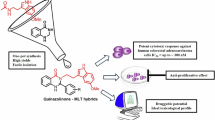
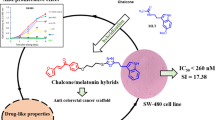
Abstract
Considering the important increase in the incidence and mortality of colorectal cancer, it is necessary to develop new strategies in the search for new alternatives against this disease. Hence, we designed and synthesized a new series of monastrol/melatonin hybrids and evaluated them in vitro and in silico to determine the potential of these new chemical entities on this type of cancer. To achieve this goal, the different compounds were evaluated in human colorectal adenocarcinoma cells SW480, while establishing the selective potential of the hybrids through the nonmalignant human colon mucosal epithelial cell line (NCM460). According to the results, hybrids 6a, 6c, 6i, and 6j displayed the best response, with IC50 values in the range of 5.2 and 6.3 μM, inducing important changes depending on concentration and time. In addition, these compounds were extremely active in comparison to the single molecules, and they were slightly more selective than the reference drug (5 fluorouracil, 5-FU). Besides, an optimal pharmacokinetic and toxicological profile was also estimated for hybrids 6a, 6c, 6i, and 6j. Altogether, novel hybrids of monastrol-MLT, in particular, 6a (-H), 6c (3-OMe), 6i (3,4-OMe), and 6j (3,5-OMe) could be addressed as starting points for further pharmacological studies concerning to combat colorectal cancer.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is considered one of the main cancers, being the third most diagnosed malignancy and the fourth leading cause of cancer death worldwide [1]. This pathology presents a great geographical distribution, and the patterns are very similar among men and women, being the third most common cancer in men and the second one in women [2]. Current treatments for CRC are effective, however, they all cause high-grade toxicity, which often results in dose limitations or cessation of the anticancer therapy [3,4,5]. Due to the widespread occurrence of the risk factors and the increase in the statistics, extensive research is ongoing to develop new potential chemopreventive agents against colorectal cancer.
Dihydropyrimidinone (DHPM) is characterized by its multi-functionalized scaffold that exhibits diverse biological activities [6,7,8,9,10] and especially anticancer activity [11]. Monastrol (Fig. 1), a DHPM, causes mitotic arrest at the G2/M phase by blocking bipolar mitotic spindle in mammalian cells due to inhibition of motility of mitotic kinesin Eg5, leading to cell apoptosis [12, 13]. On the other hand, the neurohormone melatonin (Fig. 1) is generated in several organs, mostly in the pineal gland, but also in the retina and gastrointestinal system. Numerous physiological activities, including the regulation of the immunological and endogenous antioxidant systems, are mediated by this molecule. Several in vitro and in vivo experimental models of tumors have shown that melatonin has an antiproliferative effect, suggesting that it may be an oncostatic drug in several cancer types [14]. It has been demonstrated to specifically impede the proliferation of prostate cancer cells that are both androgen-dependent and independent, through various mechanisms [15, 16].
Furthermore, molecular hybridization is a useful tool that has emerged in medicinal chemistry for the design of new drug prototypes. This strategy is based on the chemistry union of two pharmacophoric moieties of different bioactive substances to produce a new compound with distant physic-chemistry and pharmacokinetic properties. The subject of this strategy is to obtain compounds with improved affinity and efficacy, modified selectivity profile, and reduced undesired side effects [17, 18].
In this way, different authors have synthesized and evaluated different hybrids. For instance, Sashidhara and colleagues (2013) designed several coumarin-monastrol hybrids and evaluated their anti-breast cancer response. Insight this study, hybrid A showed good activity against MCF-7, T47D, and MDA-MB-231 cell lines. Besides, this compound induced apoptosis in both primary and metastatic cell lines (Fig. 1) [19]. In addition, another research showed that compound B, a monastrol-curcumin hybrid, showed maximum activity and it was found to be sensitive on different cancer cell lines as breast cancer MDA-MB-231 and HS 578T with growth percent (GP) values of 55.45 and 73.39, respectively; Prostate Cancer PC-3 (GP = 58.50); central nervous system cancer SNB-75 (GP = 59.60); leukemia RPMI-8226 (GP = 60.07), MOLT-4 (GP = 64.11) and CCRF-CEM (GP = 72.84) (Fig. 1) [20]. On the other hand, the tryptamine-salicylic acid hybrid C, exhibited a cytotoxic effect against A549 and MGC-803 causing cell-cycle arrest in the G2/M phase and apoptosis of MGC-803 cells in a concentration-dependent manner (Fig. 1) [21]. Finally, the melatonin-arylboronate hybrid D, exhibited strong toxic effects in cervical cancer cells (HeLa), without causing significant toxicity to normal NCTC-2544 cells. Besides, the cytotoxicity was accompanied by depletion of cellular glutathione (GSH) and increased ROS levels (Fig. 1) [22].
Considering all these facts and the urgent need for searching new therapeutic alternatives to treat colorectal cancer, this research was focused on the design and synthesis of a series of monastrol-melatonin hybrids (Fig. 2) together with their further biological evaluation in colorectal adenocarcinoma cells (SW480) and nonmalignant human colon mucosal epithelial cell line (NCM460) to determine the chemopreventive potential of these new molecules against this type of lethal pathology.
Results and discussion
Chemistry
As illustrated in Scheme 1, the synthesis of monastrol-melatonin hybrids uses the Biginelli multicomponent reaction method to generate the monastrol intermediates 2a–k. In this way, different aldehydes (1A) were condensed with ethyl acetoacetate (1B) and thiourea (1C), using citric acid as a catalyst. The reaction was carried out under solventless conditions [23] and obtained yields between 60–99%. These compounds have already been reported [12, 24,25,26,27,28,29].
On the other hand, the reaction of 5-methoxytryptamine (3) with chloroacetyl chloride (4) led to amide (5) with 60% yield [30]. Finally, nucleophilic substitution between 2a–k and the amide 5 led to the formation of hybrids 6a–k in 40–80% yields [24] (Table 1).
Using a combination of 1H-NMR, 13C-NMR, and HRMS-ESI (m/z) spectra analysis, the structures of all compounds were set. The molecular weights of the compounds were shown by the typical [M + H]+ peaks in the HRMS-ESI (m/z) spectra. J-constants and typical δ-values were used to allocate the signals to specific H or C-atoms. The 1H-NMR spectra of hybrids dissolved in CDCl3 showed between 5.40-5.50 ppm, a signal corresponding to CH-Ar. The CH3-C=C- and S-CH2 appear around 2.28 and 2.84 ppm, respectively. 13C-NMR spectra of the hybrids showed around 170 and 167 ppm a signal corresponding to the carbonyl groups of the amide and ester, respectively. The indole ring exhibited a signal around 122.96 ppm. Finally, the signals corresponding to NH-CH2 and S-CH2 were observed around 39.80 and 33.48 ppm, respectively.
Biological assays
The cytotoxic activity of the monastrol-melatonin hybrids 6a–k was tested against a human adenocarcinoma cell line (SW480) and nonmalignant human colon mucosal epithelial cells through the SRB assay. This research included different controls such as monastrol, melatonin, and the physical mixture of both to check the effectiveness of molecular hybridization; in addition, we included the vehicle control to establish the baseline, and the control with the reference drug (5-fluorouracil, 5-FU). The results are presented as inhibitory concentration 50 (IC50), which indicates the concentration of the compound needed the reduce the cell viability by 50%, besides, we also report the selectivity index (SI). According to the results shown in Table 2, after 48 h of treatment, all synthesized compounds 6a–k exert good cytotoxic activity against the SW480 cell line with IC50 values in the range of 2.2 ± 1.08 to 28.9 ± 1.14 µM, highlighting the importance of molecular hybridization since neither the parent compounds nor their physical mixture exhibited activity under the conditions evaluated (IC50 > 40 µM). In addition, we also observed that compounds 6a, 6c, 6i, and 6j significantly increased the selectivity over time becoming slightly more selective than the reference drug (5-FU) after 96 h of treatment, with selectivity indices of 2.1, 2.4, 3.1, and 2.1, respectively, which probably indicates that these compounds are likely to become less toxic over time against the nonmalignant cell line NCM460 without affecting the activity in SW480 cell. This effect of time was proved through two-way ANOVA, with a p-value of 0.0047.
In addition, as shown in Fig. 3 we observed that these four compounds decrease cell viability of SW480 cells in a concentration and time dependent manner. These results were also evidenced by an optical microscope. Thereon, in Fig. 4 we illustrate those changes that were clearly visible in malignant cells, altering cell number, shape, size, adhesion, and inducing granularity, while nonmalignant cell lines preserve healthy shape after the treatment with different concentrations (5, 10, and 20 µM, close to the IC50 value at the different points of evaluation) of the hybrid molecules. Different authors have also evaluated other monastrol or melatonin derivatives using in vitro models. Mervat and colleagues published in 2020 several monastrol derivatives with potential activity against a variety of cancers including the colon, finding activity through the inhibition of the Eg5 kinesin enzyme which probably leads to mitotic cell arrest at the G2/M phase, and subsequent cell death [31]. In addition, we previously published some chalcone-melatonin hybrids with remarkable cytotoxic and antiproliferative activity against SW480 cells [32]. Moreover, SanJuan et al. (2023) published for the first time the potent biological activity of Agomelatine, a naphthalene analog of melatonin, through cell-cycle arrest and caspase-dependent apoptotic death of colon cancer cells [33]. These studies support our findings about the antiproliferative effect exerted by our monastrol-melatonin hybrids on these colon cancer cell lines, highlighting the importance of realizing further studies on this matter.
Effect of the most active monastrol/melatonin hybrids (6a, 6c, 6i, and 6j) on cell proliferation of SW480 cells 48 h post treatment with different concentrations of compounds (0.6–40 µM). Data are presented as the mean ± SE of at least three independent experiments. Vehicle control (DMSO 0.5%) was assumed as 100% of viability
Structure–activity Relationship (SAR)
According to the SAR analysis illustrated in Fig. 5, the hybrid 6a without any substitution displayed moderate selectivity, even though it did not show the lowest IC50 value. In addition, we could observe that substitution at position 3 with an electron-donating group (-OCH3) seems to be a key feature in the cytotoxic activity and selectivity as evidenced in the response induced by compounds 6c, 6i, and 6j. The IC50 values in these three molecules were even lower regarding 6a. The importance of this position and methoxy group was also evidenced by the results of compound 6b, where the presence of an electron-withdrawing group (-Cl) in position 3 of the aromatic ring decreased selectivity. In addition, we could observe that the mono substitution at position 4 was not very relevant for the selectivity since compounds 6d substituted with an electron-withdrawing group (-F)) and 6e with an electron-donating group (-N(CH3)2) although displayed low IC50 values after 96 h of treatment (6.6 and 7.2 µM, respectively), they did not improve the selectivity over time. Besides, contrasting the results observed with disubstituted compounds 6g (with substitution pattern 2,4-OCH3) which decreased the selectivity, with hybrid 6i (3,4-OCH3 substitution), which displayed an important improvement in selectivity over time, we propose that the substitution in position 4 imparts an improvement in the activity when it cooperates with another substituent on the aromatic ring, being stronger the synergia between positions 3 and 4 because it has a positive effect in selectivity, while in positions 2 and 4 the selectivity decreases. Similar results were observed regarding position 2 with compounds 6f (substitution pattern 2,3-OCH3) and 6h (substitution pattern 2,5-OCH3) since these compounds displayed the lowest activity against malignant cells with the highest IC50 values after 96 h of treatment (18.9 and 14.7 µM, respectively), besides, they displayed poor selectivity. We also appreciate that the synergistic effect observed with the above-mentioned patterns is lost when we have a trisubstituted compound since 6k showed low cytotoxicity and selectivity, so it seems that the synergic effect on positions 3 and 4 are lost when the compound has three substitutions on the aromatic ring rather than having only two.
Theoretical drug-likeness and toxicological evaluation for promising hybrids 6a, 6c, 6i, and 6j
Characteristics such as drug-likeness, pharmacokinetic, and physicochemical properties can be used as efficient filters in the development of new anticancer candidates. Preclinical and clinical trials for a lead molecule could be encouraged by early biopharmaceutical profiling forecasts that increase the likelihood of success in drug discovery settings and result in a significant reduction of time and cost. In this work, pharmacokinetic parameters were computed by using the SwissADME web tool [34]. As previously discussed, among all the synthesized compounds, 6a (-H), 6c (3-OMe), 6i (3,4-OMe), and 6j (3,5-OMe) showed great ability to induce cytotoxicity in human colorectal cancer cells (SW480) with good selectivity (IS > 2), therefore, in the course of this section we focused our study only on these hybrids. Thereby, eleven crucial biopharmaceutical parameters were calculated for the selected hybrids and then compared against those major approved drugs (Table 3).
Our findings suggest suitable pharmacokinetics indices for hybrids compared to major marketed drugs, showing their therapeutic potential as orally administrable drug candidates for further pre-clinical experiments. In particular, the degree of lipophilicity (calculated as logPo/w) was predicted to be around 3.03, fitting well within the ideal range for lipid-based formulations (−2.0 to 6.0) [35]. In addition to this crucial permeability parameter, we also calculated the PSA, which similar to logP well correlates to the passive permeation of drugs through the biological cell membranes [36]. Notably, hybrids showed optimal PSA values of 130.11 and 148.57 Å2, respectively, which together with the logPo/w value would suggest good oral absorption and bioavailability. Besides, we also investigated both the fraction of carbon atoms that are sp3 hybridized (Fsp3) and the aromatic ring count (#ArRNG) which are two key new drug-likeness parameters relating to the possibility of liability or movement of a molecule through a biological barrier [37,38,39,40,41]. Major commercially available drugs have Fsp3 < 0.5. In this context, optimal 0.30 and 0.34 of Fsp3 values were found for hybrids, respectively. Further, the number of aromatic and heteroaromatic rings (#ArRNG) was counted. Current evidence has shown that molecules containing ≤3 aromatic rings (∼96% of marketed drugs meet this criterion) would have a better chance during drug development [40]. In this respect, the top-three hybrids bearing three aromatic rings would have an optimal developability profile. On the other hand, the in silico passive transmembrane permeation calculated for the title compounds using Caco-2 cell monolayers or MDCK cells as models was also considered. Both models are often recommended as a simplified in vitro model of intestinal absorption after oral administration in drug discovery [41,42,43,44]. Thus, promising compounds would possess a permeability range from 467 to 307 nm/s, respectively, across the intestinal Caco-2 cell monolayers model, and an apparent permeability (Papp) between 524 and 578 nm/s across a monolayer of MDCK cells. These in silico permeability results mean that these hybrids would be suitable to test in further studies for oral dosing. Another approach that predicted the ability of the drug candidates to bind blood plasma proteins was computed for 6a, 6c, 6i, and 6j. The most crucial factor influencing the distribution and transportation of anticancer formulations in the systemic circulation and a crucial factor in the initial stages of drug discovery is binding to human serum albumin (expressed as logKHSA) [45, 46]. According to the predictive model, compounds with positive values are expected to have a higher affinity for binding HSA, whereas compounds with negative values may exhibit a lower affinity for binding HSA. The calculated binding affinity to HSA for the top-three hybrids resulted to be positive numbers of 0.957 (for 6a), 0.950 (for 6c), 0.903 (for 6i), and 0.896 (for 6j), which fit well within the recommended range for potential oral drugs candidates (–1.5 to 1.5).
In addition to pharmacokinetic studies, ten toxicological endpoints have also been estimated for the lead-hybrids 6a, 6c, 6i, and 6j by employing several open-source chemoinformatic servers such as OSIRIS, TEST, ProTox-II, Pred-hERG, pkCSM, SwissADME, ToxTree, and ADMET-SAR (Table 4). These parameters are closely associated with adverse effects in the progress of a lead molecule, and their early estimation through artificial intelligence tools offer an attractive and rapid low-cost approach toward the design of safer pharmaceutical lead candidates [47, 48]. Regarding their in silico toxicological diagnosis, promising 6a, 6c, 6i, and 6j would have no apparent warnings, precautions and adverse events as mutagenic, tumorogenic, irritant, hepato/nephro/neuro/cardio/immunotoxic, any reproductive toxic effect, any structural alert for covalent DNA binding, and any conspicuous oral toxicity. As well, no alerts for pan-assay promiscuity (PAINS) were found for hit-hybrids.
Conclusions
Eleven monastrol-melatonin hybrids were synthesized and their structures were confirmed by means of 1H-NMR, 13C-NMR, DEPT-135 spectroscopy, and ESI-MS. The biological activity of these compounds was tested against a colorectal cancer cell line SW480 and a normal human colon mucosal epithelial cell line NCM460. The most active and selective compound 6i showed cytotoxicity against SW480 cells, with an IC50 value of 5.2 ± 1.22 µM, and the IC50 value against NCM460 was 16.4 ± 1.07 µM after 96 h of treatment, with a SI of 3.1, being much more active than parental compounds (melatonin and monastrol) and slightly more selective than the reference drug 5FU. The increase in the activity and selectivity was supposed to be attributed to the presence of electron-donating groups like methoxy at 3 and 4 positions. In addition, theoretical drug-likeness and toxicological studies would suggest that the promising hybrids 6a, 6c, 6i, and 6j exhibit optimal biopharmaceutical indices to be considered in further pre-clinical testing. Taken altogether, hybrids merging monastrol/MLT of the present study could serve as a promising scaffold in the future for the development of more effective anti-colorectal cancer agents. However, further theoretical, and experimental assays are needed to further validate these preliminary findings.
Experimental methods and materials
Chemistry
1H NMR and 13C NMR spectra were measured in CDCl3 solutions on a Varian instrument operating at 300 and 75 MHz, respectively, at 25 °C and referenced to tetramethylsilane (TMS). Chemical shifts are expressed in terms of parts per million (ppm, δ) in relation to TMS. High-resolution mass spectra of every hybrid were obtained using electrospray ionization mass spectrometry (ESI-MS). In the W-mode, a QTOF Premier apparatus (Waters, Manchester, UK) featuring an orthogonal Z-spray-electrospray interface was employed. Using a Stuart SMP10 Digital Melting Point apparatus, the melting point (uncorrected) was ascertained. Commercial sources provided all common reagents and solvents, which were utilized without additional purification. Analytical thin-layer chromatography (TLC) was used to track the progress of the chemical reactions on precoated silica gel 60 F254 (Merck), and UV light (λ = 254 nm) was used to detect the spots. On Merck 230–400 mesh silica gel, column chromatography separations were carried out under standard pressure (Merck).
General procedure for the synthesis of monastrol derivatives (2a–k)
a mixture of aldehyde 1A (1.4 mmol), ethyl acetoacetate 1B (2,1 mmol), thiourea 1C (2.4 mmol) and citric acid (0.8 mmol) was taken into a round bottom flask and the reaction mixture heated at 80 °C for 5 h. Then the reaction mixture was dissolved in ethyl acetate and it was transferred to a separatory funnel and washed twice with water, the organic layer was dried with Na2SO4 which, after filtration, was concentrated on a rotatory evaporator, and the residue was purified by column chromatography over silica gel using a mixture of hexane/ethyl acetate of different ratios to obtain the respective monastrol derivative 2a–k.
Synthesis of 2-chloro-N-(2-(5-methoxy-1H-indol-3-yl)ethyl)acetamide (5)
5-methoxytryptamine (3) (1,6 mmol) was dissolved in dry THF (7 mL) in a reaction flask equipped with a magnetic stirrer and then the system was sealed and saturated with argon. This solution was stirred in an ice-cold water bath for 15 min, then at this temperature, DIPEA (1,7 mmol) and 2-chloroacetyl chloride 4 (1,7 mmol) were added to the reaction flask using a syringe. The reaction mixture was stirred in the same water bath for 2 more hours. After completion of the reaction, the mixture is vacuum dried, and then it is re-dissolved in ethyl acetate, transferred to a separatory funnel, and washed twice initially with water and then with an HCl 10% solution. The organic layer was dried with Na2SO4 and then it was vacuum dried to acquire a crude material which was purified by silica gel chromatography column using a solution of hexane/ethyl acetate of different polarities to acquire amide 5 with a yield of 60%.
Synthesis of monastrol-melatonin hybrids 6a–k
A mixture of monastrol derivatives (2a–k) (0,2 mmol), amide 5 (0,2 mmol), Et3N (0,6 mmol), KI (0,3 mmol), and absolute EtOH (8 mL) was refluxed for 20 h. After reaction completion, the mixture was vacuum dried, and then it was re-dissolved in ethyl acetate, transferred to a separatory funnel, and washed twice with water. The organic layer was dried with Na2SO4 and then it was vacuum dried to acquire a crude material which was purified by a silica gel preparative plate using a solution of hexane/ethyl acetate in a proportion of 4/7 as eluent to yield hybrids 6a–k between 40–80%.
Ethyl-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-6-phenyl-1,6-dihydropyrimidine-5-carboxylate (6a)
Pale yellow solid; yield: 40%, mp: 116–118 °C; 1H NMR (300 MHz, CDCl3) δ 7.39–7.19 (m, 6H), 7.04 (d, J = 2.5 Hz, 1H), 6.95 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.8, 2.5 Hz, 1H), 5.53 (s, 1H, CH-Ar), 4.16 (dd, J = 7.1, 3.1 Hz, 2H, CH3-CH2-O), 3.90 (s, 3H), 3.52 (d, J = 3.7 Hz, 2H), 3.48 (s, 2H), 2.80 (s, 2H, S-CH2), 2.31 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 170.07(NH-C = O), 166.81 (O-C=O), 153.88 (Ar-O), 144.28 (Ar-CH), 131.59 (Ar-NH), 128.63 (Ar), 127.76 (Ar), 127.59 (Ar), 126.84 (Ar), 123.01 (C=CH-NH), 112.56 (CH=C-CH2), 112.17 (Ar), 111.96(C–C=O), 100.72 (Ar), 60.14 (CH2-O), 56.06(CH-Ar), 39.76 (NH-CH2), 33.48 (S-CH2), 24.94, 14.25. HRMS: m/z 507.2100, calcd. for C27H30N4O4S, [M]+ 507.2096.
Ethyl 6-(3-chlorophenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6b)
Pale yellow solid; yield: 80%, mp: 57–59 °C; 1H NMR (300 MHz, MeOD) δ 7.04–6.98 (m, 4H), 6.91 (d, J = 7.6 Hz, 1H), 6.82 (d, J = 2.4 Hz, 1H), 6.78 (s, 1H), 6.55 (dd, J = 8.7, 2.4 Hz, 1H), 5.13 (s, 1H, CH-Ar), 3.92–3.81 (m, 2H, CH3-CH2-O), 3.62 (s, 3H), 3.40 (d, J = 14.4 Hz, 2H), 3.33–3.18 (m, 2H), 3.11 (s, 2H, S-CH2), 2.08 (s, 3H), 0.96 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, MeOD) δ 170.34 (NH-C=O), 166.81 (O-C=O), 153.53 (C-S), 133.76 (Ar-NH), 131.98 (Ar), 129.67 (Ar-Cl), 127.63 (Ar), 126.69 (Ar), 125.04 (C=CH-NH), 122.80 (Ar), 111.54 (CH=C-CH2), 111.40 (Ar), 111.25 (Ar), 99.81(C–C=O), 59.57 (CH2-O), 54.95 (CH-Ar), 39.90(NH-CH2), 32.79 (S-CH2), 24.61, 13.12. HRMS: m/z 542.1716, calcd. for C27H29ClN4O4S, [M]+ 542.1711.
Ethyl 2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-6-(3-methoxyphenyl)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6c)
Pale yellow solid; yield: 42%, mp: 67–69 °C; 1H NMR (300 MHz, CDCl3) δ 7.23 (m, 3H), 7.04 (d, J = 2.4 Hz, 1H), 6.94 (d, J = 2.4 Hz, 1H), 6.88 (s, 1H), 6.85 (s, 1H), 6.83–6.76 (m, 1H), 5.52 (s, 1H, CH-Ar), 4.25–4.07 (m, 2H, CH3-CH2-O), 3.90 (s, 3H), 3.78 (s, 3H), 3.50 (d, J = 5.1 Hz, 4H), 2.81 (s, 2H, S-CH2), 2.30 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 170.00(NH-C=O), 166.78(O-C=O), 159.76 (C-S), 153.88 (Ar-O), 131.58 (Ar-CH), 129.67 (Ar-NH), 127.77 (Ar), 122.96 (C=CH-NH), 119.20 (Ar), 113.13 (Ar), 112.58 (Ar), 112.30 (CH=C-CH2), 112.17 (Ar), 111.95 (C–C=O), 100.66 (Ar), 60.16, 56.03, 55.24, 39.6. HRMS: m/z 537.2264, calcd. for C28H32N4O5S, [M]+ 537.2258.
Ethyl 6-(4-fluorophenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6d)
Off-white solid; yield: 40%, mp: 89–91 °C; 1H NMR (300 MHz, CDCl3) δ 7.32–7.24 (m, 2H), 7.23–7.14 (m, 2H), 7.06 (d, J = 2.4 Hz, 1H), 6.96 (d, J = 8.7 Hz, 2H), 6.89 (dd, J = 8.7, 2.4 Hz, 1H), 5.47 (s, 1H, CH-Ar), 4.16 (dd, J = 7.1, 4.6 Hz, 2H, CH3-CH2-O), 3.91 (s, 3H), 3.51 (m, 4H), 2.83 (s, 2H, S-CH2), 2.32 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 169.95 (NH-C=O), 166.73 (O-C=O), 153.95 (Ar-O), 131.60 (Ar-CH), 128.55 (Ar-NH), 128.44 (Ar), 127.79 (Ar), 122.96 (C=CH-NH), 115.49 (Ar), 115.20 (CH=C-CH2), 112.61 (Ar), 112.27 (Ar), 111.97 (C–C=O), 100.74(Ar), 60.16 (CH2-O), 56.07 (CH-Ar), 39.87 (NH-CH2), 33.52 (S-CH2), 24.97, 14.26. HRMS: m/z 525.2001, calcd. for C27H29FN4O4S, [M]+ 525.1995.
Ethyl 6-(4-(dimethylamino)phenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6e)
Pale yellow solid; yield: 51%, mp: 92–94 °C; 1H NMR (300 MHz, CDCl3) δ 7.25 (d, J = 8.8 Hz, 1H), 7.13 (d, J = 8.8 Hz, 2H), 7.05 (d, J = 2.5 Hz, 1H), 6.94 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 8.8, 2.5 Hz, 1H), 6.63 (d, J = 8.8 Hz, 1H), 5.42 (s, 1H, CH-Ar), 4.15 (q, J = 7.1 Hz, 2H, CH3-CH2-O), 3.90 (s, 3H), 3.53 (s, 4H), 2.88 (s, 6H), 2.81 (s, 2H, S-CH2), 2.26 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 170.19(NH-C=O), 167.11(O-C=O), 153.85 (Ar-O), 150.03 (Ar-N(CH3)2), 132.60 (Ar-CH), 131.58 (Ar-NH), 127.77 (Ar), 127.65 (2Ar), 122.96 (C=CH-NH), 112.64 (CH=C-CH2), 112.46 (Ar), 112.13 (Ar), 111.95 (C–C=O), 100.61 (Ar), 60.02 (CH2-O), 56.01 (CH-Ar), 40.52 (NH(CH3)2), 39.70 (NH-CH2), 33.51 (S-CH2), 24.97, 14.32. HRMS: m/z 550.2477, calcd. for C29H35N5O4S, [M]+ 550.2476.
Ethyl 6-(2,3-dimethoxyphenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6f)
Pale yellow solid; yield: 41%, mp: 101–102 °C; 1H NMR (600 MHz, CDCl3) δ 7.22 (d, J = 8.9 Hz, 1H), 7.04 (d, J = 2.4 Hz, 1H), 6.99–6.95 (m, 2H), 6.85–6.80 (m, 2H), 6.72 (dd, J = 7.7, 1.5 Hz, 1H), 5.81 (s, 1H, CH-Ar), 4.09 (q, J = 7.1 Hz, 2H, CH3-CH2-O), 3.90 (s, 3H), 3.88 (s, 3H), 3.82 (s, 3H), 3.53–3.42 (m, 4H), 2.82 (s, 2H, S-CH2), 2.31 (s, 3H), 1.15 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, DMSO) δ 170.01(NH-C=O), 160.72(O-C=O), 153.41 (Ar-O), 145.94 (Ar-O), 136.50 (Ar-NH), 134.73 (Ar-CH), 133.17 (Ar), 131.40 (Ar), 127.56 (C=CH-NH), 123.35 (Ar), 112.11 (CH=C-CH2), 110.66 (Ar), 108.32 (Ar), 106.88 (Ar), 100.23 (C–C=O), 99.29 (Ar), 57.41 (CH3-O), 55.78, 55.55 (CH-Ar), 42.07, 22.69. HRMS: m/z 567.2319, calcd. for C29H34N4O6S, [M]+ 567.2314.
Ethyl 6-(2,4-dimethoxyphenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6g)
Pale yellow solid; yield: 54%, mp: 90–92 °C; 1H NMR (300 MHz, CDCl3) δ 7.26 (d, J = 8.8 Hz, 1H), 7.06 (d, J = 2.4 Hz, 1H), 7.03–6.94 (m, 2H), 6.88 (dd, J = 8.8, 2.4 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 6.40 (dd, J = 8.4, 2.4 Hz, 1H), 5.79 (s, 1H, CH-Ar), 4.12 (q, J = 7.1 Hz, 2H, CH3-CH2-O), 3.91 (s, 3H), 3.85 (s, 3H), 3.77 (s, 3H), 3.58–3.40 (m, 4H), 2.86 (s, 2H, S-CH2), 2.28 (s, 3H), 1.19 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 169.87 (NH-C=O), 166.89 (O-C=O), 160.53 (Ar-O), 157.60 (C-S), 153.93 (Ar-O), 131.58 (Ar-NH), 127.78 (Ar), 122.94 (C=CH-NH), 112.72 (CH=C-CH2), 112.18 (Ar), 111.92 (Ar), 104.04 (C–C=O), 100.59 (Ar), 98.86 (Ar), 59.95 (CH2-O), 55.99 (2CH3-O), 55.57 (CH3-O), 55.35 (CH-Ar), 39.79 (NH-CH2), 33.64 (S-CH2), 25.08, 14.24. HRMS: m/z 567.2349, calcd. for C29H34N4O6S, [M]+ 567.2344.
Ethyl 6-(2,5-dimethoxyphenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6h)
Pale yellow solid; yield: 46%, mp: 97–99 °C; 1H NMR (300 MHz, CDCl3) δ 7.25 (d, J = 8.7 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.99 (d, J = 8.7 Hz, 2H), 6.87 (dd, J = 8.7, 2.5 Hz, 1H), 6.48 (d, J = 2.4 Hz, 1H), 6.39 (dd, J = 8.7, 2.4 Hz, 1H), 5.79 (s, 1H, CH-Ar), 4.12 (q, J = 7.1 Hz, 2H, CH3-CH2-O), 3.90 (s, 3H), 3.85 (s, 3H), 3.76 (s, 3H), 3.57–3.38 (m, 4H), 2.85 (s, 2H, S-CH2), 2.28 (s, 3H), 1.19 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 169.98(NH-C=O), 166.94(O-C=O), 160.49 (C-S), 157.59 (CH-CH3), 153.90 (Ar-O), 131.59 (Ar-NH), 127.76 (Ar-CH), 122.98 (C=CH-NH), 112.66 (Ar), 112.14 (CH=C-CH2), 111.94 (C–C=O), 104.05 (Ar), 100.57 (Ar), 98.85 (Ar), 59.95 (CH2-O), 55.99 (3CH3-O), 55.57 (CH3-O), 55.36 (CH-Ar), 39.80 (NH-CH2), 33.61 (S-CH2), 25.06, 14.24. HRMS: m/z 567.2342, calcd. for C29H34N4O6S, [M]+ 567.2337.
Ethyl 6-(3,4-dimethoxyphenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6i)
Pale yellow solid; yield: 47%, mp: 104–106 °C; 1H NMR (300 MHz, CDCl3) δ 7.25 (d, J = 8.8 Hz, 1H), 7.05 (d, J = 2.4 Hz, 1H), 6.96 (d, J = 2.4 Hz, 1H), 6.88 (d, J = 2.4 Hz, 1H), 6.85 (d, J = 2.1 Hz, 1H), 6.78 (d, J = 2.1 Hz, 1H), 6.76 (s, 2H), 5.49 (s, 1H, CH-Ar), 4.17 (dd, J = 7.1, 1.8 Hz, 2H, CH3-CH2-O), 3.89 (s, 3H), 3.86 (s, 3H), 3.81 (s, 3H), 3.62–3.41 (m, 4H), 2.81 (s, 2H, S-CH2), 2.32 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 170.01 (NH-C=O), 166.85 (O-C=O), 153.89 (Ar-O), 148.90 (Ar-O), 148.47 (Ar-O), 137.02 (Ar-CH), 131.58 (Ar-NH), 127.75 (Ar), 123.03 (C=CH-NH), 118.87 (Ar), 112.48 (CH=C-CH2), 112.13 (Ar), 111.96 (Ar), 111.18 (Ar), 110.40 (C–C=O), 100.65 (Ar), 60.17 (CH2-O), 55.93 (CH-Ar), 39.80 (NH-CH2), 33.48 (S-CH2), 24.96, 14.33. HRMS: m/z 567.2318, calcd. for C29H34N4O6S, [M]+ 567.2314.
Ethyl 6-(3,5-dimethoxyphenyl)-2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (6j)
Pale yellow solid; yield: 44%, mp: 104–106 °C; 1H NMR (300 MHz, CDCl3) δ 7.25 (d, J = 8.8 Hz, 1H), 7.05 (d, J = 2.4 Hz, 1H), 6.94 (d, J = 2.4 Hz, 1H), 6.87 (dd, J = 8.8, 2.4 Hz, 1H), 6.47 (d, J = 2.2 Hz, 2H), 6.37 (t, J = 2.2 Hz, 1H), 5.48 (s, 1H, CH-Ar), 4.18 (dd, J = 7.1, 2.0 Hz, 2H, CH3-CH2-O), 3.90 (s, 3H), 3.76 (s, 6H), 3.51 (s, 4H), 2.81 (s, 2H, S-CH2), 2.27 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 170.02 (NH-C=O), 166.85 (O-C=O), 160.92 (2Ar-O), 153.87 (Ar-O), 131.58 (Ar-CH), 127.77 (Ar-NH), 122.94 (C=CH-NH), 112.56 (CH=C-CH2), 112.16 (Ar), 111.94 (Ar), 105.21 (2Ar-O), 100.61 (C–C=O), 98.80 (Ar), 60.15 (CH2-O), 56.00 (CH-Ar), 55.31 (3CH3-O), 39.64 (NH-CH2), 33.58 (S-CH2), 24.94, 14.33. HRMS: m/z 567.2313, calcd. for C29H34N4O6S, [M]+ 567.2308.
Ethyl 2-((2-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-2-oxoethyl)thio)-4-methyl-6-(3,4,5-trimethoxyphenyl)-1,6-dihydropyrimidine-5-carboxylate (6k)
Pale yellow solid; yield: 42%, mp: 87–89 °C; 1H NMR (300 MHz, CDCl3) δ 7.31 (s, 1H), 7.26 (d, J = 8.7 Hz, 1H), 7.05 (d, J = 2.5 Hz, 2H), 6.97 (s, 1H), 6.88 (dd, J = 8.7, 2.5 Hz, 1H), 6.51 (s, 1H), 5.49 (s, 1H, CH-Ar), 4.27–4.12 (m, 2H, CH3-CH2-O), 3.90 (s, 2H), 3.84 (s, 2H), 3.54 (m, 4H), 2.35 (s, 2H, S-CH2), 1.84 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H, CH3-CH2-O). 13C NMR (75 MHz, CDCl3) δ 169.83 (NH-C=O), 167.00 (O-C=O), 153.91 (CH-CH3), 153.24 (3Ar-O), 131.59 (Ar-NH), 127.77 (Ar), 122.96 (C=CH-NH), 112.52 (CH=C-CH2), 112.20(Ar), 111.95(Ar), 104.02 (C–C=O), 100.62(Ar), 60.81, 60.18, 56.16, 56.02, 39.82, 33.62, 25.02, 14.39. HRMS: m/z 597.2432, calcd. for C30H36N4O7S, [M]+ 597.2426.
In vitro biological assays
Cell lines and culture medium
Biological activity was investigated using nonmalignant cells (NCM460) and human colorectal adenocarcinoma cells (SW480), obtained from the European Collection of Authenticated Cell Cultures (ECACC, UK). Dulbecco’s Modified Eagle Medium (DMEM) was used to maintain the cells, supplemented with 10% horse serum previously inactivated at 56 °C, non-essential amino acids (1%), and antibiotics (penicillin/streptomycin, 1%) (Gibco Invitrogen, Carlsbad, CA, USA). For all the experiments, the horse serum was reduced to 3%, and the culture media was additionally supplemented with ITS-defined medium (10 mg/mL insulin, 5 mg/mL transferrin, and 5 ng/mL selenium) (Gibco, Invitrogen, Carlsbad, CA, USA) [49, 50].
Cell viability
The colorimetric test with Sulforhodamine B (SRB) was used to evaluate cell viability. Cell density was adjusted to 6500 cells/well in 96-well tissue culture plates and cells were first incubated for 24 h. After this, cell lines were daily treated with increasing concentrations (0.6 to 40 µM) of the different compounds, from 24 to 96 h. In addition to the hybrid molecules, in this study, we included different controls. The first one was the vehicle used to solubilize the compounds, DMSO (0.5%), to establish the baseline of the experiments. In addition, we used the lead compounds (monastrol, melatonin, and the equimolar mixture of both), and the reference drug (5-FU). The optimum conditions were 37 °C and 5% CO2. Following the conclusion of every treatment, culture plates were kept at 4 °C for an hour in order to fix the cells. Cold trichloroacetic acid (MERCK, 50% v/v) was then added. After that, 0.4% (w/v) SRB (Sigma-Aldrich, St. Louis, MO, USA) was used to stain the cell protein content. To get rid of any unbound SRB, acetic acid (1%) was used as a wash. Protein-bound SRB was allowed to air dry before being solubilized in 10 mM Tris-base and then the absorbance at 492 nm was measured using a Mindray MR-96A microplate reader [51, 52]. Every experiment was run through at least three times.
Statistical analysis
Each experiment had a minimum of three repeats according to the experimental design. The data was presented as mean ± standard error, or SE. Tukey’s test was run after the two-way ANOVA statistical analysis. Significant p-values were those less than 0.05. Data were analyzed with GraphPad Prism version 8.0.1 for (GraphPad Software, San Diego, CA, USA).
Theoretical pharmacokinetics and toxicological studies
For promising 6a, 6c, 6i, and 6j, twelve biopharmaceutics indices together were computed by using the open-source platform SwissADME [34]. Importantly, these parameters govern oral exposure, as well as permeability, absorption, motion, and action of potential oncological drug candidates. Besides, we explored the toxicological profile for hybrids using eight in silico freeware platforms such as OSIRIS [53], T.E.S.T Version 5.1.2 [54], ProTox-II [55], Pred-Herg [56], pkCSM [57], SwissADME [34], ToxTree [58] and ADMET-SAR [59].
References
Arnold M, Sierra M, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. https://doi.org/10.1136/gutjnl-2015-310912
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660
Alam W, Bouferraa Y, Haibe Y, Mukherji D, Shamseddine A. Management of colorectal cancer in the era of COVID-19_Challenges and suggestions. Sci Prog. 2021;104:104–14. https://doi.org/10.1177/00368504211010626
Campos J, Domínguez JF, Gallo MA, Espinosa A. From a classic approach in cancer chemotherapy towards differentiation therapy: acyclic and cyclic seven-membered 5-fluorouracil O, N-acetals. Curr Pharm Des. 2000;6:1797–810. https://doi.org/10.2174/1381612003398627
McQuade RM, Bornstein JC, Nurgali K. Anti-colorectal cancer chemotherapy-induced diarrhoea: Current treatments and side effects. Int J Clin Med. 2014;5:393–406. https://doi.org/10.4236/ijcm.2014.57054
Kaur R, Chaudhary S, Kumar K, Gupta MK, Rawal RK. Recent synthetic and medicinal perspectives of dihydropyrimidinones: a review. Eur J Med Chem. 2017;132:108–34. https://doi.org/10.1016/j.ejmech.2017.03.025
Ashok M, Holla B, Kumari N. Convenient one pot synthesis of some novel derivatives of thiazolo[2,3-b] dihydropyrimidinone possessing 4- methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities. Eur J Med Chem. 2007;42:380–5.
Naidu BN, Sorenson ME, Patel M, Ueda Y, Banville J, Beaulieu F, et al. Synthesis and evaluation of C2-carbon-linked heterocyclic-5-hydroxy-6-oxo-dihydropyrimidine-4-carboxamides as HIV-1integrase inhibitors. Bioorg Med Chem Lett. 2015;25:717–20. https://doi.org/10.1016/j.bmcl.2014.11.060
de Vasconcelos A, Oliveira PS, Ritter M, Freitag A, Romano L, Quina FH, et al. Antioxidant capacity and environmentally friendly synthesis of dihydropyrimidin-(2H)-ones promoted by naturally occurring organic acids. J Biochem Mol Toxicol. 2012;26:155–61. https://doi.org/10.1002/jbt.20424
Stefani HA, Oliveira CB, Almeida RB, Pereira CMP, Braga RC, Cella R, et al. Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: a new class of potential antioxidant agents. Eur J Med Chem. 2006;41:513–8. https://doi.org/10.1016/j.ejmech.2006.01.007
Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SI, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;268:971–4. https://doi.org/10.1126/science.286.5441.971
Russowsky D, Canto RFS, Sanches SAA, D’Oca MGM, de Fatima A, Pilli RA, et al. Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: monastrol, oxo-monastrol and oxygenated analogues. Bioorg Chem. 2006;34:173–82. https://doi.org/10.1016/j.bioorg.2006.04.003
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–84. https://doi.org/10.1016/j.pneurobio.2010.12.004
Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, et al. Melatonin for the prevention and treatment of cancer. Oncotarget. 2017;8:39896–921. https://doi.org/10.18632/oncotarget.16379
Sigurdardottir LG, Markt SC, Rider JR, Haneuse S, Fall K, Schernhammer ES, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol. 2015;67:191–4. https://doi.org/10.1016/j.eururo.2014.07.008
Tai SY, Huang SP, Bao BY, Wu MT. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer: a case-control study. Sci Rep. 2016;8:29606 https://doi.org/10.1038/srep29606
Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res. 2008;41:69–77. https://doi.org/10.1021/ar7000843
De Oliveira Pedrosa M, Duarte da Cruz RM, de Oliveira Viana J, de Moura RO, Ishiki HM, Barbosa Filho JM, et al. Hybrid compounds as direct multitarget ligands: a review. Curr Top Med Chem. 2017;17:1044–79. https://doi.org/10.2174/1568026616666160927160620
Sashidhara KV, Avula SR, Sharma K, Palnati GR, Bathula SR. Discovery of coumarin-monastrol hybrid as potential antibreast tumor-specific agent. Eur J Med Chem. 2013;60:120–7. https://doi.org/10.1016/j.ejmech.2012.11.044
Sharma R, Jadav SS, Yasmin S, Bhatia S, Khalilullah H, Ahsan MJ. Simple, efficient, and improved synthesis of Biginelli-type compounds of curcumin as anticancer agents. Med Chem Res. 2015;24:636–44. https://doi.org/10.1007/s00044-014-1146-2
Xiong R, He D, Deng X, Liu J, Lei X, Xie Z, et al. Design, synthesis and biological evaluation of tryptamine salicylic acid derivatives as potential antitumor agents. Medchemcomm. 2019;10:573–83. https://doi.org/10.1039/c8md00484f
Bedini A, Fraternale A, Crinelli R, Mari M, Bartolucci S, Chiarantini L, et al. Design, synthesis, and biological activity of hydrogen peroxide responsive arylboronate melatonin hybrids. Chem Res Toxicol. 2019;32:100–12. https://doi.org/10.1021/acs.chemrestox.8b00216
Ramu E, Kotra V, Bansal N, Varala R, Adapa SR. Green approach for the efficient synthesis of biginelli compounds promoted by citric acid under solvent-free conditions. Rasayan J Chem. 2008;1:188–94. https://api.semanticscholar.org/CorpusID:98857136
Ragab FAF, Abou-Seri SM, Abdel-Aziz SA, Alfayomy AM, Aboelmagd M. Design, synthesis and anticancer activity of new monastrol analogues bearing 1,3,4-oxadiazole moiety. Eur J Med Chem. 2017;138:140–51. https://doi.org/10.1016/j.ejmech.2017.06.026
Bidram Z, Sirous H, Khodarahmi GA, Hassanzadeh F, Dana N, Hariri AA, et al. Monastrol derivatives: in silico and in vitro cytotoxicity assessments. Res Pharm Sci. 2020;15:249–62. https://doi.org/10.4103/1735-5362.288427
Elmaghraby AM, Mousa IA, Harb AA, Mahgoub MY. Three component reaction: an efficient synthesis and reactions of 3,4-dihydropyrimidin-2(1H)-ones and thiones using new natural catalyst. ISRN Org Chem. 2013;2013:1–14. https://doi.org/10.1155/2013/706437
Besoluk S, Kucukislamoglu M, Nebioglu M, Zengin M, Arslan M. Solvent-free synthesis of dihydropyrimidinones catalyzed by alumina sulfuric acid at room temperature. J Iran Chem Soc. 2008;5:62–6. https://doi.org/10.1007/BF03245816
Dai Z, Pittman CU Jr, Li T. Enantiomeric separation of racemic 4-Aryl-1,4-dihydropyridines and 4-aryl-1,2,3,4-tetrahydropyrimidines on a chiral tetraproline stationary phase. Chirality. 2013;25:238–42. https://doi.org/10.1002/chir.22135
Egorov DM, Babushkina AA, Leonenok VE, Chekalov AP, Piterskaya YL. Synthesis of 3-phosphorylated thiazolo[3,2-a]pyrimidine-6-carboxylates. Russ J Gen Chem. 2020;90:319–21. https://doi.org/10.1134/S1070363220020267
Pakhare D, Kusurkar R. Application of Horner-Wadsworth-Emmons olefination for the synthesis of granulatamide A, its: E isomer and other amides of tryptamine. N J Chem. 2016;40:5428–31. https://doi.org/10.1039/C5NJ03533C
El-Hamamsy MH, Sharafeldin NA, El-Moselhy TF, Tawfik HO. Design, synthesis, and molecular docking study of new monastrol analogues as kinesin spindle protein inhibitors. Arch Pharm (Weinheim). 2020;353:e2000060–78. https://doi.org/10.1002/ardp.202000060
Yepes AF, Arias JD, Cardona-G W, et al. New class of hybrids based on chalcone and melatonin: a promising therapeutic option for the treatment of colorectal cancer. Med Chem Res. 2021;30:2240–55. https://doi.org/10.1007/s00044-021-02805-7
Moreno-SanJuan S, Puentes-Pardo JD, Casado J, Escudero-Feliu J, Khaldy H, Arnedo J, et al. Agomelatine, a melatonin-derived drug, as a new strategy for the treatment of colorectal cancer. Antioxidants. 2023;12:926–37. https://doi.org/10.3390/antiox12040926
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717–29. https://doi.org/10.1038/srep42717
Ditzinger F, Price DJ, Ilie AR, Köhl NJ, Jankovic S, et al. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches - a PEARRL review. J Pharm Pharmacol. 2019;71:464–82. https://doi.org/10.1111/jphp.12984
Ertl P, Rohdem B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43:3714–7. https://doi.org/10.1021/jm000942e
Klein HF, Hamilton DJ, de Esch IJP, Wijtmans M, O’Brien P. Escape from planarity in fragment-based drug discovery: a synthetic strategy analysis of synthetic 3D fragment libraries. Drug Discov Today. 2022;27:2484–96. https://doi.org/10.1016/j.drudis.2022.05.021
Benardout M, Le Gresley A, ElShaer A, Wren SP. Application of fSP3 towards nonsystemic drug discovery. pre-print 2023; https://doi.org/10.20944/preprints202308.1882.v1.
Wei W, Cherukupalli S, Jing L, Liu X, Zhan P. Fsp3: a new parameter for drug-likeness. Drug Discov Today. 2020;25:1839–45. https://doi.org/10.1016/j.drudis.2020.07.017
Ward SE, Beswick P. What does the aromatic ring number mean for drug design? Expert Opin Drug Discov. 2014;9:995–1003. https://doi.org/10.1517/17460441.2014.932346
Ritchie TJ, Macdonald SJ, Young RJ, Pickett SD. The impact of aromatic ring count on compound developability: further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discov Today. 2011;16:164–71. https://doi.org/10.1016/j.drudis.2010.11.014
Pham-The H, Cabrera-Pérez MA, Nam NH, Castillo-Garit JA, Rasulev BH. In silico assessment of ADME properties: advances in Caco-2 cell monolayer permeability modeling. Curr Top Med Chem. 2018;18:2209–17. https://doi.org/10.2174/1568026619666181130140350
Broccatelli F, Salphati L, Plise E, Cheong J, Gobbi A, Lee M. Predicting passive permeability of drug-like molecules from chemical structure: where are we? Mol Pharm. 2016;13:4199–209. https://doi.org/10.1021/acs.molpharmaceut.6b00836
Press B, Di Grandi D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr Drug Metab. 2008;9:893–900. https://doi.org/10.2174/138920008786485119
Zhivkova Z. Studies on drug-human serum albumin binding: the current state of the matter. Curr Pharm Des. 2015;21:1817–30. https://doi.org/10.2174/1381612821666150302113710
Colmenarejo G. In silico prediction of drug-binding strengths to human serum albumin. Med Res Rev. 2003;23:275–301. https://doi.org/10.1002/med.10039
Pognan F, Beilmann M, Boonen HCM. The evolving role of investigative toxicology in the pharmaceutical industry. Nat Rev Drug Discov. 2023;22:317–35. https://doi.org/10.1038/s41573-022-00633-x
Tran TTV, Surya Wibowo A, Tayara H, Chong KT. Artificial intelligence in drug toxicity prediction: recent advances, challenges, and future perspectives. J Chem Inf Model. 2023;63:2628–43. https://doi.org/10.1021/acs.jcim.3c00200
Herrera-R A, Castrillón W, Otero E, Ruiz E, Carda M, Agut R, et al. Synthesis and antiproliferative activity of 3- and 7-styrylcoumarins. Med Chem Res. 2018;27:1893–905. https://doi.org/10.1007/s00044-018-2202-0
Herrera-Ramirez A, Yepes-Pérez AF, Quintero-Saumeth J, Moreno-Quintero G, Naranjo TW, Cardona-Galeano W. Colorectal cancer chemoprevention by S-allyl cysteine-caffeic acid hybrids: in vitro biological activity and in silico studies. Sci Pharm. 2022;90:40–59. https://doi.org/10.3390/scipharm90030040
Castrillón-López W, Herrera-Ramírez A, Moreno-Quintero G, Coa JC, Naranjo TW, Cardona-Galeano W. Resveratrol/hydrazone hybrids: synthesis and chemopreventive activity against colorectal cancer cells. Pharmaceutics. 2022;14:2278–97. https://doi.org/10.3390/pharmaceutics14112278
Herrera-R A, Moreno G, Araque P, Vásquez I, Naranjo E, Alzate F, et al. In vitro chemopreventive potential of a chromone from Bomarea setacea (Alstroemeriaceae) against colorectal cancer. Iran J Pharm Res. 2021;20:254–67. https://doi.org/10.22037/ijpr.2020.113745.14466
Sander T. OSIRIS property explorer. Organic Chemistry Portal. 2001. https://www.organic-chemistry.org/prog/peo
US EPA. User’s guide for T.E.S.T. (version 5.1) (toxicity estimation software tool): a program to estimate toxicity from molecular structure. Washington, D.C.: US EPA; 2020
Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46:257–63. https://doi.org/10.1093/nar/gky318
Braga RC, Alves VM, Silva MF, Muratov E, Fourches D, Lião LM, et al. Pred-hERG: a novel web-accessible computational tool for predicting cardiac toxicity. Mol Inform. 2015;34:698–701. https://doi.org/10.1002/minf.201500040
Pires DE, Blundell TL, Ascher DB. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 2015;58:4066–72. https://doi.org/10.1021/acs.jmedchem.5b00104
Patlewicz G, Jeliazkova N, Safford RJ, Worth AP, Aleksiev B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ Res. 2008;19:495–524. https://doi.org/10.1080/10629360802083871
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, et al. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model. 2012;52:3099–105. https://doi.org/10.1021/ci300367a
Acknowledgements
We thank the University of Antioquia (grant CODI 2020-32831) for financial support.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Preciado-A, D., Yepes, A.F., Herrera-R, A. et al. Novel monastrol/melatonin hybrids as a new approach for colorectal cancer intervention: design, synthesis, biological activity, and drug-likeness modeling studies. Med Chem Res (2024). https://doi.org/10.1007/s00044-024-03223-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00044-024-03223-1