Abstract
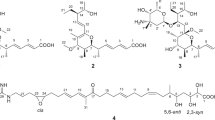
In the pursuit of discovering new active metabolites from helicosporous hyphomycetes, rice fermentation products of Neohelicosporium griseum were examined. Eight compounds were isolated from this saprophytic fungus, namely vertixanthone (1), diaportheone A (2), 1,3,6,8-tetrahydroxyanthraquinone (3), lecanoric acid (4), decarboxycitrinone (5), 6,8-dihydroxy-4-hydroxymethyl-3,5-dimethyl-isochromen-1-one (6), decarboxyhydroxycitrinone (7), and ergosterin (8). The 1D and 2D NMR characteristics of compound 1 in DMSO-d6 were detailed for the first time. Antimicrobial testing indicated that compounds 1–4 exhibited moderate activity against Pseudomonas aeruginosa, with compound 3 also showing weak activity against Staphylococcus aureus. In-vitro cytotoxicity assays revealed that compounds 1, 3, and 4 displayed cytotoxic activity against HELA cell lines, with respective IC50 values of 30.8, 13.7, and 14.1 µM. Compounds 1, 3, and 4 also showed significant cytotoxicity against A549 cell lines, with respective IC50 values of 24.7, 7.4, and 10.3 µM.




Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Tang KWK, Millar BC, Moore JE. Antimicrobial resistance (AMR). Brit J Biomed Sci. 2023;80:11387. https://doi.org/10.3389/bjbs.2023.11387.
Zhang X, Meng LH. Progress in molecularly targeted anti-tumor drugs derived from natural products or their derivatives. APSB. 2020;55:2491–500. https://doi.org/10.16438/j.0513-4870.2020-0248.
Conrado R, Gomes TC, Roque GSC, De Souza AO. Overview of bioactive fungal secondary metabolites: cytotoxic and antimicrobial compounds. Antibiotics. 2022;11:1604. https://doi.org/10.3390/antibiotics11111604.
Lu YZ, Liu JK, Hyde KD, Jeewon R, Kang JC, Fan C. et al. A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers. 2018;92:131–344. https://doi.org/10.1007/s13225-018-0411-y.
Xiao XJ, Ma J, Zhang LJ, Liu NG, Xiao YP, Tian XG. et al. Additions to the Genus Helicosporium (Tubeufiaceae, Tubeufiales) from China with an Identification Key to Helicosporium Taxa. J Fungi.2023;9:775. https://doi.org/10.20944/preprints202306.1515.v1.
Zhang LJ, Yang MF, Ma J, Xiao XJ, Ma XY, Zheng DG. et al. Neogrisphenol A, a potential ovarian cancer inhibitor from a new record fungus Neohelicosporium griseum. Metabolites. 2023;13:435. https://doi.org/10.3390/metabo13030435.
Qian SY, Zeng XB, Qian YX, Lu YZ, He ZJ, Chang JC. A Saprophytic Fungus Tubeufia rubra Produces Novel Rubracin D and E reversing multidrug resistance in cancer cells. J Fungi. 2023;9:309. https://doi.org/10.3390/jof9030309.
Yamazaki H, Rotinsulu H, Kaneko T, Murakami K, Fujiwara H, Ukai K. et al. A new dibenz [b, e] oxepine derivative, 1-hydroxy-10-methoxy-dibenz [b,e] oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar Drugs. 2012;10:2691–7. https://doi.org/10.3390/md10122691.
Wang CN, Lu HM, Gao CH, Guo L, Zhan ZY, Wang JJ. et al. Cytotoxic benzopyranone and xanthone derivatives from a coral symbiotic fungus Cladosporium halotolerans GXIMD 02502. Nat Prod Res. 2021;3:5596–603. https://doi.org/10.1080/14786419.2020.1799363.
Bungihan ME, Tan MA, Kitajima M, Kogure N, Franzblau SG, dela Cruz TEE. et al. Bioactive metabolites of Diaporthe sp. P133, an endophytic fungus isolated from Pandanus amaryllifolius. J Nat Med. 2011;65:606–9. https://doi.org/10.1007/s11418-011-0518-x.
Tan MA, Züger PP, Roggo S. Total synthesis of diaportheone A. Tetrahedron Lett. 2019;60:52–4. https://doi.org/10.1016/j.tetlet.2018.11.055.
Lum KY, Taki AC, Gasser RB, Tietjen I, Ekins MG, White JM. et al. Comatulins A–E, Taurine-Conjugated Anthraquinones from the Australian Crinoid Comatula rotalaria. J Nat Prod. 2020;83:1971–9. https://doi.org/10.1021/acs.jnatprod.0c00267.
Cheng MJ, Wu MD, Chang HS, Chen JJ, Tseng M. Secondary metabolites from the actinobacterium amycolatopsis Taiwanensis. Chem Nat Compd. 2022;58:175–7. https://link.springer.com/article/10.1007/s10600-022-03627-8.
Pavan Kumar P, Siva B, Anand A, Tiwari AK, Vekata Rao C, Boustie J, Suresh Babu K. Isolation, semi-synthesis, free-radicals scavenging, and advanced glycation end products formation inhibitory constituents from Parmotrema tinctorum. J Asian Nat Prod Res. 2020;22:976–88. https://doi.org/10.1080/10286020.2019.1628024.
Liu TT, Liao XJ, Xu SH, Zhao BX. Solieritide A, a new polyketide from the red alga Solieria sp. Nat Prod Res. 2021;35:3780–6. https://doi.org/10.1080/14786419.2020.1737057.
Jia LC. A new benzofuran from Penicillium oxalicum, an endophytic fungus isolated from Pseudostellaria heterophylla. Chin Tradit Herbal. Drugs. 2020;51:5681–6. https://doi.org/10.7501/j.issn.0253-2670.2020.22.003.
Cai Y, Rao L, Zou Y. Genome mining discovery of a C4-alkylated dihydroisocoumarin pathway in fungi. Org Lett. 2021;23:2337–41. https://doi.org/10.1021/acs.orglett.1c00458.
Tang J, Xu R, Zhao X, Wang YT, Tan HY, Shan JJ. et al. Chemical constituents and their biological activities of Lanmaoa asiatica. Mycosystema. 2023;42:1345–59. https://doi.org/10.13346/j.mycosystema.220246.
Rangsinth P, Sharika R, Pattarachotanant N, Duangjan C, Wongwan C, Sillapachaiyaporn C. et al. Potential beneficial effects and pharmacological properties of ergosterol, a common bioactive compound in edible mushrooms. Foods. 2023;12:2529. https://doi.org/10.3390/foods12132529.
Guo Z, Liu X, Wang N, Mo P, Shen J, Liu M. et al. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol. 2023;237:930–43. https://doi.org/10.1111/nph.18575.
Shao L, Marin-Felix Y, Surup F, Stchigel AM, Stadler M. Seven new cytotoxic and antimicrobial xanthoquinodins from Jugulospora vestita. J Fungi. 2020;6:188. https://doi.org/10.3390/jof6040188.
Wang KB, Jiang SS, Pu T, Fan LM, Su FW, Ye M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat Prod Res. 2018;33:1423–30. https://doi.org/10.1080/14786419.2017.1419232.
Acknowledgements
LZ would like to express her gratitude to Mae Fah Luang University for providing a scholarship for her PhD studies. RSJ thanks Eminent Scholar offered by Kyun Hee University.
Funding
The National Natural Science Foundation of China (NSFC 32060013) and the Youth Science and Technology Talent Development Project from Guizhou Provincial Department of Education (QJHKYZ [2021]263 and QJHKYZ [2022]345) provided funding for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Ma, J., Yang, M. et al. Polyketides from Neohelicosporium griseum: structure assignment and bioactivity investigation. Med Chem Res 33, 308–313 (2024). https://doi.org/10.1007/s00044-023-03172-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03172-1