM:MeOH 10:1); MP = 167 °C; λmax (ACN) nm (logε): 501 (4.99); HPLC purity: DAD 95.43%. 1H NMR (800 MHz, acetone-d6) δ 7.96 (d, J = 15.6 Hz, 2H), 7.49 (d, J = 2.0 Hz, 2H), 7.43 (dd, J = 8.4, 1.8 Hz, 2H), 7.09 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 15.6 Hz, 2H), 6.38 (s, 1H), 4.24 (t, J = 5.9 Hz, 4H), 3.92 (s, 6H), 3.63–3.62 (m, 8H), 2.81–2.80 (m, 11H), 2.57 (s, 8H); 13C NMR (201 MHz, acetone-d6) δ 180.7, 154.4, 150.0, 147.4, 128.4, 125.9, 119.8, 113.7, 112.9, 102.2, 67.9, 67.5, 58.3, 56.4, 55.0.
(1E,4Z,6E)‐5‐hydroxy‐1,7‐bis({4‐methoxy‐3‐[2‐(morpholin‐4‐yl)ethoxy]phenyl})hepta‐1,4,6‐trien‐3‐one (2b)
Yield: 99%; ESI MS recorded: 595.3010 m/z [M+H]+, expected for C33H42N2O8: 595.3014 m/z [M+H]+; Rf = 0.47 (DCM:MeOH 10:1); MP = 195 °C; λmax (ACN) nm (logε): 419 (4.73); HPLC purity: DAD 98.34%. 1H NMR (800 MHz, acetone-d6) δ 7.60 (d, J = 15.8 Hz, 2H), 7.36 (d, J = 1.8 Hz, 2H), 7.24 (dd, J = 8.3, 1.8 Hz, 2H), 7.01 (d, J = 8.3 Hz, 2H), 6.73 (d, J = 15.8 Hz, 2H), 5.99 (s, 1H), 4.20 (t, J = 5.9 Hz, 4H), 3.87 (s, 6H), 3.63–3.61 (m, 8H), 2.78 (t, J = 5.9 Hz, 4H), 2.55 (s, 8H); 13C NMR (201 MHz, acetone-d6) δ 184.5, 152.8, 149.9, 141.2, 129.0, 123.9, 122.9, 113.1, 112.9, 101.8, 67.9, 67.5, 58.4, 56.3, 55.1.
2,2‐difluoro‐4,6‐bis[(1E)‐2‐phenylethenyl]‐1,3,2‐dioxaborinine (2c-B)
Yield: 59%; ESI MS recorded: 347.1034 m/z [M+Na]+, expected for C19H15BF2O2: 347.1026 m/z [M+Na]+; Rf = 0.52 (n-hexane:EtOAc 2:1); MP = 242 °C; λmax (ACN) nm (logε): 422 (4.77); HPLC purity: DAD 99.33%. 1H NMR (800 MHz, acetone-d6) δ 8.08 (d, J = 15.8 Hz, 2H), 7.86 (d, J = 6.1 Hz, 4H), 7.57–7.49 (m, 6H), 7.16 (d, J = 15.7 Hz, 2H), 6.60 (s, 1H); 13C NMR (201 MHz, acetone-d6) δ 181.8, 147.6, 135.3, 132.7, 130.2, 130.1, 122.3, 103.0.
(1E,4Z,6E)‐5‐hydroxy‐1,7‐diphenylhepta‐1,4,6‐trien‐3‐one (2c)
Yield: 79%; ESI MS recorded: 299.1047 m/z [M+Na]+, expected for C19H16O2: 299.1043 m/z [M+Na]+; Rf = 0.83 (n-hexane:EtOAc 2:1); MP = 142 °C; λmax (ACN) nm (logε): 392 (4.76); HPLC purity: DAD 99.57%. 1H NMR (800 MHz, acetone-d6) δ 7.73–7.67 (m, 6H), 7.47–7.40 (m, 6H), 6.89 (d, J = 15.9 Hz, 2H), 6.13 (s, 1H); 13C NMR (201 MHz, acetone-d6) δ 184.5, 141.2, 136.0, 131.0, 129.9, 129.1, 125.2, 102.5.
2,2‐difluoro‐4‐methyl‐6‐phenyl‐1,3,2‐dioxaborinine (3-B)
Yield: 85%; ESI MS recorded: 211.0744 m/z [M + H]+, 233.0570 [M+Na]+, expected for C10H9BF2O2: 211.0736 m/z [M+H]+ 233.0556 [M+Na]+; Rf = 0.27 (n-hexane:EtOAc 2:1); MP = 158 °C; λmax (ACN) nm (logε): 329 (4.47); HPLC purity: DAD 97.45%. 1H NMR (800 MHz, acetone-d6) δ 8.19 (dd, J = 8.5, 1.2 Hz, 2H), 7.80 (t, J = 7.4 Hz, 1H), 7.66–7.63 (m, 2H), 7.11 (s, 1H), 2.48 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 195.2, 183.1, 136.3, 132.3, 130.3, 129.8, 98.7, 24.8.
2,2‐difluoro‐4‐phenyl‐6‐[(1E)‐2‐(3,4,5‐trimethoxyphenyl)ethenyl]‐1,3,2‐dioxaborinine (4a-B)
Yield: 89%; ESI MS recorded: 411.1210 m/z [M+Na]+, expected for C20H19BF2O5: 411.1186 m/z [M+Na]+, Rf = 0.17 (DCM:MeOH 2:1); MP = 235 °C; λmax (ACN) nm (logε): 437 (4.68); HPLC purity: DAD 99.72%. 1H NMR (800 MHz, acetone-d6) δ 8.21 (dd, J = 8.5, 1.2 Hz, 2H), 8.12 (d, J = 15.7 Hz, 1H), 7.79 (t, J = 7.4 Hz, 1H), 7.67–7.64 (m, 2H), 7.24 (s, 2H), 7.19 (d, J = 15.7 Hz, 1H), 7.17 (s, 1H), 3.93 (s, 6H), 3.83 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 183.0, 182.7, 154.9, 149.0, 143.2, 136.1, 133.2, 130.7, 130.4, 129.6, 121.5, 108.2, 98.7, 61.0, 56.8.
(2Z,4E)‐3‐hydroxy‐1‐phenyl‐5‐(3,4,5‐trimethoxyphenyl)penta‐2,4‐dien‐1‐one (4a)
Yield: 90%; ESI MS recorded: 363.1211 m/z [M+Na]+, expected for C20H20O5: 363.1203 m/z [M+Na]+; Rf = 0.58 (n-hexane:EtOAc 2:1); MP = 120 °C; λmax (ACN) nm (logε): 385 (4.66); HPLC purity: DAD 99.04%. 1H NMR (800 MHz, acetone-d6) δ 8.04 (dd, J = 8.3, 1.1 Hz, 2H), 7.67 (d, J = 15.8 Hz, 1H), 7.63 (t, J = 7.3 Hz, 1H), 7.55 (t, J = 7.7 Hz, 2H), 7.06 (s, 2H), 6.90 (d, J = 15.8 Hz, 1H), 6.65 (s, 1H), 3.90 (s, 6H), 3.78 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 189.8, 181.2, 154.7, 141.3, 141.1, 137.1, 133.6, 131.6, 129.6, 128.1, 123.6, 106.6, 98.0, 60.7, 56.5.
4‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]‐2‐methoxyphenol (4b-B)
Yield: 96%; ESI MS recorded: 367.0920 m/z [M+Na]+, expected for C18H15BF2O4: 367.0924 m/z [M+Na]+; Rf = 0.20 (DCM); MP = 211 °C; λmax (ACN) nm (logε): 446 (4.75); HPLC purity: DAD 100.0%. 1H NMR (800 MHz, acetone-d6) δ 8.66 (s, 1H), 8.18 (dd, J = 8.5, 1.2 Hz, 2H), 8.13 (d, J = 15.6 Hz, 1H), 7.76 (t, J = 7.4 Hz, 1H), 7.65–7.62 (m, 2H), 7.52 (d, J = 2.0 Hz, 1H), 7.41 (dd, J = 8.2, 1.6 Hz, 1H), 7.13 (s, 1H), 7.06 (d, J = 15.6 Hz, 1H), 6.97 (d, J = 8.2 Hz, 1H), 3.96 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 183.1, 181.4, 152.4, 149.5, 149.1, 135.6, 133.3, 130.1, 129.3, 127.5, 126.3, 119.0, 116.7, 112.7, 98.1, 56.4.
(2Z,4E)‐3‐hydroxy‐5‐(4‐hydroxy‐3‐methoxyphenyl)‐1‐phenylpenta‐2,4‐dien‐1‐one (4b)
Yield: 78%; ESI MS recorded: 319.0941 m/z [M+Na]+, expected for C18H16O4: 319.0941 m/z [M+Na]+; Rf = 0.42 (n-hexane:EtOAc 2:1); MP = 158 °C; λmax (ACN) nm (logε): 393 (4.60); HPLC purity: DAD 98.66%. 1H NMR (800 MHz, acetone-d6) δ 8.19 (s, 1H), 8.03 (dd, J = 8.2, 1.1 Hz, 2H), 7.68 (d, J = 15.8 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.54 (t, J = 7.7 Hz, 2H), 7.36 (d, J = 1.8 Hz, 1H), 7.20 (dd, J = 8.1, 1.9 Hz, 1H), 6.90 (d, J = 8.1 Hz, 1H), 6.79 (d, J = 15.8 Hz, 1H), 6.62 (s, 1H), 3.93 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 189.0, 182.2, 150.1, 148.8, 141.5, 137.1, 133.4, 129.6, 128.2, 128.0, 123.9, 121.5, 116.3, 111.5, 97.6, 56.3.
5‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]‐2‐methoxyphenol (4c-B)
Yield: 96%; ESI MS recorded: 367.0927 m/z [M+Na]+, expected for C18H15BF2O4: 367.0924 m/z [M+Na]+; Rf = 0.20 (DCM); MP = 203 °C; λmax (ACN) nm (logε): 446 (4.73); HPLC purity: DAD 100.0%. 1H NMR (800 MHz, acetone-d6) δ 8.19 (dd, J = 8.5, 1.2 Hz, 2H), 8.09 (d, J = 15.6 Hz, 1H), 8.06 (s, 1H), 7.77 (t, J = 7.4 Hz, 1H), 7.66–7.63 (m, 2H), 7.40–7.33 (m, 2H), 7.17 (s, 1H), 7.10 (d, J = 8.9 Hz, 1H), 7.03 (d, J = 15.6 Hz, 1H), 3.95 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 183.0, 181.8, 152.6, 149.1, 148.1, 135.7, 133.2, 130.2, 129.4, 128.5, 125.1, 119.7, 115.2, 112.5, 98.4, 56.5.
(2Z,4E)‐3‐hydroxy‐5‐(3‐hydroxy‐4‐methoxyphenyl)‐1‐phenylpenta‐2,4‐dien‐1‐one (4c)
Yield: 84%; ESI MS recorded: 319.0954 m/z [M+Na]+, expected for C18H16O4: 319.0941 m/z [M+Na]+; Rf = 0.38 (n-hexane:EtOAc 2:1); MP = 136 °C; λmax (ACN) nm (logε): 390 (4.65); HPLC purity: DAD 99.40%. 1H NMR (800 MHz, acetone-d6) δ 8.04 (d, J = 7.2 Hz, 2H), 7.84 (s, 1H), 7.64 (d, J = 15.8 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.54 (t, J = 7.7 Hz, 2H), 7.23 (d, J = 2.1 Hz, 1H), 7.16 (d, J = 8.3 Hz, 1H), 7.01 (d, J = 8.3 Hz, 1H), 6.76 (d, J = 15.8 Hz, 1H), 6.65 (s, 1H), 3.90 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 189.3, 181.8, 150.6, 147.9, 141.1, 137.1, 133.4, 129.6, 129.4, 128.1, 122.5, 122.1, 114.4, 112.4, 97.8, 56.3.
4‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]‐2‐ethoxyphenol (4d-B)
Yield: 80%; ESI MS recorded: 381.1083 m/z [M+Na]+, expected for C19H17BF2O4: 381.1080 m/z [M+Na]+; Rf = 0.68 (n-hexane:EtOAc 1:2); MP = 197 °C; λmax (ACN) nm (logε): 452 (4.47); HPLC purity: DAD 100.0%. 1H NMR (800 MHz, acetone-d6) δ 8.64 (s, 1H), 8.18 (dd, J = 8.5, 1.2 Hz, 2H), 8.12 (d, J = 15.6 Hz, 1H), 7.76 (t, J = 7.4, Hz, 1H), 7.65–7.62 (m, 2H), 7.50 (d, J = 2.0 Hz, 1H), 7.40 (dd, J = 8.2, 2.0 Hz, 1H), 7.12 (s, 1H), 7.05 (d, J = 15.6 Hz, 1H), 6.97 (d, J = 8.2 Hz, 1H), 4.23 (q, J = 7.0 Hz, 2H), 1.42 (t, J = 7.0 Hz, 3H); 13C NMR (201 MHz, acetone-d6) δ 183.1, 181.4, 152.5, 149.6, 148.2, 135.6, 133.3, 130.1, 129.3, 127.4, 126.2, 118.9, 116.7, 113.6, 98.1, 65.3, 14.9.
(2Z,4E)‐5‐(3‐ethoxy‐4‐hydroxyphenyl)‐3‐hydroxy‐1‐phenylpenta‐2,4‐dien‐1‐one (4d)
Yield: 90%; ESI MS recorded: 333.1099 m/z [M+Na]+, expected for C19H18O4: 333.1098 m/z [M+Na]+; Rf = 0.62 (n-hexane:EtOAc 2:1); MP = 75 °C; λmax (ACN) nm (logε): 394 (4.61); HPLC purity: DAD 98.12%. 1H NMR (800 MHz, acetone-d6) δ 8.17 (s, 1H), 8.03 (d, J = 7.1 Hz, 2H), 7.67 (d, J = 15.8 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.53 (t, J = 11.5, 4.1 Hz, 2H), 7.34 (d, J = 1.9 Hz, 1H), 7.20 (d, J = 6.3 Hz, 1H), 6.90 (d, J = 8.1 Hz, 1H), 6.78 (d, J = 15.8 Hz, 1H), 6.61 (s, 1H), 4.19 (q, J = 7.0 Hz, 2H), 1.41 (t, J = 7.0 Hz, 3H); 13C NMR (201 MHz, acetone-d6) δ 189.0, 182.2, 150.3, 148.0, 141.6, 137.1, 133.4, 129.6, 128.1, 128.0, 123.8, 121.4, 116.3, 112.4, 97.6, 65.2, 15.0.
4‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]‐2,6‐dimethoxyphenol (4e-B)
Yield: 97%; ESI MS recorded: 397.1035 m/z [M+Na]+, expected for C19H17BF2O5: 397.1030 m/z [M+Na]+; Rf = 0.56 (DCM:MeOH 50:1); MP = 170 °C; λmax (ACN) nm (logε): 461 (4.67); HPLC purity: DAD 100.0%. 1H NMR (800 MHz, acetone-d6) δ 8.18 (dd, J = 8.5, 1.2 Hz, 1H), 8.12 (d, J = 15.5 Hz, 1H), 7.76 (t, J = 7.4, Hz, 1H), 7.65–7.61 (m, 1H), 7.25 (s, 2H), 7.11 (s, 1H), 7.09 (d, J = 15.5 Hz, 1H), 3.93 (s, 6H); 13C NMR (201 MHz, acetone-d6) δ 182.9, 181.4, 149.9, 149.2, 141.9, 135.6, 133.3, 130.1, 129.3, 126.1, 119.2, 108.5, 98.2, 56.8.
(2Z,4E)‐3‐hydroxy‐5‐(4‐hydroxy‐3,5‐dimethoxyphenyl)‐1‐phenylpenta‐2,4‐dien‐1‐one (4e)
Yield: 91%; ESI MS recorded: 349.1050 m/z [M+Na]+, expected for C19H18O5: 349.1047 m/z [M+Na]+; Rf = 0.27 (n-hexane:EtOAc 2:1); MP = 133 °C; λmax (ACN) nm (logε): 399 (4.59); HPLC purity: DAD 99.37%. 1H NMR (800 MHz, acetone-d6) δ 8.03 (d, J = 7.1 Hz, 2H), 7.82 (s, 1H), 7.67 (d, J = 15.7 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.53 (t, J = 7.9, 7.4 Hz, 2H), 7.06 (s, 2H), 6.82 (d, J = 15.8 Hz, 1H), 6.61 (s, 1H), 3.90 (s, 6H); 13C NMR (201 MHz, acetone-d6) δ 189.1, 182.0, 149.0, 141.8, 139.6, 137.1, 133.4, 129.6, 128.0, 126.9, 121.7, 106.9, 97.6, 56.7.
2,2‐difluoro‐4‐phenyl‐6‐[(1E)‐2‐phenylethenyl]‐1,3,2‐dioxaborinine (4f-B)
Yield: 57%; ESI MS recorded: 321.0881 m/z [M+Na]+, expected for C17H13BF2O2: 321.0869 m/z [M+Na]+; Rf = 0.40 (n-hexane:EtOAc 2:1); MP = 184 °C; λmax (ACN) nm (logε): 396 (4.77); HPLC purity: DAD 99.34%. 1H NMR (800 MHz, acetone-d6) δ 8.22 (dd, J = 8.5, 1.2 Hz, 2H), 8.18 (d, J = 15.8 Hz, 1H), 7.87 (dd, J = 7.8, 1.2 Hz, 2H), 7.80 (t, J = 7.4, Hz, 1H), 7.66 (t, J = 7.9 Hz, 2H), 7.55–7.52 (m, 3H), 7.27 (s, 1H), 7.24 (d, J = 15.8 Hz, 1H); 13C NMR (201 MHz, acetone-d6) δ 183.2, 182.8, 148.2, 136.2, 135.2, 133.0, 132.8, 130.3, 130.3, 130.2, 129.6, 122.4, 98.8.
(2Z,4E)‐3‐hydroxy‐1,5‐diphenylpenta‐2,4‐dien‐1‐one (4f)
Yield: 79%; ESI MS recorded: 273.0893 m/z [M+Na]+, expected for C17H14O2: 273.0886 m/z [M+Na]+; Rf = 0.85 (n-hexane:EtOAc 2:1); MP = 110 °C; λmax (ACN) nm (logε): 363 (4.58); HPLC purity: DAD 99.88%. 1H NMR (800 MHz, acetone-d6) δ 8.06 (d, J = 7.2 Hz, 2H), 7.73 (d, J = 16.0 Hz, 1H), 7.71 (d, J = 7.3 Hz, 2H), 7.63 (t, J = 7.3 Hz, 1H), 7.55 (t, J = 7.8 Hz, 2H), 7.46 (t, J = 7.3 Hz, 2H), 7.42 (t, J = 7.2 Hz, 1H), 6.95 (d, J = 15.9 Hz, 1H), 6.72 (s, 1H); 13C NMR (201 MHz, acetone-d6) δ 190.3, 180.7, 140.6, 137.0, 136.1, 133.7, 130.9, 129.9, 129.7, 129.0, 128.2, 124.5, 98.3.
4‐[(1E)‐2‐(3,5‐dimethoxyphenyl)ethenyl]‐2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinine (4g-B)
Yield: 86%; ESI MS recorded: 381.1093 m/z [M+Na]+, expected for C19H17BF2O4: 381.1080 m/z [M+Na]+; Rf = 0.38 (n-hexane:EtOAc 2:1); MP = 205 °C; λmax (ACN) nm (logε): 402 (4.74); HPLC purity: DAD 99.72%. 1H NMR (800 MHz, acetone-d6) δ 8.21 (dd, J = 8.4, 1.1 Hz, 2H), 8.09 (d, J = 15.8 Hz, 1H), 7.80 (t, J = 7.4 Hz, 1H), 7.67–7.64 (m, 2H), 7.23 (t, J = 7.9 Hz, 2H), 7.04 (d, J = 2.2 Hz, 2H), 6.66 (t, J = 2.2 Hz, 1H), 3.87 (s, 6H); 13C NMR (201 MHz, acetone-d6) δ 183.2, 182.8, 162.3, 148.3, 137.1, 136.2, 132.9, 130.3, 129.6, 122.9, 107.9, 105.1, 98.8, 55.9.
(2Z,4E)‐5‐(3,5‐dimethoxyphenyl)‐3‐hydroxy‐1‐phenylpenta‐2,4‐dien‐1‐one (4g)
Yield: 86%; ESI MS recorded: 333.1102 m/z [M+Na]+, expected for C19H18O4: 333.1098 m/z [M+Na]+; Rf = 0.69 (n-hexane:EtOAc 2:1); MP = 104 °C; λmax (ACN) nm (logε): 374 (4.64); HPLC purity: DAD 99.61%. 1H NMR (800 MHz, acetone-d6) δ 8.05 (dd, J = 8.2, 1.1 Hz, 1H), 7.68–7.61 (m, J = 19.3, 11.8 Hz, 2H), 7.55 (t, J = 7.8 Hz, 2H), 6.95 (d, J = 15.9 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 6.69 (s, 1H), 6.55 (t, J = 2.2 Hz, 1H), 3.84 (s, 6H); 13C NMR (201 MHz, acetone-d6) δ 190.4, 180.6, 162.2, 140.7, 138.0, 137.1, 133.7, 129.7, 128.2, 124.9, 106.8, 103.2, 98.4, 55.8.
2,2‐difluoro‐4‐phenyl‐6‐[(1E)‐2‐(2,4,6‐trimethoxyphenyl)ethenyl]‐1,3,2‐dioxaborinine (4h-B)
Yield: 99%; ESI MS recorded: 411.1188 m/z [M+Na]+, expected for C20H19BF2O5: 411.1186 m/z [M+Na]+; Rf = 0.10 (n-hexane:EtOAc 2:1); MP = 210 °C; λmax (ACN) nm (logε): 472 (4.63); HPLC purity: DAD 98.48%. 1H NMR (800 MHz, acetone-d6) δ 8.60 (d, J = 15.6 Hz, 1H), 8.16 (dd, J = 8.3, 1.1 Hz, 2H), 7.73 (t, J = 7.4 Hz, 1H), 7.62 (t, J = 7.8 Hz, 2H), 7.41 (d, J = 15.6 Hz, 1H), 7.02 (s, 1H), 6.36 (s, 2H), 4.01 (s, 6H), 3.95 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 184.2, 179.9, 167.1, 164.0, 140.5, 135.1, 133.6, 130.0, 129.1, 120.1, 107.0, 98.7, 91.9, 56.7, 56.3.
(2Z,4E)‐3‐hydroxy‐1‐phenyl‐5‐(2,4,6‐trimethoxyphenyl)penta‐2,4‐dien‐1‐one (4h)
Yield: 51%; ESI MS recorded: 363.1206 m/z [M+Na]+, expected for C20H20O5: 363.1203 m/z [M+Na]+; Rf = 0.48 (n-hexane:EtOAc 2:1); MP = 143 °C; λmax (ACN) nm (logε): 405 (4.67); HPLC purity: DAD 98.55%. 1H NMR (800 MHz, acetone-d6) δ 8.17 (d, J = 16.0 Hz, 1H), 8.02 (dd, J = 8.3, 1.1 Hz, 1H), 7.59 (t, J = 7.3 Hz, 1H), 7.52 (t, J = 7.7 Hz, 1H), 7.16 (d, J = 16.0 Hz, 1H), 6.52 (s, 1H), 6.31 (s, 2H), 3.95 (s, 6H), 3.89 (s, 3H); 13C NMR (201 MHz, acetone-d6) δ 188.5, 183.9, 164.4, 162.4, 137.3, 133.1, 132.6, 129.5, 128.0, 123.5, 106.8, 97.8, 91.7, 56.3, 55.9.
4‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]phenol (4i-B)
Yield: 93%; ESI MS recorded: 337.0825 m/z [M+Na]+, expected for C17H13BF2O3: 337.0818 m/z [M+Na]+; Rf = 0.59 (n-hexane:EtOAc 1:2); MP = 179 °C; λmax (ACN) nm (logε): 440 (4.87); HPLC purity: DAD 97.63%. 1H NMR (800 MHz, acetone-d6) δ 9.36 (s, 1H), 8.18 (d, J = 7.3 Hz, 2H), 8.14 (d, J = 15.6 Hz, 1H), 7.79–7.74 (m, 3H), 7.63 (t, J = 7.9 Hz, 2H), 7.15 (s, 1H), 7.02 (d, J = 15.6 Hz, 1H), 6.99 (d, J = 8.6 Hz, 2H); 13C NMR (201 MHz, acetone-d6) δ 183.2, 181.5, 162.6, 149.1, 135.6, 133.2, 132.9, 130.1, 129.3, 127.0, 118.8, 117.3, 98.2.
(2Z,4E)‐3‐hydroxy‐5‐(4‐methoxyphenyl)‐1‐phenylpenta‐2,4‐dien‐1‐one (4i)
Yield: 58%; ESI MS recorded: 289.0837 m/z [M+Na]+, expected for C17H14O3: 289.0835 m/z [M+Na]+; Rf = 0.41 (n-hexane:EtOAc 2:1); MP = 173 °C; λmax (ACN) nm (logε): 419 (4.66); HPLC purity: DAD 98.34%. 1H NMR (800 MHz, acetone-d6) δ 8.93 (s, 1H), 8.03 (d, J = 7.0 Hz, 2H), 7.69 (d, J = 15.8 Hz, 1H), 7.61 (t, J = 7.4 Hz, 1H), 7.59 (d, J = 8.6 Hz, 2H), 7.54 (t, J = 7.8 Hz, 2H), 6.92 (d, J = 8.6 Hz, 2H), 6.75 (d, J = 15.8 Hz, 1H), 6.64 (s, 1H); 13C NMR (201 MHz, acetone-d6) δ 189.0, 182.2, 160.6, 141.1, 137.1, 133.4, 131.0, 129.6, 128.1, 127.7, 121.3, 116.8, 97.6.
4‐(2‐{5‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]‐2‐methoxyphenoxy}ethyl)morpholine (4j-B)
Yield: 99%; ESI MS recorded: 458.1952 m/z [M + H]+, expected for C24H26BF2NO5: 458.1945 m/z [M + H]+; Rf = 0.40 (DCM:MeOH 20:1); MP = 210 °C; λmax (ACN) nm (logε): 451 (4.76); HPLC purity: DAD 99.28%. 1H NMR (800 MHz, acetone-d6) δ 8.19 (dd, J = 8.4, 1.1 Hz, 2H), 8.13 (d, J = 15.7 Hz, 1H), 7.77 (t, J = 7.4 Hz, 1H), 7.64 (t, J = 7.9 Hz, 2H), 7.53 (d, J = 2.0 Hz, 1H), 7.48 (dd, J = 8.3, 2.0 Hz, 1H), 7.13 (s, 1H), 7.12 (d, J = 8.3 Hz, 1H), 7.08 (d, J = 15.6 Hz, 1H), 4.26 (t, J = 5.8 Hz, 2H), 3.94 (s, 3H), 3.65–3.62 (m, 4H), 2.83 (t, J = 5.8 Hz, 2H), 2.59 (s, 4H); 13C NMR (201 MHz, acetone-d6) δ 183.2, 182.0, 154.9, 150.2, 149.1, 135.7, 133.4, 130.2, 129.4, 128.3, 126.3, 119.9, 114.3, 113.2, 98.3, 68.1, 67.5, 58.4, 56.6, 55.1.
4‐(2‐{4‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]‐2‐methoxyphenoxy}ethyl)morpholine (4k-B)
Yield: 73%; ESI MS recorded: 458.1959 m/z [M + H]+, expected for C24H26BF2NO5: 458.1945 m/z [M + H]+; Rf = 0.34 (DCM:MeOH 20:1); MP = 110 °C; λmax (ACN) nm (logε): 452 (4.62); HPLC purity: DAD 99.24%. 1H NMR (800 MHz, acetone-d6) δ 8.19 (dd, J = 8.3, 1.0 Hz, 2H), 8.14 (d, J = 15.6 Hz, 1H), 7.77 (t, J = 7.4 Hz, 1H), 7.65 (t, J = 7.9 Hz, 2H), 7.51 (d, J = 1.9 Hz, 1H), 7.46 (dd, J = 8.3, 2.0 Hz, 1H), 7.15 (s, 1H), 7.14 (d, J = 8.3 Hz, 1H), 7.11 (d, J = 15.6 Hz, 1H), 4.32 (t, J = 5.6 Hz, 2H), 3.93 (s, 3H), 3.69 (t, J = 4.5 Hz, 4H), 2.97 (s, 2H), 2.73 (s, 4H); 13C NMR (201 MHz, acetone-d6) δ 183.0, 181.8, 153.4, 151.0, 149.0, 135.8, 133.2, 130.2, 129.4, 128.5, 126.0, 119.8, 114.1, 112.5, 98.3, 67.5, 67.0, 58.0, 56.4, 54.9.
4‐(2‐{4‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]phenoxy}ethyl)morpholine (4l-B)
Yield: 79%; ESI MS recorded: 428.1850 m/z [M + H]+, expected for C23H24BF2NO4: 428.1839 m/z [M + H]+; Rf = 0.18 (DCM:MeOH 35:1); MP = 154 °C; λmax (ACN) nm (logε): 440 (4.72); HPLC purity: DAD 98.02%. 1H NMR (800 MHz, acetone-d6) δ 8.19 (dd, J = 8.5, 1.2 Hz, 2H), 8.15 (d, J = 15.7 Hz, 1H), 7.84 (d, J = 8.6 Hz, 2H), 7.77 (t, J = 7.4 Hz, 1H), 7.66–7.63 (m, 2H), 7.17 (s, 1H), 7.10 (d, J = 8.8 Hz, 2H), 7.08 (d, J = 15.7 Hz, 1H), 4.30 (t, J = 5.7 Hz, 2H), 3.66 (t, J = 4.6 Hz, 4H), 2.90 (t, J = 5.4 Hz, 2H), 2.65 (s, 4H); 13C NMR (201 MHz, acetone-d6) δ 183.1, 181.9, 163.3, 148.6, 135.8, 133.2, 132.6, 130.2, 129.4, 128.1, 119.6, 116.3, 98.4, 67.1, 66.7, 58.1, 54.8.
4‐(2‐{2‐[(1E)‐2‐(2,2‐difluoro‐6‐phenyl‐1,3,2‐dioxaborinin‐4‐yl)ethenyl]phenoxy}ethyl)morpholine (4m-B)
Yield: 32%; ESI MS recorded: 428.1843 m/z [M + H]+, expected for C23H24BF2NO4: 428.1839 m/z [M + H]+; Rf = 0.18 (DCM:MeOH 35:1); MP = 192 °C; λmax (ACN) nm (logε): 418 (4.53); HPLC purity: DAD 96.52%. 1H NMR (800 MHz, acetone-d6) δ 8.44 (d, J = 15.9 Hz, 1H), 8.23 (dd, J = 8.5, 1.2 Hz, 2H), 7.88 (dd, J = 7.7, 1.6 Hz, 1H), 7.80 (t, J = 7.4 Hz, 1H), 7.68–7.65 (m, 2H), 7.55 (t, J = 8.7 Hz, 1H), 7.30 (d, J = 15.9 Hz, 1H), 7.26–7.24 (m, 2H), 7.15 (t, J = 7.5 Hz, 1H), 4.73 (t, 2H), 4.03 (s, 4H), 3.94 (s, 2H), 3.65 (s, 4H); 13C NMR (201 MHz, acetone-d6) δ 183.1, 182.8, 158.5, 142.7, 136.2, 134.7, 132.9, 130.3, 130.2, 129.7, 124.1, 122.9, 122.8, 113.9, 99.1, 65.1, 64.7, 57.7, 54.5.
The absorbance scan
The compounds 2a-B and 2a were dissolved in DMSO to a final concentration of 10 µM. The absorbance and emission scans were done using the Infinite® M Plex microplate reader (Tecan Trading AG, Männedorf, Switzerland).
Microtox assay
Acute toxicity of the prepared compounds was assessed on Modern Water Microtox Model 500 apparatus equipped with Modern Water MicrotoxOmni 4.2 software, according to the 81.9% Screening test procedure provided by the supplier. The change of the bioluminescence of the bacterial suspension was monitored upon adding the sample solution compared to the negative control. For all the experiments, the solutions of appropriate concentrations were prepared using DMSO and deionized water. The concentration of DMSO was ≤1% v/v. Each compound was tested at three concentrations (1, 10, and 100 µM) at two-time points: 5 min and 15 min.
Cell culture
The human bladder cancer cell lines 5637 (human bladder grade II carcinoma) and SCaBER (human bladder squamous cell carcinoma) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The MRC-5 cell line (normal human lung fibroblasts) was obtained from the European Collection of Authenticated Cell Cultures (ECACC) distributed by Sigma-Aldrich. SCaBER cells were cultured in EMEM, 5637 cells were maintained in RPMI 1640 medium, and MRC-5 were grown in DMEM. All media were supplemented with 10% (v/v) FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 10 mg/mL streptomycin. Cells were incubated in a humidified atmosphere containing 5% CO2 at 37 °C. Tested compounds were dissolved in DMSO at a concentration of 10 mM and stored at −20 °C in dark conditions. The final concentration of DMSO in the cell culture medium did not exceed 0.1%.
MTT assay
The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed to evaluate the cytotoxic activity of tested derivatives. The cells were seeded at a density of 15 × 103 cells/well (5637 cells), 10 × 103 cells/well (SCaBER cells), and 20 × 103 cells/well (MRC-5 cells) on a 96-well plate. Screening experiments were performed for 5637 and SCaBER cells treated with tested compounds at a concentration of 1, and 10 µM. The most active compounds were selected for further studies employing 5637, SCaBER, and MRC-5 cells. Cells were seeded and incubated overnight at standard cell culture conditions. Cells were treated with a series of different concentrations (0.3, 0.6, 1.2, 2.5, 5, and 10 µM) of each compound. DMSO at a concentration of 0.1% was used as a control. Cells were incubated with tested compounds for 24 and 48 h. Then, cells were washed twice with PBS (100 µL), and MTT solution at a final concentration of 0.59 mg/mL in a cell culture medium was added to each well. After incubation lasting 1.5 h, the formazan crystals were solubilized by adding 200 μL of DMSO. The absorbance was measured at 570 nm using a microplate reader (Biotek Instruments, Elx-800, Winooski, VT, USA). The relative cell viability was expressed as the mean percentage of viable cells relative to the untreated cells. The IC50 values were calculated using GraphPad Prism 8 software. The results were presented as mean ± standard deviation from three independent experiments.
The intracellular uptake
Quantitative method
The 5637 cells were seeded at a density of 100 × 103 cells/well on a 24-well plate and incubated overnight under standard cell culture conditions. Then, the cells were treated with 2a-B and 2a at 2 and 8 µM concentrations. The cells were incubated with tested compounds for 0.5, 1, 2, 4, 6, and 8 h. DMSO was used as a control, and the concentration did not exceed 0.1% in the cell culture medium. After incubation, cells were washed twice with PBS. Cells were lysed in 80 µL of RIPA buffer (Thermofisher, Waltham, MA, USA). Standard curves were prepared in RIPA buffer for both compounds. Lysates were then transferred to a 96-well black microplate, and fluorescence intensity was measured at an excitation of 520 nm and emission of 605 nm for 2a-B, and 430 nm and 520 nm for compound 2a using Infinite® M Plex microplate reader (Tecan Trading AG, Männedorf, Switzerland). Additionally, the total protein concentration of each sample was measured using the Pierce™ BCA Protein Assay Kit (Thermofisher, Waltham, MA, USA). Data were presented as mean values for at least two experiments.
Fluorescence microscopy
The 5637 cells were seeded at a density of 45 × 103 cells/well on a Falcon® 8-well culture glass slide (Corning, New York, USA) and incubated overnight under standard cell culture conditions. Then, the cells were treated with 2a-B and 2a at a concentration of 8 µM for 0.5, 2, and 4 h. DMSO was used as a control, and the concentration did not exceed 0.1% in the cell culture medium. After incubation, cells were washed three times with PBS and fixed with 4% paraformaldehyde. Then, cells were washed three times with PBS, and stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, at a concentration of 0.5 µg/mL). The slides were mounted with Dako mounting medium (Agilent, Santa Clara, CA, USA) and images were captured using the Leica model DM4 B Fluorescence Microscope.
References
Thomford N, Senthebane D, Rowe A, Munro D, Seele P, Maroyi A, et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. IJMS. 2018;19:1578. https://doi.org/10.3390/ijms19061578
Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Chemistry and biological activities of C. Longa. Trends Food Sci Technol. 2005;16:533–48. https://doi.org/10.1016/j.tifs.2005.08.006
Oglah MK, Mustafa YF, Bashir MK, Jasim MH. Curcumin and its derivatives: a review of their biological activities. Syst Rev Pharm. 2020;11:472–81. https://doi.org/10.5530/srp.2020.3.60
Oliveira S, Monteiro-Alfredo T, Silva S, Matafome P. Curcumin derivatives for type 2 diabetes management and prevention of complications. Arch Pharm Res. 2020;43:567–81. https://doi.org/10.1007/s12272-020-01240-3
Xu X-Y, Meng X, Li S, Gan R-Y, Li Y, Li H-B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018;10:1553. https://doi.org/10.3390/nu10101553
Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78:1305–15. https://doi.org/10.1016/j.bcp.2009.06.105
Sindhwani P, Hampton JA, Baig M, Keck R, Selman SH. Curcumin: a food spice with cytotoxic activity against urinary bladder cancer. J Am Coll Surg. 2000;191:S94–S95. https://doi.org/10.1016/S1072-7515(00)00670-0
Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-κb and nuclear factor-κB–regulated gene products in IFN-α–sensitive and IFN-α–resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–30. https://doi.org/10.1158/1535-7163.MCT-06-0545
Sindhwani P, Hampton JA, Baig MM, Keck R, Selman SH. Curcumin prevents intravesical tumor implantation of the MBT-2 tumor cell line in C3H mice. J Urol. 2001;166:1498–501.
Boone CW, Steele VE, Kelloff GJ. Screening for Chemopreventive (Anticarcinogenic) compounds in rodents. Muta Res/Fundamental Mol Mech Mutagenesis. 1992;267:251–5. https://doi.org/10.1016/0027-5107(92)90069-E
Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010;30:818–60. https://doi.org/10.1002/med.20188
Mazumder A, Neamati N, Sunder S, Schulz J, Pertz H, Eich E, et al. Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J Med Chem 1997;40:3057–63. https://doi.org/10.1021/jm970190x
Nurfina AN, Reksohadiprodjo MS, Timmerman H, Jenie UA, Sugiyanto D, van der Goot H. Synthesis of some symmetrical curcumin derivatives and their antiinflammatory activity. Eur J Med Chem. 1997;32:321–8. https://doi.org/10.1016/S0223-5234(97)89084-8
Pröhl M, Schubert US, Weigand W, Gottschaldt M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Coord Chem Rev. 2016;307:32–41. https://doi.org/10.1016/j.ccr.2015.09.001
Ferrari E, Pignedoli F, Imbriano C, Marverti G, Basile V, Venturi E, et al. Newly synthesized curcumin derivatives: crosstalk between chemico-physical properties and biological activity. J Med Chem 2011;54:8066–77. https://doi.org/10.1021/jm200872q
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. https://doi.org/10.1021/mp700113r
Kharat M, Du Z, Zhang G, McClements DJ. Physical and Chemical stability of curcumin in aqueous solutions and emulsions: impact of pH, temperature, and molecular environment. J Agric Food Chem 2017;65:1525–32. https://doi.org/10.1021/acs.jafc.6b04815
Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Critic Rev Food Sci Nutr. 2004;44:97–111. https://doi.org/10.1080/10408690490424702
Nagaraju GP, Benton L, Bethi SR, Shoji M, El‐Rayes BF. Curcumin analogs: their roles in pancreatic cancer growth and metastasis. Int J Cancer. 2019;145:10–19. https://doi.org/10.1002/ijc.31867
Weiss H, Reichel J, Görls H, Schneider KRA, Micheel M, Pröhl M, et al. Curcuminoid–BF2 complexes: synthesis, fluorescence and optimization of BF2 group cleavage. Beilstein J Org Chem. 2017;13:2264–72. https://doi.org/10.3762/bjoc.13.223
Bai G, Yu C, Cheng C, Hao E, Wei Y, Mu X, et al. Syntheses and photophysical properties of BF 2 complexes of curcumin analogues. Org Biomol Chem 2014;12:1618–26. https://doi.org/10.1039/C3OB42201A
Kucinska M, Skupin-Mrugalska P, Szczolko W, Sobotta L, Sciepura M, Tykarska E, et al. Phthalocyanine derivatives possessing 2-(Morpholin-4-Yl)Ethoxy groups as potential agents for photodynamic therapy. J Med Chem. 2015;58:2240–55. https://doi.org/10.1021/acs.jmedchem.5b00052
Popov AB, Krstulović L, Koštrun S, Jelić D, Bokulić A, Stojković MR, et al. Design, synthesis, antitrypanosomal activity, DNA/RNA binding and in vitro ADME profiling of novel imidazoline-substituted 2-Arylbenzimidazoles. Eur J Med Chem. 2020;207:112802. https://doi.org/10.1016/j.ejmech.2020.112802
Liu K, Chen J, Chojnacki J, Zhang S. BF3·OEt2-promoted concise synthesis of difluoroboron-derivatized curcumins from aldehydes and 2,4-Pentanedione. Tetrahedron Lett. 2013;54:2070–3. https://doi.org/10.1016/j.tetlet.2013.02.015
Laali KK, Rathman BM, Bunge SD, Qi X, Borosky GL. Fluoro-curcuminoids and curcuminoid-BF2 adducts: synthesis, X-Ray structures, bioassay, and computational/docking study. J Fluorine Chem. 2016;191:29–41. https://doi.org/10.1016/j.jfluchem.2016.09.009
Okrągła E, Chraniuk M, Wolska L. Microtox test as a tool to assess antimicrobial properties of herbal infusions used in urinary tract infections. Acta Poloniae Pharm - Drug Res. 2017;74:895–901.
Maliński MP, Budzianowski J, Kikowska M, Derda M, Jaworska MM, Mlynarczyk DT, et al. Two ecdysteroids isolated from micropropagated lychnis flos-cuculi and the biological activity of plant material. Molecules. 2021;26:904. https://doi.org/10.3390/molecules26040904
Drzymała J, Kalka J. Elimination of the hormesis phenomenon by the use of synthetic sea water in a toxicity test towards Aliivibrio Fischeri. Chemosphere. 2020;248:126085. https://doi.org/10.1016/j.chemosphere.2020.126085
Telesiński A, Pawłowska B, Biczak R, Śnieg M, Wróbel J, Dunikowska D, et al. Enzymatic activity and its relationship with organic matter characterization and ecotoxicity to Aliivibrio Fischeri of soil samples exposed to tetrabutylphosphonium bromide. Sensors. 2021;21:1565. https://doi.org/10.3390/s21051565
Lazewski D, Kucinska M, Potapskiy E, Kuzminska J, Popenda L, Tezyk A, et al. Enhanced cytotoxic activity of PEGylated curcumin derivatives: synthesis, structure–activity evaluation, and biological activity. Int J Mol Sci. 2023;24:1467. https://doi.org/10.3390/ijms24021467
Indrayanto, G; Putra, GS; Suhud, F. Validation of in-vitro bioassay methods: application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier, 2021;46:273–307.
Weerapreeyakul N, Nonpunya A, Barusrux S, Thitimetharoch T, Sripanidkulchai B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin Med. 2012;7:15. https://doi.org/10.1186/1749-8546-7-15
Carvalho DDM, Takeuchi KP, Geraldine RM, Moura CJD, Torres MCL. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci Technol. 2015;35:115–9. https://doi.org/10.1590/1678-457X.6515
Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. ASSAY Drug Dev Technol. 2007;5:567–76. https://doi.org/10.1089/adt.2007.064
Yadav S, Singh AK, Agrahari AK, Sharma K, Singh AS, Gupta MK, et al. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci Rep. 2020;10:14204. https://doi.org/10.1038/s41598-020-70921-2
Suresh K, Nangia A. Curcumin: pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm. 2018;20:3277–96. https://doi.org/10.1039/C8CE00469B
Gál E, Nagy LC. Photophysical properties and electronic structure of symmetrical curcumin analogues and their BF2 complexes, including a phenothiazine substituted derivative. Symmetry. 2021;13:2299. https://doi.org/10.3390/sym13122299
Huang C, Lu H-F, Chen Y-H, Chen J-C, Chou W-H, Huang H-C. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin induced caspase-dependent and –independent apoptosis via Smad or Akt signaling pathways in HOS Cells. BMC Complement Med Ther. 2020;20:68. https://doi.org/10.1186/s12906-020-2857-1
Basile V, Belluti S, Ferrari E, Gozzoli C, Ganassi S, Quaglino D, et al. Bis-dehydroxy-curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy. PLOS ONE. 2013;8:e53664. https://doi.org/10.1371/journal.pone.0053664
Kumari A, Singh RK. Morpholine as ubiquitous pharmacophore in medicinal chemistry: deep insight into the Structure-Activity Relationship (SAR). Bioorg Chem. 2020;96:103578. https://doi.org/10.1016/j.bioorg.2020.103578
Kourounakis AP, Xanthopoulos D, Tzara A. Morpholine as a privileged structure: a review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med Res Rev. 2020;40:709–52. https://doi.org/10.1002/med.21634
Roy S, Mitra P, Acharya S, Roy SS, Ghosh S, Maji M, et al. A photoactive lysosome targeting ruii complex downregulates stemness genes in oral squamous cell carcinoma. Inorg Chem Front. 2022;9:5840–52. https://doi.org/10.1039/D2QI01079H
Wu L, Li X, Ling Y, Huang C, Jia N. Morpholine derivative-functionalized carbon dots-based fluorescent probe for highly selective lysosomal imaging in living cells. ACS Appl Mater Interfaces. 2017;9:28222–32. https://doi.org/10.1021/acsami.7b08148
Sudheesh KV, Jayaram PS, Samanta A, Bejoymohandas KS, Jayasree RS, Ajayaghosh A. A cyclometalated iriii complex as a lysosome-targeted photodynamic therapeutic agent for integrated imaging and therapy in cancer cells. Chemistry. 2018;24:10999–1007. https://doi.org/10.1002/chem.201801918
Štefane B, Požgan F, Kim E, Choi E, Ribierre J-C, Wu JW, et al. Ethynylene-analogues of hemicurcuminoids: synthesis and ground- and excited properties of their boron difluoride complexes. Dyes Pigments. 2017;141:38–47. https://doi.org/10.1016/j.dyepig.2017.02.005
Pedersen U, Rasmussen PB, Lawesson S. Synthesis of naturally occurring curcuminoids and related compounds. Liebigs Ann Chem. 1985;1985:1557–69. https://doi.org/10.1002/jlac.198519850805
Caldarelli A, Penucchini E, Caprioglio D, Genazzani AA, Minassi A. Synthesis and tubulin-binding properties of non-symmetrical Click C5-curcuminoids. Bioorg Med Chem. 2013;21:5510–7. https://doi.org/10.1016/j.bmc.2013.05.053
Venugopalan P, Krishnankutty K. Potentiometric studies on Chelation of Cobalt-, Nickel- and Copper(II) with Some 5-Aryl-1-Phenyl-4-Pentene-1,3-Diones. J Indian Chem Soc. 1998;75:98–99.
Alves LV, Canto-Cavalheiro MMD, Cysne-Finkelstein L, Leon L. In vitro antiproliferative effects of several diaryl derivatives on leishmania Spp. Biol Pharm Bull. 2003;26:453–6. https://doi.org/10.1248/bpb.26.453
Rees DD, Gunning PJ, Orsi A, Howson PA, Barraclough P, Callizot N. WO2008003978A2 World Intellectual Property Organization. 2008.
Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60:1620–37. https://doi.org/10.1021/acs.jmedchem.6b00975
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. https://doi.org/10.1038/srep42717
Egan WJ, Merz KM, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43:3867–77. https://doi.org/10.1021/jm000292e
Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1:55–68. https://doi.org/10.1021/cc9800071
Muegge I, Heald SL, Brittelli D. Simple selection criteria for drug-like chemical matter. J Med Chem 2001;44:1841–6. https://doi.org/10.1021/jm015507e
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23. https://doi.org/10.1021/jm020017n
Palm K, Luthman K, Unge A-L, Strandlund G, Artursson P. Correlation of drug absorption with molecular surface properties. J Pharm Sci. 1996;85:32–39. https://doi.org/10.1021/js950285r
Kralj S, Jukič M, Bren U. Molecular filters in medicinal chemistry. Encyclopedia. 2023;3:501–11. https://doi.org/10.3390/encyclopedia3020035
Pathania S, Singh PK. Analyzing FDA-approved drugs for compliance of pharmacokinetic principles: should there be a critical screening parameter in drug designing protocols? Expert Opin Drug Metabol Toxicol. 2021;17:351–4. https://doi.org/10.1080/17425255.2021.1865309
Xue M, Cheng Y, Xu L, Zhang L. Study of the intestinal absorption characteristics of curcumin in vivo and in vitro. J Appl Pharm. 2017;9:1000246. https://doi.org/10.21065/1920-4159.1000246
Acknowledgements
This work was supported by the Polish National Science Centre under grant no. 2019/35/B/NZ7/01165. The authors thank Assoc Prof. Łukasz Sobotta, DSc, PhD and MSc Marcin Wysocki for their help in the calculations related to determining the solubility of the most active compounds as well as Mrs Beata Kwiatkowska and Ms Rita Kuba for their excellent technical assistance.
Author contributions
Conceptualization: PB and TG; Investigation: PB, PK, JK, DTM, LP, KG, M. Kucinska and M. Kasperkowiak. Resources: PB, TK, M. Kucinska, and TG; Writing—original draft preparation: PB, PK, JK, TK, M. Kucinska, and DTM; Writing—review and editing: PB, DTM, LP, M. Kucinska, TK, M. Kasperkowiak, MM, AJ, and TG; Visualization: PB, PK, and M. Kucinska; Supervision: TG, AJ, and MM; Project administration: PB and TG; Funding acquisition: TG. All authors have read and agreed to the published version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakun, P., Kucinska, M., Kobyłka, P. et al. Morpholinated curcuminoids against urinary bladder cancer cells: synthesis and anticancer evaluation. Med Chem Res 33, 944–963 (2024). https://doi.org/10.1007/s00044-024-03233-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-024-03233-z