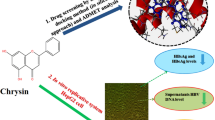
Abstract
Previous in vivo and in vitro studies revealed that esculetin (Fig. 1) has anti-hepatitis B virus (anti-HBV) activity as well as a protective effect on liver damage caused by duck hepatitis B virus. We designed and synthesized a series of esculetin derivatives, introduced side chains containing various amino groups into site 7 of the parent structure, and synthesized C-4 and C-8 substituted derivatives with the goal of investigating their anti-HBV activities. In vitro anti-HBV activity was performed against HepG2.2.15 cells by using Enzyme-Linked Immunosorbent Assay(ELISA) kit and cytotoxicity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay with lamivudine as the positive control. The results demonstrated that several compounds showed moderate anti-HBV activity, while the introduction of morpholine groups could significantly inhibit the expression of hepatitis B e antigen (HBeAg) and the introduction of the 2-methylimidazole group could significantly inhibit the expression of Hepatitis B surface antigen (HBsAg). Among all tested compounds, compound 4a demonstrated the best anti-HBeAg activity (IC50 = 15.8 ± 4.2 μM), while compound 6d demonstrated the best anti-HBsAg activity (IC50 = 21.4 ± 2.8 μM). Compounds 6b and 6c showed moderate anti-HBV activity and HBsAg inhibition. Compounds 4b showed moderate anti-HBV activity and an inhibitory effect on HBeAg. In addition, compounds 4a, 4c, 4d, 6b, 6c and 6d showed improved metabolic stability. This study provides useful guidance for the discovery of anti-HBV drugs, which merits further investigation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis B is a disease that affects people all over the world and is caused by the hepatitis B virus (HBV). Nearly 257 million people worldwide are infected with hepatitis B, and the disease causes 686,000 deaths each year. HBV infection may cause chronic hepatitis, cirrhosis, and liver cancer. Although the number of hepatitis B infections among children is currently declining, the number of deaths among adults who were infected prior to vaccination will continue to rise if they are not treated [1, 2]. The prevention and treatment of hepatitis B are serious all around the world [3]. Currently, the main anti-HBV drugs are interferons [4], lamivudine [5], telbivudine and so on [6]. However, existing drugs commonly have defects like virus resistance [7], adverse reactions and toxic side effect [8]. Therefore, it is necessary to develop new anti-HBV drugs.
Hydroxyphenol and coumarin compounds, widely found in nature [9], have good activity against a variety of viruses, such as HBV and coronavirus [10]. Among them, esculetin is an important coumarin derivative with a common structure of 6,7-dihydroxy-2-H-1-benzopyran-2-one. Studies have shown that esculetin has a variety of biological activities, such as anti-inflammatory [11, 12], antibacterial [13, 14], antiviral [15], antitumor [16], liver protection [17,18,19], antifungal [20]. In vivo studies of esculetin showed improvement in hepatic fibrosis by increasing phospho-Forkhead box protein O1 (FOXO1) expression at concentrations of 50 mg/kg or 100 mg/kg [19]. In vitro antiviral experiments with esculetin revealed that esculetin concentrations of 32 μM inhibited Newcastle disease virus replication well [15]. Thus, investigations on novel esculetin derivatives may provide an important platform for seeking compounds with potential anti-HBV effects.
The previous study of our research group revealed that esculetin showed good anti-HBV activity. In vitro studies, it was found that escinolactone had a dose-effect and time-sensitive relationship on Hepatitis B surface antigen (HBsAg) and HBeAg cell inhibition, and TI > 2; In in vivo experiments,esculetin can reduce the levels of HBsAg and hepatitis B e antigen (HBeAg) in duck serum [21]. However, we find that although esculetin can inhibit the secretion of HBsAg and HBeAg, it also has strong toxicity to cells. Moreover, some studies have shown that the introduction of side chains containing amino groups can increase the activity of the compounds [22]. Meanwhile, amine groups such as piperidine, diethylamine, morpholine and so on have a wide range of activities and have received much attention [23,24,25]. Based on the previous studies, we planned to synthesize a series of esculetin with C-7 substituted amino side chains and C-4, C-8 substituted derivatives. The anti-HBV activity was investigated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay and Enzyme-Linked Immunosorbent Assay(ELISA) kit. HepG2.2.15 cells were used as HBV vectors to detect the inhibition of HBsAg and HBeAg by compounds 1–8. This work represents the first report on the synthesis and in vitro anti-HBV activity of esculetin derivatives bearing amino side chains.
Results and discussion
Synthesis
The etherification of the hydroxyl group in the C-7 position of esculetin with 1, ω-dibromoalkane was carried out in the presence of acetonitrile (ACN) and a small amount of triethylamine (TEA) to offer the key intermediates 1a–1d, which possessed various side chains with different length. The synthetic route of derivatives was described in Fig. 2. Then, compounds 1a–1d were treated with appropriate different amines in acetonitrile to yield the target compounds 2–6. Among them, the synthesis of compounds 2a–2d and 4a–4d take TEA as the catalyst and react at room temperature, the compounds 3a–3d take K2CO3 and KI as the catalyst and react under room temperature, the compounds 5a–5d and 6a–6d take K2CO3 and KI as the catalyst and reacts at 65 °C. The synthesis of compounds 7a–c and 8a–c (Fig. 3) was carried out according to the method we have published previously [26, 27]. The target compounds were characterized by 1H-nuclear magnetic resonance (1H NMR), carbon nuclear magnetic resonance (13C NMR) and high-resolution mass spectroscopy (HRMS). In particular, according to the HMBC correlation between H-11 and C-7 and the ROESY correlation between H-11 and H-8 in the 2D-NMR spectrum, it can be determined that C-11 is linked to the hydroxyl group of C-7, that is, the synthetic group is linked to the hydroxyl group of C-7.
Biological evaluations
Anti-HBV activity analysis
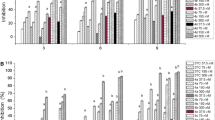
All the compounds were tested for anti-HBV activity, which inhibits the expression of HBsAg and HBeAg by using lamivudine as a positive control. The anti-HBV activity of each compound was expressed by the concentration of compounds that achieved 50% inhibition (IC50) to the secretion of HBsAg and HBeAg. The cytotoxicity of the compounds was expressed as the concentration of compound required to kill 50% (CC50) of the HepG2.2.15 cells. The selectivity index (SI), a major pharmaceutical parameter to evaluate the action and toxicity of the drug, was determined as the ratio of CC50 to IC50. The result of anti-HBV activity and cytotoxicity were shown in Table 1.
In the preliminary screening of anti-HBV activity, we found that compounds 2a–2d and 3a–3d, either with a piperidine or diethylamine side chain showed no obvious anti-HBV activity, and C-4, C-8 substituted derivatives 7–8 also showed little anti-HBV activity.
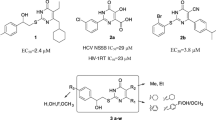
Among the synthesized derivatives, 6b, 6c and 6d showed better activity than lamivudine in inhibiting the secretion of HBsAg, and 4a and 4b showed better activity in inhibiting the secretion of HBeAg. The compounds containing the morpholine group had an obvious inhibition effect on HBeAg, among which compound 4a had a better inhibition effect on HBeAg with IC50 and SI of 15.8 ± 4.2 μM and 13.1, respectively. Among the compounds with the imidazole group, the compounds had no obvious anti-hepatitis B virus activity. The compounds with 2-methylimidazole showed potential activity against HBsAg, and compound 6d showed a better inhibitory effect on HBsAg (IC50 = 21.4 ± 2.8 μM, SI = 20.3) than HBeAg. In summary, compounds 6c and 6d showed better anti-HBsag activity than esculetin. Compounds 4a and 4b showed better anti-HBeAg activity than esculetin.
Structure-activity relationship
The intermediates 1a–1d showed low anti-HBV activity which means the introduction of bromine atoms has little use for anti-HBV, while a part of the derivatives showed significant potency against HBsAg or HBeAg as shown in Table 1. The derivative 4a–4d with morpholine group showed significant activity against HBeAg. Moreover, compound 4a showed the best activity against HBeAg (IC50 = 15.8 ± 4.2 μM), and the next was 4b (IC50 = 52.4 ± 8.7 μM), followed by 4c (IC50 = 81.6 ± 7.0 μM), the last was 4d (IC50 = 91.2 ± 3.1 μM).
Among the intermediate products 4a–4d, only 4a and 4b have a good inhibitory effect on HBeAg, which cannot prove the anti-HBV effect of introducing bromine atom, while some derivatives have good anti-HBV activity on HBsAg or HBeAg, as shown in Table 1. Derivatives of morpholine group 4a–4d, compound 4a showed the strongest activity against HBeAg (IC50 = 15.8 ± 4.2 μM), followed by compound 4b (IC50 = 52.4 ± 8.7 μM), while 4c and 4d showed no significant anti-HBV activity. To sum up, the activity of these compounds was correlated with the length of the side chains, and the shorter length of the side chain, the more significant in inhibition to HBeAg, and compound 4a showed the best activity. Compounds 5a–5d in the imidazole group had no good anti-HBV activity, suggesting that the introduction of the imidazole group was not greatly helpful for the activity against HBV. Compounds 6a–6d with the 2-methylimidazole group could enhance the activity against HBsAg. Compound 6d showed the best activity against HBsAg (IC50 = 21.4 ± 2.8 μM), the next was 6c (IC50 = 34.2 ± 6.8 μM) and the last was 6b (IC50 = 108.5 ± 30.8 μM). Obviously, the activity of these compounds was related to the length of side chains, the longer the length of the side chain, the more significant the inhibition of HBsAg. Thus, the introduction of the morpholine group could enhance the activity against HBeAg and the introduction of the 2-methylimidazole group could enhance the activity against HBsAg, and both of their activities were related to the length of the side chain. Compounds 7a–7c showed no obvious activity against HBV, which means that the introduction of different groups on the C-4 position makes compounds lose their original activity against HBV instead of increasing it. Meanwhile, compounds 8a–8c also showed little activity against HBV, which means that the introduction of amino groups at the C-8 position did not help to enhance the activity of the compounds Fig. 4.
Metabolic stability assays
The metabolic stability of compounds 4a, 4c, 4d, 6b, 6c and 6d in human liver microsomes were tested, while testosterone and 7-hydroxycoumarin were used as the reference drugs for phase I and phase II metabolism, respectively. As shown in Table 2, the metabolic half-lives of 4a, 4c, 6b, 6c and 6d in Cytochromes P450 (CYP) and uridine diphosphate glucuronosyltransferase (UGT) were more than 300 min, respectively, and the metabolic half-lives of 4d in CYP and UGT were more than 300 min and 97.8 min, which were much more stable than that of testosterone (24 min) and 7-hydroxycoumarin (15.8 min). This finding demonstrated that compounds 4a, 4c, 4d, 6b, 6c and 6d exhibited good metabolic stability, suggesting that these compounds could sever as a promising drug candidates for the development of novel anti-HBV drugs. In this study, several anti-hepatitis B virus compounds with good stability were screened out. In the future, the anti-hepatitis B virus activity and plasma stability of these compounds can be further studied, which is expected to replace anti-hepatitis B drugs with poor stability.
Conclusions
In conclusion, a series of esculetin derivatives have been synthesized and their potential anti-HBV activity has been evaluated. The results demonstrated that several amino-containing side chains showed potential activity against HBV with the SIHBeAg from 5.8 to 13.1 and SIHBsAg from 4.6 to 20.3. Compared to the synthetic materials esculetin, some of the derivatives have lower cytotoxicity with lower SI values, while 3TC showed less activity against HBsAg and HBeAg secretion. The analysis of the structure-activity relationship indicated that the morpholine group could enhance the activity against HBeAg and the 2-methylimidazole group could enhance the activity against HBsAg. Meanwhile, the length of the side chain showed an obvious influence on the activity: with the morpholine group the shorter side chain the better activity against HBsAg, while with the 2-methylimidazole group the longer side chain the better activity against HBsAg. This study presented a new class of potential anti-HBV agents and offered valuable information for seeking anti-HBV drug candidates. By further modifying the structure of esculetin derivatives, the development of safe esculetin drugs with antiviral activities has a broad prospect. Compounds 4a, 4c, 4d, 6b, 6c and 6d showed great metabolic stability. We speculate that this is due to the introduction of hydrophilic heterocycles by the compounds which could increase the solubility of the compounds. Meanwhile, these structures could increase the steric hindrance of the compound and close the metabolic site, thus slowing down the metabolic rate. After structural modification, the druggability of these compounds was improved. These results indicate that the introduction of morpholine or 2-methylimidazole groups at the 7 sites of esculetin can appropriately improve the anti-hepatitis B virus activity and metabolic stability of drugs. This study provides a new direction for the development of effective and stable anti-hepatitis B drugs in the future.
Experimental section
Instruments and materials
All the organic solvents used for the synthesis were of analytical grade. Unless otherwise stated, all chemicals were purchased from Aladdin (Shanghai, China). Melting points were measured on a WRX-4 micro melting point instrument (Shanghai YiCe Equipment, China) and were uncorrected. 1H NMR and 13C NMR spectra were recorded on BRUKER DPX-400 and DPX-600 spectrometers (Bruker Company, Germany), using TMS as an internal standard and CDCl3, MeOD-d4, Pyridine-d5 and DMSO-d6 as solvents. Chemical shifts (δ values) and coupling constants (J values) are given in ppm and Hz, respectively. All the solvents and reactants were of analytical grade and were used without further purification unless noted. Column chromatography was accomplished on silica gel (100–200 or 200–300 mesh, Qingdao, China). The purity of compounds was confirmed to be over 95% and was determined by high performance liquid chromatography (HPLC) with a ZORBAX SB-Aq column (250 mm × 4.6 mm, 5 μm, Agilent) using ACN/water (10%, v/v) as the mobile phase (1.0 mL/min).HepG2.2.15 cells were provided by Beijing 302 Hospital and presented by Prof.Su of Guilin Medical College. HepG2.2.15 cells are hepatoma cell lines capable of expressing HBV antigen and secreting complete HBV particles.
Synthesis
General procedure of compounds 1a-1d
Esculetin (0.4 g, 2.2 mmoL) was dissolved in 10 mL of ACN, and 0.66 mL triethylamine (4.4 mmoL) was added and stirred for 10 minutes. Then 4.4 mmoL different 1, ω-dibromoalkane (1, 3-dibromopropane, 1, 4-dibromobutane, 1, 5-dibromopentane, 1, 6-dibromohexane) were added into the mixture, stirring for 6 h at 65 °C. Finally, when the reaction was completed (monitored by TLC analysis), the solvent was removed under reduced pressure to afford the crude product. The residue was applied to ash column chromatography (silica gel) to afford compounds 1a–1d. The physical properties, 1H NMR and 13C NMR data of the compounds are as follows.
General procedure of compounds 2a–2d, 3a–3d and 4a–4d
0.6 mmoL of 1a–1d was dissolved in 10 mL of ACN, and 1.2 mmoL of K2CO3 was added and stirred for 10 minutes. Then 1.2 mmoL of different amines (piperidine, diethylamine and morpholine) were added into the mixture and stirred for 8 hours at room temperature. After the completion of the reaction (monitored by TLC analysis), the reaction was quenched with water. The resulting reaction mixture was extracted with dichloromethane and the dichloromethane was removed under reduced pressure to afford the crude product. The residue was applied to ash column chromatography (silica gel) to afford the compounds 2a–2d, 3a–3d and 4a–4d. The physical properties, 1H NMR and 13C NMR data of the compounds are as follows.
General procedure of compounds 5–6
0.6 mmoL of 1a–1d was dissolved in 10 mL of ACN, 1.2 mmoL of K2CO3 and 0.3 mmoL of KI were added into the reaction mixture, stirred and reacted for 10 minutes, then 1.2 mmoL of different amine (imidazole, 2-methylimidazole) were added into the mixture, stirring at 65 °C for 8 hours. After the reaction was completed (monitored by TLC analysis), the reaction was quenched with water. The resulting reaction mixture was extracted with dichloromethane and the dichloromethane was removed under reduced pressure to afford the crude product. The residue was applied to ash column chromatography (silica gel) to afford the compounds 5a–5d and 6a–6d. The physical properties, 1H NMR and 13C NMR data of the compounds are as follows.
General procedure of compounds 7–8
The synthesis of compounds 7a–c and 8a–c (Fig. 2) was carried out according to the method we have published previously [26, 27].
Physical properties, HRMS, 1H NMR and 13C NMR data of compounds 1–8
6-Hydroxyl-7-(3-bromopropoxy)-2H-benzopyran-2-one (1a). White powder, yield: 23%, MP: 157–160 °C. 1H NMR (400 MHz, DMSO) δ 7.93(d, J = 9.5 Hz, 1H, Pyran-H), 7.34 (s, 1H, Benzene-H), 7.01 (s, 1H, Benzene-H), 6.36 (d, J = 9.5 Hz, 1H, Pyran-H), 4.27–4.24 (m, 2H, CH2), 4.18–4.15 (t, J = 5.7 Hz, 2H, CH2), 2.16 (m, 2H, CH2). 13C NMR (101 MHz, DMSO) δ 160.7, 155.0, 150.2, 148.2, 144.2, 120.3, 114.6, 109.1, 71.3, 71.1, 31.4. HRMS(ESI): Calcd. for C12H11BrO4 [M-H]-: 296.9762, found: 296.9765.
6-Hydroxyl-7-(4-bromobutoxy)-2H-benzopyran-2-one (1b). White powder, yield: 44%, MP: 117–121 °C. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 9.5 Hz, 1H, Pyran-H), 6.91 (s, 1H, Benzene-H), 6.74 (s, 1H, Benzene-H), 6.22 (d, J = 9.5 Hz, 1H, Pyran-H), 4.09 (t, J = 5.7 Hz, 2H, CH2), 3.44 (t, J = 6.1 Hz, 2H, CH2), 2.01 (m, 4H, 2CH2). 13C NMR (101 MHz, CDCl3) δ 165.5, 159.7, 155.0, 153.0, 148.9, 125.1, 119.3, 113.9, 76.0, 75.9, 36.2. HRMS(ESI): Calcd. for C13H13BrO4 [M-H]-: 310.9919, found: 310.9924.
6-Hydroxyl-7-(5-bromopentyloxy)-2H-benzopyran-2-one (1c). White powder, yield: 55%, MP: 114–119 °C. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 9.5 Hz, 1H, Pyran-H), 6.90 (s, 1H, Benzene-H), 6.74 (s, 1H, Benzene-H), 6.22 (d, J = 9.5 Hz, 1H, Pyran-H), 4.06 (t, J = 6.4 Hz, 2H, CH2), 3.39 (t, J = 6.6 Hz, 2H, CH2), 1.92–1.81 (m, 4H, 2CH2), 1.63–1.58 (m, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 161.5, 149.3, 149.2, 143.4, 142.7, 113.9, 112.2, 111.1, 99.9, 69.1, 33.4, 32.2, 28.0, 24.6. HRMS(ESI): Calcd. for C14H15BrO4 [M-H]-: 325.0075, found: 325.0076.
6-Hydroxyl-7-(6-bromohexyloxy)-2H-benzopyran-2-one (1d). White powder, yield: 59%, MP: 107–114 °C. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 9.5 Hz, 1H, Pyran-H), 6.90 (s, 1H, ArH), 6.74 (s, 1H, ArH), 6.22(d, J = 9.5 Hz, 1H, Pyran-H), 4.05 (t, J = 6.5 Hz, 2H, CH2), 3.37 (t, J = 6.7 Hz, 2H, CH2), 1.81–1.86 (m, 4H, 2CH2), 1.48–1.46 (m, 4H, 2CH2). 13C NMR (101 MHz, CDCl3) δ 160.4, 148.3, 148.2, 142.4, 141.7, 112.8, 111.1, 110.0, 98.9, 68.3, 32.6, 31.5, 27.7, 26.8, 24.2. HRMS(ESI): Calcd. for C15H17BrO4 [M-H]-: 339.0232, found: 339.0235.
6-Hydroxyl-7-(3-(piperidine-1-yl)propoxy)-2H-benzopyran-2-one (2a). Brown powder, yield: 18%, MP: 130–132 °C. 1H NMR (400 MHz, MeOD) δ 7.80 (d, J = 9.5 Hz, 1H, Pyran-H), 6.96 (d, J = 4.3 Hz, 2H, ArH), 6.24 (d, J = 9.5 Hz, 1H, Pyran-H), 4.15 (t, J = 6.0 Hz, 2H, CH2), 3.31 (dt, J = 3.3, 1.6 Hz, 2H, CH2), 2.73–2.69 (m, 2H, CH2), 2.12–2.02 (m, 2H, CH2), 1.54 (dt, J = 11.2, 5.7 Hz, 4H, 2CH2), 1.34 (m, 2H, CH2), 1.29 (m, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 162.6, 151.4, 148.4, 145.2, 144.5, 112.6, 112.3, 111.9, 101.1, 68.0, 55.6, 53.9, 25.2, 24.6, 23.3. HRMS(ESI): Calcd. for C17H21NO4 [M-H]-: 302.1392, found: 302.1401.
6-Hydroxyl-7-(4-(piperidine-1-yl)butoxy)-2H-benzopyran-2-one (2b). Brown powder, yield: 19%, MP: 85–88 °C. 1H NMR (400 MHz, MeOD) δ 7.71 (d, J = 9.4 Hz, 1H, Pyran-H), 6.86 (d, J = 14.4 Hz, 2H, ArH), 6.14 (d, J = 9.4 Hz, 1H, Pyran-H), 4.06 (d, J = 5.8 Hz, 2H, CH2), 2.61 (dd, J = 16.5, 8.0 Hz, 6H, 3CH2), 1.80–1.73 (m, 4H, 2CH2), 1.61 (d, J = 5.0 Hz, 4H, 2CH2), 1.45 (s, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 162.7, 151.4, 148.7, 144.5, 144.2, 112.0, 111.6, 100.1, 100.0, 68.6, 57.9, 53.6, 26.3, 24.3, 23.0, 21.9. HRMS(ESI): Calcd. for C18H23NO4 [M-H]-: 316.1549, found: 316.1555.
6-Hydroxyl-7-(5-(piperidine-1-yl)pentyloxy)-2H-benzopyran-2-one (2c). Brown powder, yield: 15%, MP: 115–121 °C. 1H NMR (400 MHz, MeOD) δ 7.70 (d, J = 9.5 Hz, 1H, Pyran-H), 6.87 (s, 1H, ArH), 6.82 (s, 1H, ArH), 6.13 (d, J = 9.5 Hz, 1H, Pyran-H), 4.03 (t, J = 6.3 Hz, 2H, CH2), 2.84 (s, 4H, 2CH2), 2.75–2.71 (m, 2H, CH2), 1.83–1.80 (m, 2H, CH2), 1.67–1.66 (m, 6H, 3CH2), 1.51–1.47 (m, 4H, 2CH2). 13C NMR (101 MHz, MeOD) δ 163.9, 152.8, 150.1, 145.8, 145.4, 113.4,113.3, 112.9, 101.3, 70.0, 58.9, 54.6, 29.4, 25.5, 25.0, 24.6, 23.6. HRMS(ESI): Calcd. for C19H25NO4 [M-H]-: 330.1705, found: 330.1713.
6-Hydroxyl-7-(6-(piperidine-1-yl)hexyloxy)-2H-benzopyran-2-one (2d). Brown powder, yield: 16%, MP: 51–54 °C. 1H NMR(400 MHz, MeOD) δ 7.71 (d, J = 9.5 Hz, 1H, Pyran-H), 6.88 (s, 1H, ArH), 6.84 (s, 1H, ArH), 6.14 (d, J = 9.5 Hz, 1H, Pyran-H), 4.03 (t, J = 6.4 Hz, 2H, CH2), 2.92 (s, 4H, 2CH2), 2.79 (dd, J = 9.8, 6.8 Hz, 2H, CH2), 1.70 (m, 2H, CH2), 1.62–1.61 (m, 4H, 2CH2), 1.53–1.52 (m, 2H, CH2), 1.51–1.49 (m, 4H, 2CH2), 1.47–1.34 (d, J = 8.2 Hz, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 162.6, 151.5, 148.9, 144.5, 144.0, 112.1, 112.0, 111.5, 100.0, 68.8, 57.4, 53.2, 28.2, 26.2, 25.2, 24.1, 23.4, 22.0. HRMS(ESI): Calcd. for C20H27NO4 [M-H]-: 344.1862, found: 344.1874.
6-Hydroxyl-7-(3-(diethylamino-1-yl)propoxy)-2H-benzopyran-2-one (3a). Yellow powder, yield: 19%, MP: 76–79 °C. 1H NMR (400 MHz, MeOD) δ 7.71 (d, J = 9.5 Hz, 1H, Pyran-H), 6.86 (s, 2H, ArH), 6.14 (d, J = 9.5 Hz, 1H, Pyran-H), 4.08 (t, J = 5.9 Hz, 2H, CH2), 2.85–2.82 (m, 2H, CH2), 2.77–2.71 (q, J = 7.2 Hz, 4H, 2CH2), 2.02–1.99 (m, 2H, CH2), 1.07 (t, J = 7.2 Hz, 6H, 2CH3). 13C NMR (101 MHz, MeOD) δ 162.6, 151.5, 148.3, 145.55, 144.6, 112.7, 112.3, 112.0, 101.2, 67.8, 49.4, 46.5, 29.4 24.8, 9.0. HRMS(ESI): Calcd. for C16H21NO4 [M-H]-: 290.1392, found: 290.1400.
6-Hydroxyl-7-(4-(diethylamino-1-yl)butoxy)-2H-benzopyran-2-one (3b). Yellow powder, yield: 20%, MP: 84–87 °C. 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 9.5 Hz, 1H, Pyran-H), 6.96 (s, 1H, ArH), 6.80 (s, 1H, ArH), 6.26 (d, J = 9.5 Hz, 1H, Pyran-H), 4.13 (t, J = 5.9 Hz, 2H, CH2), 2.70 (dt, J = 13.1, 7.1 Hz, 6H, 3CH2), 1.91 (dt, J = 12.5, 6.2 Hz, 2H, CH2), 1.80 (dd, J = 13.9, 7.0 Hz, 2H, CH2), 1.12 (t, J = 7.2 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 161.6, 150.2, 148.9, 143.8, 143.5, 113.6, 112.4, 111.9, 100.7, 69.5, 52.1, 46.6, 26.8, 23.0, 10.3. HRMS(ESI): Calcd. for C17H23NO4 [M-H]-: 304.1549, found: 304.1564.
6-Hydroxyl-7-(5-(diethylamino-1-yl)pentyloxy)-2H-benzopyran-2-one (3c). Yellow powder, yield: 16%, MP: 56–58 °C. 1H NMR(400 MHz, CDCl3) δ 7.51 (d, J = 9.5 Hz, 1H, Pyran-H), 6.89 (s, 1H, ArH), 6.70 (s, 1H, ArH), 6.17 (d, J = 9.5 Hz, 1H, Pyran-H), 4.03 (t, J = 5.9 Hz, 2H, CH2), 2.72 (q, J = 7.2 Hz, 4H, 2CH2), 2.65–2.58 (m, 2H, CH2), 1.84 (m, 2H, CH2), 1.64 (dt, J = 14.9, 7.3 Hz, 2H, CH2), 1.53–1.49 (m, 2H, CH2), 1.09 (t, J = 7.2 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 160.7, 149.4, 148.0, 142.61, 142.5, 112.4, 111.2, 111.1, 99.0, 68.2, 50.3, 45.2, 26.7, 24.0, 23.0, 8.6. HRMS(ESI): Calcd. for C18H25NO4 [M-H]-: 318.1705, found: 318.1712.
6-Hydroxyl-7-(6-(diethylamino-1-yl)hexyloxy)-2H-benzopyran-2-one (3d). Yellow powder, yield: 35%, MP: 72–75 °C. 1H NMR(400 MHz, CDCl3) δ 7.57 (d, J = 9.5 Hz, 1H, Pyran-H), 6.95 (s, 1H, ArH), 6.77 (s, 1H, ArH), 6.24 (d, J = 9.5 Hz, 1H, Pyran-H), 4.07 (t, J = 6.1 Hz, 2H, CH2), 2.77 (q, J = 7.1 Hz, 4H, 2CH2), 2.67–2.63 (m, 2H, CH2), 1.90–1.80 (m, 2H, CH2), 1.63 (dt, J = 15.0, 7.4 Hz, 2H, CH2), 1.56–1.50 (m, 2H, CH2), 1.40 (td, J = 14.1, 6.4 Hz, 2H, CH2), 1.14 (t, J = 7.2 Hz, 6H, 2CH3). 13C NMR (101 MHz, CDCl3) δ 160.6, 149.2, 148.1, 142.5, 142.3, 112.5, 111.0, 110.6, 99.0, 68.3, 50.7, 45.1, 28.7, 27.4, 25.6, 24.5, 8.9. HRMS(ESI): Calcd. for C19H27NO4 [M-H]-: 332.1862, found: 332.1874.
6-Hydroxyl-7-(3-(morpholine-1-yl)propoxy)-2H-benzopyran-2-one (4a). Brown powder, yield: 46%, MP: 170–171 °C. 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 9.5 Hz, 1H, Pyran-H), 6.94 (d, J = 7.6 Hz, 2H, ArH), 6.30 (d, J = 9.5 Hz, 1H, Pyran-H), 4.09 (t, J = 5.3 Hz, 2H, CH2), 3.79 (t, J = 4.6 Hz, 4H, 2CH2), 2.64 (t, J = 6.0 Hz, 2H, CH2), 2.58 (s, 4H, 2CH2), 2.05–2.00 (m, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 161.5, 150.2, 148.0, 146.6, 143.4, 114.8, 114.7, 112.8, 106.9, 72.4, 66.0, 57.1, 53.7, 25.1. HRMS(ESI): Calcd. for C16H19NO5 [M-H]-: 304.1185, found: 304.1194.
6-Hydroxyl-7-(4-(morpholine-1-yl)butoxy)-2H-benzopyran-2-one (4b). Brown powder, yield: 53%, MP: 92–95 °C.1H NMR (400 MHz, MeOD) δ 7.79 (d, J = 9.5 Hz, 1H, Pyran-H), 6.94 (d, J = 14.3 Hz, 2H, ArH), 6.22 (d, J = 9.5 Hz, 1H, Pyran-H), 4.14 (t, J = 6.3 Hz, 2H, CH2), 3.71 (m, 4H, 2CH2), 2.54 (s, 4H, 2CH2), 2.50 (d, J = 7.7 Hz, 2H, CH2), 1.90–1.85 (m, 2H, CH2), 1.79–1.71 (m, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 162.6, 151.3, 148.9, 144.4, 144.0, 112.1, 112.0, 111.6, 100.0, 68.7, 66.0, 58.1, 53.2, 29.4, 22.1. HRMS(ESI): Calcd. for C17H21NO5 [M-H]-: 318.1341, found: 318.1352.
6-Hydroxyl-7-(5-(morpholine-1-yl)pentyloxy)-2H-benzopyran-2-one (4c). Brown powder, yield: 48%, MP: 131–133 °C.1H NMR(400 MHz, CDCl3) δ 7.57 (d, J = 9.5 Hz, 1H, Pyran-H), 6.93 (s, 1H, ArH), 6.76 (s, 1H, ArH), 6.24 (d, J = 9.5 Hz, 1H, Pyran-H), 4.09 (t, J = 6.2 Hz, 2H, CH2), 3.74–3.71 (m, 4H, 2CH2), 2.45 (s, 4H, 2CH2), 2.41–2.37 (m, 2H, CH2), 1.89 (m, 2H, CH2), 1.85 (m, 2H, CH2), 1.64–1.48 (dt, J = 13.8, 6.8 Hz, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 160.6, 148.9, 148.1, 142.5, 142.0, 112.6, 111.1, 110.7, 98.9, 68.4, 65.6, 57.1, 52.6, 26.9, 24.8, 22.9. HRMS(ESI): Calcd. for C18H23NO5 [M-H]-: 332.1498, found: 332.1508.
6-Hydroxyl-7-(6-(morpholine-1-yl)hexyloxy)-2H-benzopyran-2-one (4d). Brown powder, yield: 51%, MP: 41–43 °C.1H NMR(400 MHz, CDCl3) δ 7.57 (d, J = 9.5 Hz, 1H, Pyran-H), 6.93 (s, 1H, ArH), 6.77 (s, 1H, ArH), 6.25 (d, J = 9.5 Hz, 1H, Pyran-H), 4.07 (t, J = 6.5 Hz, 2H, CH2), 3.72–3.69 (m, 4H, 2CH2), 2.44 (s, 4H, 2CH2), 2.36–2.32 (m, 2H, CH2), 1.85–1.81 (m, 2H, CH2), 1.56–1.50 (dt, J = 16.5, 8.2 Hz, 4H, 2CH2), 1.48–1.36 (m, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 161.6, 149.8, 149.2, 143.5, 142.9, 113.6, 112.1, 111.3, 100.0, 69.4, 66.8, 58.9, 53.7, 28.7, 27.1, 26.2, 25.8. HRMS(ESI): Calcd. for C19H25NO5 [M-H]-: 346.1654, found: 346.1663.
6-Hydroxyl-7-(3-(imidazole-1-yl)propoxy)-2H-benzopyran-2-one (5a). Brown powder, yield: 32%, MP: 100–102 °C. 1H NMR (400 MHz, MeOD) δ 7.83 (s, 1H, CH), 7.82 (d, J = 9.5 Hz, 1H, Pyran-H), 7.25 (s, 1H, CH), 7.06 (s, 1H, CH), 7.01 (s, 1H, ArH), 6.93 (s, 1H, ArH), 6.25 (d, J = 9.5 Hz, 1H, Pyran-H), 4.35 (t, J = 7.1 Hz, 2H, CH2), 4.08 (t, J = 5.8 Hz, 2H, CH2), 2.35 (dt, J = 12.8, 6.3 Hz, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 162.5, 150.9, 148.8, 144.3, 143.9, 126.6, 119.7, 112.4, 112.35, 111.8, 100.3, 65.5, 43.6, 29.9. HRMS(ESI): Calcd. for C15H14N2O4 [M-H]-: 285.0875, found: 285.0883.
6-Hydroxyl-7-(4-(imidazole-1-yl)butoxy)-2H-benzopyran-2-one (5b). Brown powder, yield: 47%, MP: 142–144 °C. 1H NMR(400 MHz, MeOD) δ 7.61 (d, J = 9.5 Hz, 1H, Pyran-H), 7.58 (s, 1H, CH), 7.56 (d, J = 1.2 Hz, 1H, CH), 6.97 (d, J = 1.2 Hz, 1H, CH), 6.94 (s, 1H, ArH), 6.78 (s, 1H, ArH), 6.26 (d, J = 9.5 Hz, 1H, Pyran-H), 4.08 (t, J = 4.9 Hz, 2H, CH2), 4.01 (t, J = 3.9 Hz, 2H, CH2), 1.89 (dt, J = 19.8, 7.2 Hz, 2H, CH2), 1.52–1.48 (m, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 161.6, 150.0, 149.0, 143.3, 137.1, 128.9, 118.9, 113.6, 111.5, 100.1, 68.8, 46.9, 30.6, 28.2, 23.1. HRMS(ESI): Calcd. for C16H16N2O4 [M-H]-: 299.1032, found: 299.1038.
6-Hydroxyl-7-(5-(imidazole -1-yl)pentyloxy)-2H-benzopyran-2-one (5c). Brown powder, yield: 56%, MP: 145–148 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 1H, CH), 7.58 (d, J = 9.5 Hz, 1H, Pyran-H), 7.56 (s, 1H, CH), 6.97 (s, 1H, ArH), 6.94 (s, 1H, CH), 6.78 (s, 1H, ArH), 6.26 (d, J = 9.5 Hz, 1H, Pyran-H), 4.08 (t, J = 6.2 Hz, 2H, CH2), 4.01 (t, J = 7.0 Hz, 2H, CH2), 1.90–1.86 (m, 4H, 2CH2), 1.52–1.48 (m, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 161.6, 150.0, 149.0, 143.3, 137.1, 128.9, 118.9, 113.6, 111.5, 100.1, 68.8, 46.9, 30.6, 28.2, 23.1. HRMS(ESI): Calcd. for C17H18N2O4 [M-H]-: 313.1188, found: 313.1195.
6-Hydroxyl-7-(6-(imidazole-1-yl)hexyloxy)-2H-benzopyran-2-one (5d). Brown powder, yield: 49%, MP: 133–135 °C. 1H NMR(400 MHz, CDCl3) δ 7.61 (d, J = 9.4 Hz, 1H, Pyran-H), 7.11 (s, 1H, CH), 7.11 (s, 1H, CH), 7.01 (s, 1H, ArH), 6.97 (s, 1H, CH), 6.81 (s, 1H, ArH), 6.28 (d, J = 9.4 Hz, 1H, Pyran-H), 4.09 (t, J = 6.3 Hz, 2H, CH2), 4.01 (t, J = 6.9 Hz, 2H, CH2), 1.85 (dd, J =z 12.5, 6.5 Hz, 4H, CH2, CH2), 1.53–1.42 (m, 2H, CH2), 1.39 (dd, J = 17.7, 10.9 Hz, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 160.7, 149.3, 148.0, 142.5, 142.5, 135.9, 127.7, 118.0, 112.4, 110.6, 99.1, 68.0, 46.2, 29.7, 27.6, 25.3, 24.5. HRMS(ESI): Calcd. for C18H20N2O4 [M-H]-: 327.1345, found: 327.1352.
6-Hydroxyl-7-(3-(2-methylimidazole-1-yl)propoxy)-2H-benzopyran-2-one (6a). Brown powder, yield: 37%, MP: 110–112 °C. 1H NMR (400 MHz, MeOD) δ 7.82 (d, J = 9.5 Hz, 1H, Pyran-H), 7.06 (s, 1H, CH), 7.02 (s, 1H, ArH), 6.93 (s, 1H, ArH), 6.85 (s, 1H, CH), 6.25 (d, J = 9.5 Hz, 1H, Pyran-H), 4.23 (t, J = 6.9 Hz, 2H, CH2), 4.05 (t, J = 5.7 Hz, 2H, CH2), 2.35 (s, 3H, CH3), 2.30 (dt, J = 12.7, 6.5 Hz, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 162.5, 150.9, 148.8, 144.4, 144.0, 125.4, 119.4, 112.4, 111.8, 100.2, 65.2, 42.1, 29.4, 11.8. HRMS(ESI): Calcd. for C16H16N2O4 [M-H]-: 299.1032, found: 299.1038.
6-Hydroxyl-7-(4-(2-methylimidazole-1-yl)butoxy)-2H-benzopyran-2-one (6b). Brown powder, yield: 41%, MP: 70–74 °C.1H NMR(400 MHz, MeOD) δ 7.81 (d, J = 9.4 Hz, 1H, Pyran-H), 7.13 (d, J = 1.4 Hz, 1H, CH), 6.98 (d, J = 2.7 Hz, 2H, ArH), 6.90 (d, J = 1.3 Hz, 1H, CH), 6.24 (d, J = 9.4 Hz, 1H, Pyran-H), 4.15 (t, J = 6.0 Hz, 2H, CH2), 4.06 (t, J = 7.2 Hz, 2H, CH2), 1.37 (s, 3H, CH3), 0.89 (t, J = 6.8 Hz, 4H, 2CH2). 13C NMR (101 MHz, MeOD) δ 162.6, 151.3, 148.9, 144.4, 144.0, 124.2, 122.9, 119.8, 112.2, 111.6, 100.2, 78.1, 68.5, 29.4, 13.1. HRMS(ESI): Calcd. for C17H18N2O4 [M-H]-: 313.1188, found: 313.1196.
6-Hydroxyl-7-(5-(2-methylimidazole-1-yl)pentyloxy)-2H-benzopyran-2-one (6c). Brown powder, yield: 46%, MP: 62–65 °C.1H NMR(400 MHz, MeOD) δ 7.80 (d, J = 9.4 Hz, 1H, Pyran-H), 7.05 (d, J = 1.5 Hz, 1H, CH), 6.97 (s, 1H, ArH), 6.93 (s, 1H, ArH), 6.85 (d, J = 1.4 Hz, 1H, CH), 6.23 (d, J = 9.4 Hz, 1H, Pyran-H), 4.11 (t, J = 6.3 Hz, 2H, CH2), 3.97 (t, J = 7.2 Hz, 2H, CH2), 1.40 (s, 2H, CH2), 1.26 (s, 3H, CH3), 0.89 (t, J = 6.8 Hz, 4H, 2CH2). 13C NMR (101 MHz, MeOD) δ 164.0, 152.7, 150.2, 145.8, 145.3, 125.9, 124.2, 120.9, 114.6, 113.4, 112.8, 101.4, 70.1, 47.0, 30.7, 24.1, 23.7, 14.4. HRMS(ESI): Calcd. for C18H20N2O4 [M-H]-: 327.1345, found: 327.1352.
6-Hydroxyl-7-(6-(2-methylimidazole-1-yl)hexyloxy)-2H-benzopyran-2-one (6d). Yellow powder, yield: 57%, MP: 62–65 °C.1H NMR(600 MHz, Pyr) δ 7.72 (d, J = 9.4 Hz, 1H, Pyran-H), 7.32 (s, 1H, ArH), 7.26 (d, J = 1.0 Hz, 1H, CH), 7.09 (d, J = 1.0 Hz, 1H, CH), 7.05 (s, 1H, ArH), 6.38 (d, J = 9.4 Hz, 1H, Pyran-H), 4.02 (t, J = 6.4 Hz, 2H, CH2), 3.89 (q, J = 7.0 Hz, 2H, CH2), 3.69 (t, J = 7.2 Hz, 2H, CH2), 2.42 (s, 3H, CH3), 1.60–1.45 (m, 2H, CH2), 1.31 (dd, J = 15.1, 7.5 Hz, 2H, CH2), 1.10 (dt, J = 15.4, 7.9 Hz, 2H, CH2). 13C NMR (151 MHz, Pyr) δ 162.5, 153.0, 146.2, 145.3, 144.9, 127.5, 125.0, 120.7, 114.3, 114.0, 113.4, 101.9, 70.2, 58.3, 50.6, 46.9, 27.3, 20.1, 13.7. HRMS(ESI): Calcd. for C19H22N2O4 [M-H]-: 341.1501, found: 341.1507.
6,7-Dihydroxy-4-(hydroxymethyl)-2H-benzopyran-2-one (7a). Brown solid, yield: 83%, MP: > 300 °C. 1H NMR (400 MHz, MeOD) δ 6.96 (s, 1H, ArH), 6.76 (s, 1H, ArH), 6.36 (t, J = 1.4 Hz, 1H, Pyran-H), 4.76 (d, J = 1.4 Hz, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 163.3, 156.9, 150.3, 148.4, 143.1, 109.5, 107.4, 106.5, 102.4, 59.5. HRMS(ESI): Calcd. for C10H8O5 [M-H]-: 207.0293, found: 207.0298.
6,7-Dihydroxy-4-phenyl-2H-benzopyran-2-one (7b). White solid, yield: 91%, MP: 54–56 °C. 1H NMR (400 MHz, MeOD) δ 7.55–7.47 (m, 5H, ArH), 6.85 (s, 1H, ArH), 6.83 (s, 1H, ArH), 6.13 (s, 1H, Pyran-H). 13C NMR (101 MHz, MeOD) δ 162.7, 157.0, 150.7, 149.2, 143.0, 135.9, 129.3, 128.6, 128.1, 110.9, 110.6, 109.9, 102.7. HRMS(ESI): Calcd. for C15H10O4 [M-H]-: 253.0501, found:253.0508.
6,7-Dihydroxy-4-carboxymethyl-2H-benzopyran-2-one (7c). White solid, yield: 70%, MP: 210–212 °C. 1H NMR (400 MHz, MeOD) δ 7.04 (s, 1H, ArH), 6.76 (s, 1H, ArH), 6.21 (s, 1H, Pyran-H), 3.78 (d, J = 7.2 Hz, 2H, CH2). 13C NMR (101 MHz, MeOD) δ 171.3, 162.7, 150.5, 148.6, 143.1, 111.9, 111.4, 108.7, 102.5, 37.4. HRMS(ESI): Calcd. for C11H8O6 [M-H]-: 236.0243, found: 236.0300.
6,7-Dihydroxy-8-((4-hydroxypiperidine-1-yl) methyl)-2H-benzopyran-2-one (8a). Brown powder, yield: 12%, MP: 129–131 °C. 1H NMR (600 MHz, MeOD) δ 7.76 (d, J = 9.3 Hz, 1H, Pyran-H), 6.86 (s, 1H, ArH), 6.04 (d, J = 9.3 Hz, 1H, Pyran-H), 4.23 (s, 2H, CH2), 3.34 (d, J = 8.3 Hz, 1H, CHOH)), 3.23–3.21 (m, 2H, CH2), 2.86 (s, 2H, CH2), 1.99 (s, 2H, CH2), 1.73 (m, 2H, CH2). 13 C NMR (151 MHz, MeOD) δ 163.2, 148.6, 145.6, 144.8, 109.3, 108.2, 107.6, 104.9, 51.8, 48.5, 48.2, 32.0. HRMS(ESI): Calcd. for C15H17NO5 [M-H]-: 290.1028, found: 290.1033.
6,7-Dihydroxy-8-(pyrrolidin-1-ylmethyl)-2H-benzopyran-2-one (8b). Light brown solid, yield: 18%, MP: 125–127 °C. 1H NMR (600 MHz, MeOD) δ 7.73 (d, J = 9.2 Hz, 1H, Pyran-H), 6.81 (s, 1H, ArH), 5.94 (d, J = 9.2 Hz, 1H, Pyran-H), 4.44 (d, J = 11.6 Hz, 2H, CH2), 3.31–3.30 (m, 4H, 2CH2), 2.08–2.06 (m, 4H, 2CH2). 13 C NMR (151 MHz, MeOD) δ 163.8, 162.2, 149.7, 146.2, 145.9, 108.1, 106.2, 105.2, 103.7, 53.3, 49.0, 22.7. HRMS(ESI): Calcd. for C14H15NO4 [M-H]-: 260.0923, found: 260.0927.
6,7-Dihydroxy-8-((dimethylamino)methyl)-2H-benzopyran-2-one(8c). Brown powder, yield: 17%, MP: 131–133 °C. 1H NMR (600 MHz, MeOD) δ 8.40 (s, 1H, ArOH), 7.81 (d, J = 9.4 Hz, 1H, Pyran-H), 7.01 (s, 1H, ArH), 6.19 (d, J = 9.4 Hz, 1H, Pyran-H), 4.46 (s, 2H, CH2), 3.31 (s, 6H, 2CH3). 13 C NMR (151 MHz, MeOD) δ 167.3, 162.2, 148.2, 145.0, 143.7, 111.6, 110.0, 109.8, 104.0, 50.6, 42.2. HRMS(ESI): Calcd. for C12H13NO4 [M-H]-: 234.0766, found: 234.0772.
Cell cytotoxicity test
Cytotoxicity of esculetin derivatives was measured by MTT assay [28]. Take the cells in the logarithmic growth phase to prepare a cell suspension with a density of 2 × 104 cells/mL, inoculate 200 μL per well in a 96-well plate, and culture at 37 °C. Lamivudine was used as the positive control and the concentration gradient was 20, 40, 80, 160 and 320 μM. After 24 h, 200 μL of the drug-containing medium was added to each well, and the culture was continued for 9 days. On the ninth day, discard the supernatant and add 180 μL of medium and 20 μL MTT (5 mg/ml) per well, protected from light. After 4 hours of incubation at 37 °C, discard the supernatant, add 150 μL DMSO to each well, and shake for 10 minutes to fully dissolve the crystals, Finally, the absorbance value (OD) was measured using a microplate reader at a wavelength of 490 nm. The reed-Muench method was used to calculate half the cytotoxicity level (CC50).
Evaluation of HBsAg and HBeAg expression
Cell supernatant was collected on day 6 and day 9. The levels of HBsAg and HBeAg were simultaneously detected using ELISA kits according to the manufacturer’s instructions, and then the absorbance value (OD) was measured using a microplate reader at a wavelength of 450 nm. The IC50 and selected index (SI) of each compound were calculated, respectively [29].
Metabolic stability assays of compounds in both phase I and phase II metabolic systems
For phase I metabolism, the incubation mixture (0.6 mL) was consisted of human liver microsomes (0.1 mg/mL), MgCl2 (5 mM), glucose-6-phosphate (1 mM), glucose-6-phosphate dehydrogenase (1 U/mL), target compounds, testosterone (10 mM) and 0.1 M potassium phosphate buffer (pH = 7.4). The mixture was pre-incubated at 37 °C for 3 min and then added with NADP+ (1 mM) to initiate the oxidative reaction. At the different time points (0-, 15-, 30-, 60-, 90-min), 100 mL of the aliquots were transferred to a tube containing 100 mL of ice-cold ACN to stop the reaction. The mixtures were then vortexed for 30 s at room temperature and centrifuged at 20,000 g at 4 °C for 20 min. The supernatants were analyzed by liquid chromatography coupled with a UV detector.
For phase II metabolism, the incubation mixture (0.6 mL) was consisted of human liver microsomes (0.1 mg/mL), MgCl2 (5 mM), target compounds, reference drug (10 mM), and 50mMTris-HCl buffer (pH = 7.4). The reaction mixture was incubated at 37 °C for 3 min and then added with UDPGA (2 mM) to initiate the reaction. At the different time points (0-, 15-, 30-, 60-, 90-min), 100 mL of the aliquots were transferred to a tube containing 100 mL of ice-cold ACN to stop the reaction. The mixtures were then vortexed for 30 s at room temperature and centrifuged at 20,000 g at 4 °C for 20 min. The supernatants were analyzed by liquid chromatography coupled with a UV detector.
References
Hill A, Gotham D, Cooke G, Bhagani S, Andrieux-Meyer I, Cohn J, et al. Analysis of minimum target prices for production of entecavir to treat hepatitis B in high- and low-income countries. J Virus Erad. 2015;1:103–10.
World Health Organization. Global hepatitis report 2017. Geneva, Switzerland: World Health Organization; 2017.
Goyal A, Murray JM. Roadmap to control HBV and HDV epidemics in China. J Theor Biol. 2017;423:41–52.
Yuen MF, Schiefke I, Yoon JH, Ahn SH, Heo J, Kim JH, et al. RNA Interference Therapy With ARC-520 results in prolonged hepatitis B surface antigen response in patients with chronic hepatitis B infection. Hepatology. 2020;72:19–31.
Jiang XW, Ye JZ, Li YT, Li LJ. Hepatitis B reactivation in patients receiving direct-acting antiviral therapy or interferon-based therapy for hepatitis C: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:3181–91.
Ying Y, Hu YK, Jin JL, Zhang JM, Zhang WH, Huang YX. Case report: Lactic acidosis and rhabdomyolysis during telbivudine and tenofovir treatment for chronic hepatitis B. BMC Gastroenterol. 2018;18:45.
Xianyu JB, Feng JF, Yang YW, Tang J, Xie G, Fan LY. Correlation of oxidative stress in patients with HBV-induced liver disease with HBV genotypes and drug resistance mutations. Clin Biochem. 2018;55:21–7.
Ding GJ, Liu Y. Study on the serum HBV-DNA load change and adverse reactions of adefovir dipivoxil in the treatment of patients with chronic hepatitis B. Chin Comm Doc. 2016;35:54–5.
Hroboňová K, Sádecká J, imárik J. HPLC separation and determination of dicoumarol and other simple coumarins in sweet clover. Nov Biotechnol Chim. 2018;1:95–102.
Rabie AM. Improved synthesis of the anti-SARS-CoV-2 investigational agent (E)-N-(4-cyanobenzylidene)-6-fluoro-3-hydroxypyrazine-2-carboxamide (cyanorona-20). Rev Chim. 2022;73:69–75.
Jayakumar T, Huang CJ, Yen TL, Hsia CW, Sheu JR, Bhavan PS, et al. Activation of Nrf2 by esculetin mitigates inflammatory responses through suppression of NF-kB signaling cascade in RAW 264.7 cells. Molecules. 2022;27:5143.
Sun B, Wang B, Xu M. Esculetin inhibits histamine-induced expression of inflammatory cytokines and mucin in nasal epithelial cells. Clin Exp Pharmacol Physiol. 2019;46:821–27.
Pawar A, Konwar C, Jha P, Chopra M, Chaudhry U, Saluja D. P651 Elucidating the effect of esculetin against glutamate racemase-a novel drug target of Neisseria gonorrhoeae. Sex Transm Infect. 2019;95:A287.
Yang L, Ding W, Xu YQ, Wu DS, Li S, Chen JN, et al. New insights into the antibacterial activity of hydroxycoumarins against ralstonia solanacearum. Molecules. 2016;21:468.
Galabov AS, Iosifova T, Vassileva E, Kostova I. Antiviral activity of some hydroxycoumarin derivatives. Z Naturforsch C. 1996;51:558–62.
Jiang RQ, Su GF, Chen X, Chen S, Li QH, Xie BM, et al. Esculetin inhibits endometrial cancer proliferation and promotes apoptosis via hnRNPA1 to downregulate BCLXL and XIAP. Cancer Lett. 2021;521:308–21.
Lee J, Yang J, Jeon J, Jeong J, Sang H, Sung J. Hepatoprotective effect of esculetin on ethanol-induced liver injury in human HepG2 cells and C57BL/6J mice. J Funct Foods. 2018;40:536–43.
Choi RY, Ham JR, Lee MK. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem Biol Interact. 2016;260:13–21.
Pandey A, Raj P, Goru SK, Kadakol A, Malek V, Sharma N, et al. Esculetin ameliorates hepatic fibrosis in high fat diet induced non-alcoholic fatty liver disease by regulation of FoxO1 mediated pathway. Pharmacol Rep. 2017;69:666–72.
Wang B, Li P, Xu S, Liu LY, Xu YN, Feng X, et al. Inhibitory effects of the natural product esculetin on Phytophthora capsici and its possible mechanism. Plant Dis. 2021;105:1814–22.
Huang SX, Mou JF, Luo Q, Mo QH, Zhou XL, Huang X, et al. Anti-hepatitis B virus activity of esculetin from Microsorium fortunei in vitro and in vivo. Molecules. 2019;19:3475.
Yang ZM, Huang J, Qin JK, Dai ZK, Lan WL, Su GF, et al. Design, synthesis and biological evaluation of novel 1-hydroxyl-3-aminoalkoxy xanthone derivatives as potent anticancer agents. Eur J Med Chem. 2014;85:487–97.
Li LH, Wang AP, Wang B, Liu ML, Lv K, Tao ZY, et al. N-(2-Phenoxy)ethyl imidazo[1,2-a]pyridine-3-carboxamides containing various amine moieties: Design, synthesis and antitubercular activity. Chin Chem Lett. 2020;31:409–12.
Rabie AM, Tantawy AS, Badr SMI. Design, synthesis, and biological evaluation of new 5-substituted-1,3,4-thiadiazole-2-thiols as potent antioxidants. Eur Res. 2018;10:21–43.
Rabie AM, Tantawy AS, Badr SMI. Design, synthesis, and biological evaluation of novel 5-substituted-2-(3,4,5-trihydroxyphenyl)-1,3,4-oxadiazoles as potent antioxidants. Am J Org Chem. 2016;6:54–80.
Wang P, Xia YL, Yu Y, Lu JX, Zou LW, Feng L, et al. Design, synthesis and biological evaluation of esculetin derivatives as anti-tumour agents. Rsc Adv. 2015;66:53477–483.
Xia YL, Wang JJ, Li SY, Liu Y, Gonzalez FJ, Wang P, et al. Synthesis and structure-activity relationship of coumarins as potent Mcl-1 inhibitors for cancer treatment. Bioorg Med Chem. 2021;29:115851
Iravani S, Sajjadi SE, Rafieian-Kopaei M, Zolfaghari B. Gas Chromatography-Mass Spectrometry analysis of Anbarnesa smoke and its antiviral activity. Adv Biomed Res. 2022;11:91.
Aytaç Ö, Toyran A, Aksoy A. HBsAg nötralizasyon testinin hepatit B hastalığının tanı algoritmasindaki yeri ve önemi [The significance and place of HBsAg neutralization test in the diagnosis and algorithm of hepatitis B infection]. Mikrobiyol Bul. 2017;51:136–144.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82060787, 31560100), the Natural Science Foundation of Guangxi Province (No. 2018GXNSFBA281079), and the Open Research Fund program of the Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (No. CHEMR2017-B15).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Z., Zhao, TSY., Li, SB. et al. Synthesis and biological evaluation of esculetin derivatives as potential anti-HBV agents. Med Chem Res 32, 899–909 (2023). https://doi.org/10.1007/s00044-023-03045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03045-7