Abstract
Thienopyrimidine derivatives hold a unique place between fused pyrimidine compounds. They are important and widely represented in medicinal chemistry as they are structural analogs of purines. Thienopyrimidine derivatives have various biological activities. The current review discusses different synthetic methods for the preparation of heterocyclic thienopyrimidine derivatives. It also highlights the most recent research on the anticancer effects of thienopyrimidines through the inhibition of various enzymes and pathways, which was published within the last 9 years.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
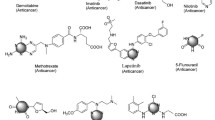
Cancer is the most significant health problem with increasing frequency and mortality rates globally. Due to the large number of cancer patients, effective treatment approaches and prompt diagnosis are imperative [1]. Cell proliferation and differentiation of cancer cells are originated from the action of some enzymes including protein kinases (PKs) [2]. As a result of mutations in PKs, oncogenesis can occur, and these mutations are critical to the progression of cancer [3]. As a result, the use of PK inhibitors has become increasingly important in the last two decades since PKs are one of the pathways that can be inhibited in cancer treatment to solve a variety of cellular communication problems [4]. In clinical oncology, PKs are frequently used as molecular therapeutic targets because they play key roles in several signal transduction pathways, which can lead to metastasis and drug resistance. [5]. Developing kinase inhibitors as anticancer medicines continue to be a crucial research priority to improve tumor selectivity, efficiency, and safety of anticancer medicines. Furthermore, there are other targets that can be inhibited to give effective anticancer drugs such as topoisomerases [6, 7], tubulin polymerization [8], and histone deacetylase (HDAC) [9]. Thienopyrimidine scaffold is one of the most frequently used chemical scaffolds in drug development. The structural and isoelectronic characteristics of thienopyrimidine-containing compounds are similar to those of purine and they have become an attractive structural feature in the production of pharmaceutical drugs [10, 11]. Thienopyrimidines have been demonstrated to have significant and various pharmacological properties, such as antibacterial [12,13,14], antiviral [15, 16], anti-inflammatory [17, 18], antiprotozoal [19], and anticancer activities [20,21,22,23]. Figure 1 represents some thienopyrimidine-containing drugs with varying profiles of biological activity. Relugolix (TAK-385), is a thienopyrimidine derivative that has completed phase III clinical trials and is being studied for its capacity to treat endometriosis and prostate carcinoma by acting as a gonadotropin-releasing hormone receptor (GnRHR) antagonist [24, 25]. DDP-225 is another thienopyrimidine drug that entered phase II clinical trials and was designed to cure irritable bowel syndrome (IBS) and gastrointestinal tract (GIT) diseases by acting as a serotonin receptor (5-HT3) antagonist and noradrenaline reuptake inhibitor [26, 27]. Moreover, pictilisib (GDC-0941) is a thieno[3,2-d]pyrimidine derivative which inhibits phosphatidylinositol 3-kinase (PI3K) and is in clinical trials and was clinically investigated for the treatment of advanced solid tumors [28]. In addition, olmutinib is a marketed drug that inhibits epidermal growth factor receptor (EGFR) and is used to treat NSC lung cancer [29, 30].
Synthetic strategy
In the literature, several synthetic pathways are reported that involve the construction of either the pyrimidine ring or the thiophene ring to obtain the polysubstituted thienopyrimidines. Thienopyrimidines have been prepared using 2-amino-3-substituted thiophene derivatives as important starting compounds. Gewald’s procedure was used in the traditional synthesis of these derivatives [31,32,33]. The synthesis of thieno[2,3-d]pyrimidine scaffolds has been described using a variety of methods [34,35,36,37]. Our goal is to report the synthesis of thienopyrimidine derivatives using two main strategies. Either pyrimidine ring closure in aminothiophene derivatives or thiophene ring closure in pyrimidine derivatives.
Starting from thiophene derivatives
Starting from 2-aminothiophene-3-carboxylate derivatives
Phoujdar et al. reported a microwave-based synthesis of novel thienopyrimidine derivatives that were designed as gefitinib bioisosteres in high yield [38]. The synthesis of compound 2 via the reaction of 2-aminoester derivative 1 with formamide using microwave irradiation (MWI) took 25 minutes, whereas the previous traditional method took from 8–10 hours [39] (Scheme 1). Moreover, chloro derivative 3 was produced when compound 2 reacted with phosphorus oxychloride under MWI for 12 minutes. In addition to improved yield and purity, the reaction time for this method was reduced from 14 h to 12 min.
Moreover, Mavrova et al. synthesized thiosemicarbazide and 1,3,4-thiadiazole thieno[2,3-d]pyrimidine derivatives [40] (Scheme 2). The formation of the pyrimidine ring of compound 4 was achieved by cyclocondensation of the 2-aminoester derivative 1 with ethyl isothiocyanate in presence of NaOH.
Starting from amino cyanothiophene derivatives
In 2014, Kerru et al. synthesized derivatives of thienopyrimidines that contain 1,2,4-triazoles and 1,3,4-oxadiazoles [41] (Scheme 3). Compound 6 was obtained by refluxing 5-amino-4-cyanothiophene derivatives 5 and triethyl orthoformate. Moreover, triazolo derivatives 7 were produced from the reaction of compound 6 with substituted aryl hydrazides in toluene under reflux.
On the other hand, Gao et al. demonstrated that thienopyrimidine derivative 9 was produced by the reaction of substituted 2-aminothiophene-3-carbonitrile 8 with trifluoroacetic acid (TFA) in presence of toluene and phosphorus oxychloride [42] (Scheme 4).
Starting from 2-aminothiophene-3-carboxamide derivatives
Kassab et al. synthesized a series of hexahydrocyclooctathieno[2,3-d]pyrimidines [43] (Scheme 5). Cyclocondensation of 2-aminothiophene-3-carboxamide derivative 10 with aromatic aldehydes in dry dimethylformamide provided thienopyrimidine derivatives 11. Refluxing derivatives 11 and phosphorus pentasulfide in presence of xylene, produced 4-thioxo derivatives 12.
Meanwhile, Rashad et al. synthesized thienopyrimidine derivatives [44] (Scheme 6). Compound 13 produced the equivalent 2-thioxo derivative 14 when heated with carbon disulfide under reflux.
Starting from pyrimidine derivatives
Thienopyrimidines could be synthesized from pyrimidine derivatives. Brough et al. reported the production of compound 16 by the reaction of compound 15 with ethyl-2-mercaptoacetate in presence of potassium carbonate as a base. Upon reaction of the ester 16 with aqueous ammonia and microwave irradiation at 130 °C, compound 17 was produced [45] (Scheme 7).
Saddik et al. produced thieno[2,3-d]pyrimidine derivatives in 2018 [46] (Scheme 8). Compound 18 reacted with thiourea in ethanol, followed by treatment with sodium hydroxide solution and acidification with diluted HCl, to yield 4-mercapto-2-morpholino-6-phenylpyrimidine-5-carbonitrile 19. Moreover, compound 19 undergoes cyclization to give derivatives 20 through alkylation with chloroacetonitrile, chloroacetamide, and ethyl chloroacetate in ethanol and in the presence of potassium carbonate.
Anticancer activity
Nowadays cancer is the most dangerous life-threatening disease in our life. it is suggested to be the first reason for mortality in the future. The number of cancer patients globally is supposed to increase during the next years [47]. Biological studies of thieno[2,3-d]pyrimidines have demonstrated that the replacement of different groups on this important core confers antineoplastic activity via inhibition of various kinases [48,49,50].
Thienopyrimidine derivatives as protein kinase inhibitors
Thienopyrimidine derivatives as epidermal growth factor receptor (EGFR) inhibitors
In small molecular cancer therapy, epidermal growth factor receptor tyrosine kinase (EGFR TK) is an important target [51, 52]. It is a cell-surface tyrosine kinase receptor that is stimulated by the alpha transforming growth factor (TGFα), extracellular protein ligands, and members of the epidermal growth factor (EGF) family [53]. EGFR overexpression has been linked to uncontrolled cell division in a variety of cancers, including multiform glioblastoma, lung and anal carcinoma [54]. In 2014, Yang et al. produced a series of thienopyrimidine derivatives with α,β-unsaturated amide side chains at position 6 (Fig. 2) [55]. Compound 21 was of great interest because it was found to be better than the marketed drug lapatinib as an EGFR inhibitor. Moreover, it showed better activity than lapatinib against breast carcinoma (SK-BR-3) cell line with IC50 = 0.13 µM. It displayed irreversible inhibition of the EGFR enzyme due to the existence of an amide side chain that creates a covalent bond with Cys773 placed in the ATP pocket of the EGFR enzyme [55].
A novel series of 6-cinnamoyl-4-arylaminothienopyrimidines was synthesized in 2020. They were evaluated as anticancer agents and displayed highly potent cytotoxic activity in comparison to erlotinib (Fig. 2) [56]. Thieno[2,3-d]pyrimidine derivatives 22a-h and thieno[3,2-d]pyrimidine derivatives 23a-f were created. The antineoplastic activity of these compounds against prostate cancer (PC3) showed that all of the compounds demonstrated excellent activity with IC50 values in the submicromolar values from 0.1 to 0.79 µM. All of the derivatives 23a-f demonstrated a significant effect on prostate cancer PC3; breast cancer MDA-MB-231 and hepatocellular cancer cell line (HepG2) with IC50 from 0.10 to 15.90 µM, and moderate activity on lung cancer cell line (A549) with IC50 from 6.67 to 26.24 µM.
In the two series 22a-h and 23a-f, the presence of an ethynyl group connected to the aryl amine group at C-3 resulted in good cytotoxic effects on PC3 and breast cancer (MDA-MB-231) cell lines when compared to other cell lines. 3,5-Dichloro substituted derivative 22e was the most potent derivative against all of the examined cell lines, followed by derivative 22g with 3,4-dichloro substitution. The most powerful compounds 22e and 22g were evaluated on colorectal cancer (HCT-116 and SW480); breast cancer (SKBR3); ovarian cancer (SKOV3) and glioblastoma cell (U87) cell lines. They demonstrated IC50 ranging from 3.83 to 11.94 µM, compared to erlotinib which exhibited lower effectiveness against these cell lines with IC50 from 22.99 to 61.78 µM. In addition, western blot analysis revealed that compound 22e inhibited the phosphorylation of EGFR and downstream molecule ERK1/2. Furthermore, the effects of compounds 22e and 22g on cell cycle distribution and apoptosis were investigated, and the results revealed that most cells remained in the G0 phase and that cell growth was arrested. Moreover, it was revealed that for 22e and 22g, the percentages of early and late apoptosis were 11%, 15.5%, and 42.8%, 15.7%, correspondingly so they stimulated cell death. Molecular docking study showed that compound 22e formed hydrogen binding interactions with Asp 831, Met 769, and Lys 721. In addition to π-π and π-alkyl interactions with Phe 699 and Val 702, respectively in the hydrophobic pocket. Moreover, in the solvent region, the cinnamamide part created Van der Waals interactions with Gly 772 and Pro 770 (Fig. 3). From this study we can summarize the presence of dichloro substitution on aniline ring may enhance the anticancer activity and the cinnamamide moiety is favorable to interact with the active site of EGFR enzyme.
Thienopyrimidine derivatives as vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitors
The VEGF receptors are a kind of receptor tyrosine kinase (RTK) that is important for vascular development and hematopoiesis [57]. The three VEGFR members are VEGFR-1, VEGFR-2, and VEGFR-3 [58]. VEGFR performs a crucial starring role in the proliferation, migration, and angiogenesis of vascular endothelial cells when it is activated by VEGF [59, 60]. The significance of VEGFR-2 in tumor angiogenesis has prompted attention to the progress of VEGFR-2 inhibitors [61,62,63]. Sorafenib [64], sunitinib [65, 66], lenvatinib [67], and linifanib [68] are examples of VEGFR-2 inhibitors. In 2015, Abdel Aziz et al. synthesized tricyclic pyrido[3’,2’:4,5]thieno[3,2-d]pyrimidin-4-amine derivatives as VEGFR-2 inhibitors (Fig. 4) [69]. It was found that compound 24a with thienopyridine ring demonstrated the strongest inhibition against VEGFR-2 by 67% with an IC50 value of 2.6 μM, whereas compound 24b with pyrazolopyridine and 24c with isoxazolepyridine inhibited VEGFR-2 by 12% and 18%, respectively. Additionally, the molecular docking of 24a revealed that Cys917 in the adenine region of the ATP binding site generated the crucial hydrogen bonding connection with its core structure and hydrophobic interactions between the 4,6-dimethylthieno[2,3-b]pyridine group and Val897, Cys1043, and Leu1033 at the other end of the ATP binding site. Moreover, 7,9-dimethyl substituted pyrido[3’,2’:4,5]thieno[3,2-d]pyrimidine was participated in interactions with Ala864 and Val914 (Fig. 5). From the previous findings, we conclude that the existence of thieno[3,2-d]pyrimidine as a core structure is important to interact with the ATP binding site of VEGFR-2.
In 2021, new hybrid compounds of thieno[2,3-d]pyrimidine with aryl aminothiazole were designed and evaluated as VEGFR-2 kinase inhibitors (Fig. 4) [70]. In relation to the synthesized compounds, it was discovered that the addition of a weak electron-withdrawing halogen atom, such as 4-chloro, 3-bromo, or 4-bromo, results in more effective carcinogenic agents. However, the compound with unsubstituted phenyl 25a and the compound substituted with a strong electron withdrawing as the 3-nitro group 25b displayed little cytotoxic activity against all investigated cell lines. Moreover, it was found that the replacement of 4-chloro atom in compound 25c with a 4-bromo atom in compound 25e, decreased the anticancer activity with mean inhibition of 18.30% and 4.35%, correspondingly. Additionally, the position of the bromo group in 25e was changed from para to meta in 25d, which resulted in an increase in the mean inhibition from 4.35% (25e) to 16.83% (25d). Therefore, the 4-chloro derivative 25c showed highly powerful VEGFR-2 kinase inhibition with an IC50 value of 62.48 nM in comparison to sorafenib. Additionally, compound 25c was the most effective derivative versus CNS cancer (SNB-75 and SF-295), and renal cancer (CAKI-1) cell lines with IC50 values of 7.12 ± 0.33, 7.36 ± 0.39, and 4.84 ± 0.22 μM, respectively. Furthermore, the results of the flow cytometric study demonstrated that 25c exhibited cytotoxic activity by inhibiting cellular growth and causing cell cycle arrest at the G2/M phase. Moreover, molecular modeling showed the ability of compound 25c to form interactions with essential amino acids in the VEGFR-2 binding site as demonstrated in Fig. 5.
From the previous results, we can assume the substitution of terminal phenyl ring with halogen is more beneficial in anticancer activity than substitution with a strong electron-withdrawing group.
Moreover, in 2021, new derivatives of thieno[2,3-d]pyrimidines were synthesized as VEGFR-2 kinase inhibitors [71]. Among the synthesized compounds, 26a-d and 27 showed the most potent inhibition against VEGFR-2 kinase with IC50 values ranging from 0.23 ± 0.03 to 0.37 ± 0.04 µM. Additionally, compound 26b with 4-chlorophenyl showed the most powerful VEGFR-2 kinase inhibition with an IC50 value of 0.23 ± 0.03 µM. For compound 26b, it was found to have greater anticancer activity than sorafenib against colorectal carcinoma HCT-116 and hepatocellular carcinoma HepG2 cell lines with IC50 of 2.80 ± 0.16 and 4.10 ± 0.45 µM, respectively. It was found that the replacement of an electron-withdrawing group (4-Cl) in 26b with an electron donating group (4-OCH3) in 26f, resulted in a loss of activity. However, compound 26e with a 2-methoxyphenyl group showed enhanced biological activity with VEGFR-2 inhibition IC50 of 0.69 ± 0.06 µM. Molecular docking of compound 26b revealed that hydrogen bonds were created between the hydrazide group and Glu883 and Asp1044. In addition, Cys1043 and Val897 formed two hydrophobic interactions with the phenyl ring (spacer). Moreover, 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine moiety created hydrophobic interactions with Leu1033, Leu838, Ala864, Cys917 and Val846 and a hydrogen bond with Cys917. Finally, the terminal phenyl ring interacted with Ile886 through a hydrophobic bond (Fig. 5). From the previous study, we can adopt the presence of thieno[2,3-d]pyrimidine as a core structure is important to interact with the ATP binding site of VEGFR-2 and the substitution of terminal phenyl ring with electron-withdrawing group is more useful in anticancer activity than substitution with electron donating group.
Thienopyrimidines derivatives as PI3K/AKT/mTOR pathway inhibitors
The phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (AKT)/ mammalian target of rapamycin (mTOR) (PI3K/AKT/mTOR) signaling system stands as a major mechanism that controls cell existence, proliferation, glucose metabolism, migration, and death [72, 73]. Throughout the last few decades, it has been widely explored to develop new cancer therapies. There are now several medications that target this route in clinical studies, the most predominant of which is class I PI3K inhibitors [74, 75]. In 2021, Sun et al. synthesized and evaluated a novel series of thieno[2,3-d]pyrimidine derivatives as PI3K inhibitors [76]. Compound 28 (Fig. 6) with unsubstituted morpholine moiety showed extraordinary PI3K inhibition with an IC50 value equal to 7.2 nM. In addition to good pharmacokinetics properties, and significant anticancer activity against gastric cancer cell line HGC-27 with IC50 of 0.39 µM. Compound 28 formed a hydrogen bond with Val851 in the hinge region of the PI3K active site. Additionally, compound 28 created a hydrogen bond network with Tyr836, Asp801, and the conserved water molecule in the affinity pocket, Also, Lys802 and deprotonated sulfonamide exhibited a charged interaction (Fig. 7).
A Interactions of compound 28 inside PI3K binding site (PDB: 4ZOP); B Interactions of compound 30 inside PI3Kβ binding site (PDB: 2Y3A); C Interactions of compound 30 inside PI3Kγ binding site (PDB: 3DBS); D Interactions of compound 33c inside PI3Kγ binding site (PDB: 3DBS); E Interactions of compound 33c inside mTOR binding site (PDB: 3L16)
Recently in 2022, Elmenier et al. synthesized and evaluated a series of 2-aryl-4-morpholinothieno[2,3-d]pyrimidine derivatives as PI3K inhibitors against various isomers PI3Kα, β, and γ in addition to their anticancer activity versus NCI 60 cell lines (Fig. 6) [77]. The enzymatic activity of compounds 29 and 30 with a 3-hydroxyphenyl ring was good for PI3Kβ (62% and 72%) and PI3Kγ (70% and 84%), correspondingly. Furthermore, derivatives containing tetramethylene substitution at positions 5 and 6 of the thienopyrimidine 30 and 32 mainly revealed improved activity contrasted to 5-methyl-6-carboxylate derivatives 29 and 31. Moreover, compounds 31 and 32 also showed reduced inhibitory activity when the hydroxyl position was changed from 3 to 4 with PI3Kβ % inhibition (39% and 50%) and PI3Kγ % inhibition (33% and 36%), respectively. Therefore, compound 30 was demonstrated as the strongest inhibitor (72% and 84% on PI3Kβ and PI3Kγ, respectively). Additionally, a molecular docking study of compound 30 with PI3Kβ showed that morpholine moiety formed a hydrogen bond with Val848 and the 3-hydroxyl group formed two hydrogen bond interactions with Lys799 and Asp931. Moreover, the thienopyrimidine ring formed hydrophobic interaction with Met773. On the other hand, docking of 30 with PI3Kγ demonstrated the formation of three hydrogen bonds with Val882, Asp964, and Asp841 and hydrophobic interaction between the tetramethylene ring and Met953. Furthermore, the morpholine moiety created hydrophobic interaction with Ile881 (Fig. 7). Finally, according to this study, maintaining the morpholine part that binds to Val residue at the hinge region is one of the most important factors to take into account in the design of an efficient PI3K inhibitor.
In 2020, Han et al. synthesized a new series of thieno[3,2-d]pyrimidine derivatives that contain aryl hydrazide part which were evaluated as PI3K/mTOR dual inhibitors (Fig. 6) [78]. This study showed that the aryl hydrazide on C-6 was most beneficial since the hydrazide moiety is strongly desired by the solvent-exposed region of PI3Kα and the presence of methoxy group on the terminal phenyl ring enhanced the activity. Moreover, the inhibition activity of compounds substituted with indazole 33b or 2-aminopyrimidine 33c groups on C-2 was greater than the inhibition activity of morpholino-substituted derivative 33a. Therefore, compound 33c with a 4-methoxybenzohydrazide group at C-6 and 2-aminopyrimidine group at C-2 of the thieno[3,2-d]pyrimidine backbone demonstrated the most effective PI3Kα and mTOR inhibitory activities with IC50 values of 0.46 and 12 nM, respectively. Also, 33c inhibits PI3Kγ with an IC50 value of 13 nM. Moreover, the cell cycle study of 33c revealed cell cycle arrest in the G1/S stages, which caused the HCT-116 cells to undergo apoptosis. The docking study of compound 33c with PI3Kγ displayed that the oxygen atom of morpholine moiety formed a hydrogen bond with Val882 and the NH of aryl hydrazide formed two H-bonds with Asp950. Moreover, Thr 887 created a hydrogen bond with the carbonyl group of the aryl hydrazide. Additionally, at the terminal phenyl ring, a 4-methoxy group formed a hydrogen bond with Lys 890, while the 2-aminopyrimidine fragment at the C-2 formed hydrogen bonds with Asp 841 and Asp 964. When compound 33c was docked in the mTOR active site, comparable hydrogen bond interactions were detected whereas the morpholine moiety formed a hydrogen bond with Val882, the 2-aminopyrimidine fragment at the C-2 also formed hydrogen bonds with Asp 964 and Asp 841 (Fig. 7). From the previous three studies, we can assume the importance of the presence of unsubstituted morpholine moiety while designing effective PI3K inhibitors as it binds to Val residue at the hinge region of PI3K active site.
Thienopyrimidine derivatives as EGFR/ HER2 dual inhibitors
The ErbB family consists of four members: EGFR (ErbB-1), human epidermal growth factor receptors (HER2, ErbB-2), catalytically inactive ErbB-3, and ErbB-4 [79]. When a ligand interacts with the extracellular domain of either EGFR or HER2, kinase-active homodimers and heterodimers are formed [80, 81]. EGFR TK inhibitors have been successfully used as a treatment for NSC lung cancer patients [82]. The main disadvantage of administering EGFR TK inhibitors is the development of secondary or acquired resistance [83]. Because overactive EGFR and HER2 tyrosine kinases are essential hallmarks of various cancers, including colorectal, lung, pancreatic, head, and neck cancers, dual blocking of the EGFR and HER2 pathway is an excellent strategy for effective anticancer therapy [84,85,86].
In 2016, Abd El Hadi et al. reported the synthesis of two series of 4-anilinothieno[2,3-d] pyrimidines which were assessed as dual EGFR/HER2 kinase inhibitors (Fig. 8) [87]. Series A contains compounds that were less potent inhibitors for EGFR/HER2 kinase than Series B. Hence, the replacement of the 5-methyl-6-carboxylate groups in series A with the more hydrophobic 5,6-tetramethylene moiety in series B led to a significant increase in EGFR/HER2 inhibition. On the other hand, 3-chloroaniline-containing derivatives were more active than derivatives with m-unsubstituted aniline. Five derivatives of the tested compounds from series B (34-38) showed significant EGFR/HER2 inhibitory action, as measured by their IC50 values as demonstrated in Table 1. Therefore, compound 37 exhibited the highest inhibitory activity on both kinases. Regarding the molecular docking of compound 37 with EGFR active site, it showed hydrogen bond interaction between N1 of thienopyrimidine ring and Met793 and it formed a water-mediated hydrogen bond through N3 with Thr854. Additionally, 37 created π–cation and π–sigma interactions with Lys745 and Phe856, respectively. On the other hand, compound 37 demonstrated crucial interactions with HER2 binding site as N1 of thienopyrimidine created hydrogen bond interaction with Met801 as well as the formation of π–cation interaction with Lys753 (Fig. 9). From the mentioned work we conclude that, the presence of a more hydrophobic moiety attached to the thienopyrimidine ring is more effective towards EGFR/HER2 inhibition.
In 2018, Milik et al. prepared a series of thieno[2,3-d]pyrimidine as dual EGFR/HER2 kinase inhibitors built on the 6-phenylthieno[2,3-d]pyrimidine as a core scaffold (Fig. 8) [88]. Compounds 39a-c provided potent dual EGFR/HER2 inhibitory activity with IC50 values of 21.4, 47.7, and 91.7 nM and 1.5, 0.879, and 1.2 µM, respectively. This study showed that these compounds are equipped with bulky aniline head groups able to penetrate into the EGFR and HER2 back pockets. Moreover, compounds 39a-c inhibited breast cancer SKBR3 cell line with IC50 equals 6.0, 4.7, and 4.83 µM, respectively. Also, with IC50 of 4.2 µM, 39c greatly inhibited the growth of the non-small cell lung cancer NCI-H1975 cell line and enhanced the percentages of apoptotic and necrotic cells. Molecular docking and interactions of compound 39a with key amino acids inside EGFR and HER2 active sites are presented in Fig. 9. We determine from the previous work the significance of the presence of bulky aniline group at position 4 of the thienopyrimidine ring in designing EGFR and HER2 inhibitors.
Thienopyrimidine derivatives as VEGFR-2/ BRAF kinases dual inhibitors
Rapidly accelerated fibrosarcoma (RAF) kinases are serine/threonine protein kinases (PKs) that show a significant role in cell survival and proliferation. ARAF, BRAF, and CRAF are members of the RAF family whereas, in human malignancies, the BRAF valine 600 residue (V600E) mutation is the most common [89]. Melanomas, colorectal, thyroid cancer, and other human cancers can all be treated with BRaf inhibitors [90,91,92]. Consequently, dual inhibition of BRAF /VEGFR-2 is seen as a viable cancer therapeutic technique [93, 94]. Recently in 2022, Hassan et al. synthesized new hexahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivatives as dual VEGFR-2/BRAF inhibitors (Fig. 10) [95]. Compounds 40 and 41 were elongated with the crucial urea insertion to have distal moieties, which increased antiproliferative action. Additionally, adding a p-chloro group to the terminal phenyl ring of compound 41 slightly increased its antiproliferative activity as compared to derivative 40. Therefore, compounds 40 and 41 demonstrated significant anticancer activity against most cancer cell lines. In addition, compounds 40 and 41 effectively inhibited VEGFR-2, BRAFV600E, and BRAFWT with IC50 values of 0.111 ± 0.006 and 0.049 ± 0.003 µM, 0.089 ± 0.005 and 0.063 ± 0.003 µM, and 0.071 ± 0.004 and 0.05 ± 0.003 µM, respectively, compared to sorafenib. Furthermore, compounds 40 and 41 increased the overall apoptotic proportion in the breast cancer MCF7 cell line by 22.82 and 25.81 fold, correspondingly. Additionally, the examination of the cell cycle revealed that compounds 40 and 41 primarily arrested the cell cycle in the G1 and G1/S phases, correspondingly. When compounds 40 and 41 docked inside VEGFR-2 and BRAF binding sites, they exhibited comparable interactions. Regarding VEGFR-2, 4-chlorophenyl cycloalkylthieno[2,3-d]pyrimidine formed a hydrogen bond with Cys919 through N1 as well as π-π and π-H interactions with Phe1047 and Leu840, respectively. Moreover, Glu885 and Asp1046 demonstrated hydrogen bond interactions with the urea group, and a sulfur-dipole interaction took place between the sulfur atom in 2-thioacetamide moiety and Glu917. Also, in 41 the chloro atom at the terminal phenyl group formed halogen bonding with Ile1025. On the other hand, docking of 40 and 41 inside BRAF showed hydrogen bond interactions between urea moiety with Glu500 and Asp593. Furthermore, the 2-thioacetamide spacer made a hydrogen bond with Thr528. Finally, 4-chlorophenyl cycloalkylthieno[2,3-d]pyrimidine formed π-H interaction with Val470 and sulfur-dipole bond with Cys531(Fig. 11). From the mentioned data, the insertion of urea moiety in the previous compounds was crucial for VEGFR-2/BRAF activity due to the important interactions with VEGFR-2 and BRAF binding sites.
Thienopyrimidine derivatives as FMS-like tyrosine kinase-3 (FLT3) inhibitors
Early hematopoietic progenitor cells express FLT3, a type III receptor tyrosine kinase, which is essential for the survival and proliferation of hematopoietic stem cells [96, 97]. Acute myeloid leukemia (AML) is a clonal hematopoietic stem cell disease characterized by aberrant blast cell differentiation and proliferation in the bone marrow and peripheral circulation [98]. FLT3 overexpression is prevalent in AML patients, as well as other patients with FLT3 mutations [99].
Park et al. created thienopyrimidine-based analogs by modifying SPC-839, the well-known inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ) to prepare derivatives 42a-e, and then tested them in 2014 (Fig. 12) [100]. It was found that the synthesized analogs revealed no inhibitory effect against IKKβ but they demonstrated good inhibition against FLT3 kinase. Compounds 42a-e which contain aliphatic or aromatic substituents at C-5 of thienopyrimidine revealed good FLT3 kinase inhibition with IC50 ranging from 0.065 to 0.750 µM. In addition, compound 42b with a methyl group at C-5 showed the highest inhibitory activity on FLT3 kinase with IC50 equals 0.065 µM. Furthermore, compound 42c with unsubstituted phenyl showed more inhibitory activity than compounds 42d and 42e with 3-hydroxy and 4-hydroxy phenyl substitutions. In 2016, Kim et al. developed thieno[2,3-d]pyrimidine derivatives to potentially act as FLT3 inhibitors for the treatment of AML (Fig. 12) [101]. Effective antiproliferative activity against the leukemia cell line MV4-11 was shown by compounds 43a–d, with GI50 of 0.366, 0.585, 0.540, and 0.278 µM, respectively. These compounds contain methyl group at the C-5 and cycloaminoalkoxy or elongated aminoethoxy groups in the para position of the phenyl group at C-6 which provided successful FLT3 inhibition. Therefore, compounds 43a–d inhibited FLT3 kinase with IC50 values of 3.769, 6.427, 8.026, and 2.495 nM, correspondingly. Furthermore, these compounds had improved metabolic stabilities. From the previous two studies, we can design FLT3 inhibitors upon some modification on SPC-839 structure as well as the presence of methyl group at C-5 of thienopyrimidine ring is valuable towards FLT3 inhibition.
Recently, in 2022 Elmongy et al. synthesized thienopyrimidine compounds that target FLT3 kinase (Fig. 12) [102]. Whereas, upon FLT3 enzyme assay, it was found that compound 44a had the highest FLT3 inhibitory activity of the investigated compounds, with IC50 of 17.83 ± 3.8 µM, followed by derivatives 44b and 45, which had IC50 values of 20.4 ± 2.8 and 27.22 ± 5.6 µM, correspondingly. Moreover, compound 44c demonstrated moderate inhibition of FLT3 with IC50 of 47.64 ± 9.3 µM. On the other hand, the molecular docking study of compound 44a inside the FLT3 active site exhibited three interactions with Leu616, Cys694, and Asp698 (Fig. 13).
Thienopyrimidine derivatives as topoisomerase II inhibitors
Topoisomerases I and II are enzymes that govern supercoiling and prevent DNA tangling, making them crucial for cancer cell proliferation. Topoisomerases have been identified as a key target for anticancer medicines [103]. Topoisomerase inhibitors have been developed to limit the function of topoisomerases (I and II) or to reduce their expression either or both their protein content [6, 104]. Doxorubicin, etoposide, and mitoxantrone are examples of the most well-known topoisomerase II inhibitors, and they serve as models for future research [105]. Abdelhaleem et al. synthesized a variety of new tetrahydrobenzothieno[2,3-d]pyrimidine urea derivatives (Fig. 14). Whereas, the C-4 position of thieno[2,3-d]pyrimidine had phenyl urea or phenyl thiourea group [106]. Consequently, compounds 46a-c with electron-withdrawing groups showed the most powerful anticancer activity against breast cancer MCF-7 cell line with IC50 equals 7.10, 10.33, and 9.55 µM, respectively which were more effective than doxorubicin with IC50 equals 10.60 µM. Additionally, compounds 46a and 46c were found to be more efficient than 46b when the electron-withdrawing group was present in the p-position. In addition to topoisomerase inhibition, compound 46a inhibited many enzymes. Whereas the inhibitory activity against topoisomerase II with IC50 equals 9.29 µM and VEGFR-2 with IC50 equals 0.2 µM which was more effective than sorafenib. Moreover, compound 46a significantly increased the proportion of cells in the pre-G1 and G2/M phases in comparison to control by 15.1 and 2.2 times, correspondingly, suggesting a potential role for apoptosis in compound 46a. Furthermore, molecular modeling of 46a showed that it could interact with essential amino acids in topoisomerase II binding site as NH of diaryl urea formed a hydrogen bond with Thr 744 and the carbonyl group of urea formed a hydrogen bond Tyr 734 (Fig. 15). From the previous work we can summarize the substitution of the terminal phenyl ring with electron-withdrawing group at para position is significant in anticancer activity. Recently in 2020, El-Metwally et al. synthesized and assessed thieno[2,3-d]pyrimidine derivatives as topoisomerase II inhibitors (Fig. 14) [107]. Compounds 47-50 which contain various substituents at C-4 of thienopyrimidine showed the most potent anticancer activity against liver cancer (HepG2) and breast cancer (MCF7) cell lines with IC50 ranging from 4.38 to 6.71 and 3.96 to 9.19 µM, respectively. In addition, semicarbazide compound 50 significantly reduced topoisomerase II expression by about 60% compared to doxorubicin which reduced topoisomerase II expression by about 40%. Additionally, the docking of compound 50 inside the DNA binding site of topoisomerase II demonstrated that it interacted through the formation of two hydrogen bonds between the two amidic NH groups and AspA479. In addition, thienopyrimidine moiety created hydrophobic interactions with LysA739, ThrA783, and TyrA773. Moreover, aromatic stacking interaction was presented between LysA456 and ArgA503 and the benzene ring (Fig. 15). From the previous two studies, we assume the significant role of urea moiety in interaction with the topoisomerase II active site.
Thienopyrimidine derivatives as tubulin polymerization inhibitors
The cytoskeleton consists of microtubules, which play an important role in all eukaryotic cells [108]. Among their functions are cell division, mitosis, maintaining the shape of cells, motility regulation, and cell signaling [109, 110]. A successful cancer treatment approach involves creating small molecules that disrupt tubulin dynamics [111]. Tian et al. designed thieno[3,2-d]pyrimidine compounds as tubulin polymerization inhibitors (Fig. 16) [112]. Regarding compounds 51a-d, it was found that the substitution of compounds 51c (3-methyl) and 51d (3-methoxy) with electron‒donating groups enhanced the antineoplastic activity while the substitution of 51a (3-F) and 51b (3-Br) with electron-withdrawing groups decreased the antineoplastic activity. Therefore, compound 51c revealed effective tubulin polymerization inhibition with IC50 of 4.1 ± 0.1 µM. Additionally, a cell cycle study of 51c revealed that it caused G2/M arrest in Hela cells. According to molecular modeling of compound 51c in tubulin, the 3-methyl group on the phenyl ring was tightly positioned within a sub-pocket shaped by Val181 (α-monomer) and Val315 (β-monomer) (Fig. 17). From this study, we can conclude that the presence of methyl group in position 3 of the phenyl ring significantly increased the antiproliferative action and it was important for tubulin polymerization inhibition due to its interaction with the active site of tubulin. In 2018, Yang et al. synthesized thienopyrimidine derivatives having dithiocarbamate moiety at C2 (Fig. 16) [113]. The results showed that compounds substituted with strong electron-withdrawing groups at postion 4 of terminal phenyl ring as 52b (CN) and 52c (NO2) were more powerful than compound 52a with unsubstituted phenyl ring. On the other side, compounds substituted with electron-donating groups at postion 4 of the phenyl ring as 52d (methyl) and 52e (methoxy) demonstrated comparable anticancer activity with 52a. Hence, compound 52b presented the highest antineoplastic activity against A549 cell line with IC50 of 4.87 µM. As a result of 52b, caused cell cycle arrest at the G2/M phase and the spindle assembly checkpoint (SAC) is activated. Furthermore, compound 52b showed tubulin polymerization inhibition in a dose-dependent manner. From this study, we can highlight the importance of the substitution of phenyl ring with a strong electron-withdrawing group to achieve the maximum anticancer activity.
Thienopyrimidine derivatives as histone deacetylase inhibitors
It has been well-documented that HDACs play an important role in epigenetic regulation [114]. Histone acetylation is regulated by two enzymes: HDACs and histone acetyltransferases (HATs) [115]. Normally, HATs and HDACs are balanced in cells. Alternatively, cancer cells have an imbalance between histone acetylation and deacetylation due to overexpression of HDACs or suppression of HATs, resulting in oncogene activation and tumor progression [116]. Therefore, Developing novel anticancer agents through the inhibition of HDACs has proven successful [117]. In 2017, Wang et al. designed thienopyrimidine derivatives containing hydroxamic acid as HDAC inhibitors (Fig. 18) [118]. The design of the synthesized compounds (53a-c) is based on the presence of hydroxamic acid which is an essential functional group for HDACs inhibition and act as zinc binding group (ZBC) at the HDACs active site. The results of tested compounds demonstrated that the introduction of a bulky group at position 3 or 4 of phenyl ring as in 53b (3-tertbutyl) and 53c (4-isopropyl) decreased HDACs inhibition. Finally, compound 53a (3-ethynyl) was observed to be an effective inhibitor of HDAC1, HDAC3, and HDAC6 with IC50 values of 29.81 nM, 24.71 nM, and 21.29 nM, respectively. Mohamed et al. synthesized thienopyrimidine compounds which were evaluated as HDAC inhibitors (Fig. 18) [119]. Consequently, compounds containing hydroxamic acid as a zinc-binding group (ZBC) either with an aliphatic or aromatic linker (54a and 55) exhibited high inhibitory activity against HDAC while replacement of hydroxamic acid moiety with hydrazide (54b) or 2-aminoanilide (54c) groups reduced HDAC inhibition. Moreover, it was observed that compounds with aliphatic linkers were more effective against HDAC than compounds with aromatic linkers as illustrated in. Therefore, compound 54a revealed the most effective HDACs inhibition with IC50 against HDAC1, HDAC2, HDAC6, and HDAC8 equals 0.028 µM, 0.078 µM, 0.471 µM, and 1.903 µM, respectively. Additionally, a molecular docking study of compound 54a with HDAC2 active site exhibited that the hydroxamic acid group (ZBG) formed hydrogen bonds with His145, His146, and Tyr308. Besides, it showed metallic bonds with Zn ion and hydrophobic interaction with His33 (Fig. 19). From the previous research, we can assume that the presence of a hydroxamic acid group with an aliphatic linker achieved significant HDAC inhibition and it is a favorable feature for designing HDAC inhibitors.
Conclusion
Thienopyrimidine derivatives perform a significant role in the production of drugs that have different pharmacological activities particularly, anticancer activity. Thienopyrimidines act as anticancer agents through diverse enzyme inhibition as (EGFR, VEGFR-2, BRAF, etc.). So in this review, the most recent publications on thienopyrimidine scaffold synthesis and anticancer evaluation have been reviewed. The current review can help scientists and researchers from around the world select precisely the goals for the future development of powerful lead compounds as antineoplastic medicines.
References
Hanjani NA, Esmaelizad N, Zanganeh S, Gharavi AT, Heidarizadeh P, Radfar M, et al. Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit Rev Oncol Hematol. 2022;169:103565.
Adil MS, Khulood D, Somanath PR. Targeting Akt-associated microRNAs for cancer therapeutics. Biochem Pharm. 2021;189:114384.
Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39.
Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharm Res. 2021;165:105463.
Soltan OM, Shoman ME, Abdel-Aziz SA, Narumi A, Konno H, Abdel-Aziz M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur J Med Chem. 2021;225:113768.
Liang X, Wu Q, Luan S, Yin Z, He C, Yin L. et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur J Med Chem. 2019;171:129–68. https://doi.org/10.1016/j.ejmech.2019.03.034.
Nagaraju B, Kovvuri J, Kumar CG, Routhu SR, Shareef MA, Kadagathur M. et al. Synthesis and biological evaluation of pyrazole linked benzothiazole-β-naphthol derivatives as topoisomerase I inhibitors with DNA binding ability. Bioorg Med Chem. 2019;27:708–20. https://doi.org/10.1016/j.bmc.2019.01.011.
Kamal A, Shaik AB, Jain N, Kishor C, Nagabhushana A, Supriya B. et al. Design and synthesis of pyrazole-oxindole conjugates targeting tubulin polymerization as new anticancer agents. Eur J Med Chem. 2015;92:501–13. https://doi.org/10.1016/j.ejmech.2013.10.077.
Yang W, Li L, Ji X, Wu X, Su M, Sheng L. et al. Design, synthesis and biological evaluation of 4-anilinothieno[2,3-d]pyrimidine-based hydroxamic acid derivatives as novel histone deacetylase inhibitors. Bioorg Med Chem. 2014;22:6146–55. https://doi.org/10.1016/j.bmc.2014.08.030.
Litvinov VP. Thienopyrimidines: Synthesis, properties, and biological activity. Russ Chem Bull. 2004;53:487–516.
Elrazaz EZ, Serya RAT, Ismail NSM, Abou El Ella DA, Abouzid KAM. Thieno[2,3-d]pyrimidine based derivatives as kinase inhibitors and anticancer agents. Futur J Pharm Sci. 2015;1:33–41.
Malasala S, Polomoni A, Ahmad MN, Shukla M, Kaul G, Dasgupta A. et al. Structure based design, synthesis and evaluation of new thienopyrimidine derivatives as anti-bacterial agents. J Mol Struct. 2021;1234:130168. https://doi.org/10.1016/j.molstruc.2021.130168.
Abdel Hamid AM, Shehta W. Synthesis of some novel furan-tagged thienopyrimidine derivatives as antibacterial agents. J Heterocycl Chem. 2019;56:485–92.
Ahmed M, Sayed M, Saber AF, Hassanien R, Kamal El-Dean AM, Tolba MS. Synthesis, characterization, and antimicrobial activity of new thienopyrimidine derivatives. Polycycl Aroma Compd. 2022;42:3079–88. https://doi.org/10.1080/10406638.2020.1852587.
Bassetto M, Leyssen P, Neyts J, Yerukhimovich MM, Frick DN, Brancale A. Computer-aided identification, synthesis and evaluation of substituted thienopyrimidines as novel inhibitors of HCV replication. Eur J Med Chem. 2016;123:31–47. https://doi.org/10.1016/j.ejmech.2016.07.035.
Khattab RR, Hassan AA, Kutkat OM, Abuzeid KM, Hassan NA. Synthesis and antiviral activity of novel thieno[2,3-d]pyrimidine hydrazones and their C-nucleosides. Russ J Gen Chem. 2019;89:1707–17.
El-Shoukrofy MS, Abd El Razik HA, AboulWafa OM, Bayad AE, El-Ashmawy IM. Pyrazoles containing thiophene, thienopyrimidine and thienotriazolopyrimidine as COX-2 selective inhibitors: Design, synthesis, in vivo anti-inflammatory activity, docking and in silico chemo-informatic studies. Bioorg Chem. 2019;85:541–57. https://doi.org/10.1016/j.bioorg.2019.02.036.
Tolba MS, Ahmed M, Kamal El-Dean AM, Hassanien R, Farouk M. Synthesis of new fused thienopyrimidines derivatives as anti-inflammatory agents. J Heterocycl Chem. 2018;55:408–18.
Leeza Zaidi S, Agarwal SM, Chavalitshewinkoon-Petmitr P, Suksangpleng T, Ahmad K, Avecilla F, et al. Thienopyrimidine sulphonamide hybrids: Design, synthesis, antiprotozoal activity and molecular docking studies. RSC Adv. 2016;6:90371–83.
Bozorov K, Zhao JY, Elmuradov B, Pataer A, Aisa HA. Recent developments regarding the use of thieno[2,3-d]pyrimidin-4-one derivatives in medicinal chemistry, with a focus on their synthesis and anticancer properties. Eur J Med Chem. 2015;102:552–73. https://doi.org/10.1016/j.ejmech.2015.08.018.
Bugge S, Buene AF, Jurisch-Yaksi N, Moen IU, Skjønsfjell EM, Sundby E. et al. Extended structure-activity study of thienopyrimidine-based EGFR inhibitors with evaluation of drug-like properties. Eur J Med Chem. 2016;107:255–74. https://doi.org/10.1016/j.ejmech.2015.11.012.
Shyyka O, Pokhodylo N, Finiuk N, Matiychuk V, Stoika R, Obushak M. Anticancer activity evaluation of new thieno[2,3-d]pyrimidin-4(3H)-ones and thieno[3,2-d]pyrimidin-4(3H)-one derivatives. Sci Pharm. 2018;86:28.
Khattab RR, Alshamari AK, Hassan AA, Elganzory HH, El-Sayed WA, Awad HM. et al. Click chemistry based synthesis, cytotoxic activity and molecular docking of novel triazole-thienopyrimidine hybrid glycosides targeting EGFR. J Enzym Inhib Med Chem. 2021;36:504–16. https://doi.org/10.1080/14756366.2020.1871335.
Miwa K, Hitaka T, Imada T, Sasaki S, Yoshimatsu M, Kusaka M. et al. Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gona. J Med Chem. 2011;54:4998–5012.
Liu YF, Fu SQ, Yan YC, Gong BB, Xie WJ, Yang XR. et al. Progress in clinical research on gonadotropin- releasing hormone receptor antagonists for the treatment of prostate cancer. Drug Des Devel Ther. 2021;15:639–49. https://doi.org/10.2147/DDDT.S291369.
Hasler WL. Serotonin and the GI tract. Curr Gastroenterol Rep. 2009;11:383–91.
Ali EMH, Abdel-Maksoud MS, Oh CH. Thieno[2,3-d]pyrimidine as a promising scaffold in medicinal chemistry: Recent advances. Bioorg Med Chem. 2019;27:1159–94. https://doi.org/10.1016/j.bmc.2019.02.044.
Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) Inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:77–86.
Liao BC, Lin CC, Lee JH, Yang JCH. Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptor tyrosine kinase inhibitors. J Biomed Sci. 2016;23:1–10. https://doi.org/10.1186/s12929-016-0305-9.
Kim DW, Lee DH, Han JY, Lee J, Cho BC, Kang JH, et al. Safety, tolerability, and anti-tumor activity of olmutinib in non-small cell lung cancer with T790M mutation: A single arm, open label, phase 1/2 trial. Lung Cancer. 2019;135:66–72. https://doi.org/10.1016/j.lungcan.2019.07.007.
Aurelio L, Valant C, Figler H, Flynn BL, Linden J, Sexton PM, et al. 3- and 6-Substituted 2-amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridines as A1 adenosine receptor allosteric modulators and antagonists. Bioorg Med Chem. 2009;17:7353–61.
Mohareb RM, Ibrahim RA, Wardakhan WW. Synthesis of pyridine, pyran and thiazole containing thiophene derivatives and their anti-tumor evaluations. Med Chem Res. 2016;25:2187–204. https://doi.org/10.1007/s00044-016-1654-3.
Kathiravan MK, Shishoo CJ, Chitre TS, Mahadik KR, Jain KS. Efficient synthesis of substituted 2-amino-3-carbethoxythiophenes. Synth Commun. 2007;37:4273–9.
Kandeel MM, Refaat HM, Kassab AE, Shahin IG, Abdelghany TM. Synthesis, anticancer activity and effects on cell cycle profile and apoptosis of novel thieno[2,3-d]pyrimidine and thieno[3,2-e]triazolo[4,3-c]pyrimidine derivatives. Eur J Med Chem. 2015;90:620–32. https://doi.org/10.1016/j.ejmech.2014.12.009.
Salib SB, Khalil OM, Kamel MM, El-Dash Y. Synthesis and antitumor activity of novel thienopyrimidine derivatives containing thiosemicarbazide moiety. OALib. 2016;03:e2876.
Wu CH, Coumar MS, Chu CY, Lin WH, Chen YR, Chen CT, et al. Design and synthesis of tetrahydropyridothieno[2,3-d]pyrimidine scaffold based epidermal growth factor receptor (EGFR) kinase inhibitors: The role of side chain chirality and michael acceptor group for maximal potency. J Med Chem. 2010;53:7316–26.
Lagardère P, Fersing C, Masurier N, Lisowski V. Thienopyrimidine: A promising scaffold to access anti-infective agents. Pharmaceuticals 2022;15:35.
Phoujdar MS, Kathiravan MK, Bariwal JB, Shah AK, Jain KS. Microwave-based synthesis of novel thienopyrimidine bioisosteres of gefitinib. Tetrahedron Lett. 2008;49:1269–73.
Nirogi RVS, Kambhampati RS. Convenient and efficient synthesis of some novel fused thieno pyrimidines using gewald’s reaction. Synth Commun. 2011;41:2835–51.
Mavrova AT, Dimov S, Yancheva D, Rangelov M, Wesselinova D, Tsenov JA. Synthesis, anticancer activity and photostability of novel 3-ethyl-2-mercapto-thieno[2,3-d]pyrimidin-4(3H)-ones. Eur J Med Chem. 2016;123:69–79. https://doi.org/10.1016/j.ejmech.2016.07.022.
Kerru N, Settypalli T, Nallapaneni HCV. Novel thienopyrimidine derivatives Containing 1,2,4-triazoles and 1,3,4-oxadiazoles as potent antimicrobial activity. Med Chem (Los Angeles). 2014;4:623–9.
Gao H, Fu J, Zhao MJ, Song XJ, Yang P, Zheng Y. Syntheses, crystal structures, and biological activities of two enantiomeric 2-trifluoromethylthieno[2,3-d]pyrimidin-4-amine derivatives. Chin J Struct Chem. 2015;34:1224–30.
Kassab AE, Gedawy EM, El-Malah AA, Abdelghany TM, Abdel-Bakky MS. Synthesis, anticancer activity, effect on cell cycle profile, and apoptosis-inducing ability of novel hexahydrocyclooctathieno[2,3-d]pyrimidine derivatives. Chem Pharm Bull. 2016;64:490–6.
Rashad AE, Shamroukh AH, Abdel-Megeid RE, El-Sayed WA. Synthesis, reactions, and antimicrobial evaluation of some polycondensed thienopyrimidine derivatives. Synth Commun. 2010;40:1149–60.
Brough PA, Barril X, Borgognoni J, Chene P, Davies NGM, Davis B, et al. Combining hit identification strategies: Fragment-based and in silico approaches to orally active 2-aminothieno[2,3-d]pyrimidine inhibitors of the Hsp90 molecular chaperone. J Med Chem. 2009;52:4794–809.
Saddik AA, Kamal El-Dean AM, El-Said WA, Hassan KM, Abbady MS. Synthesis, antimicrobial, and anticancer activities of a new series of thieno[2,3-d] pyrimidine derivatives. J Heterocycl Chem. 2018;55:2111–22.
Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol. 2021;18:663–72. https://doi.org/10.1038/s41571-021-00514-z.
Elmongy EI, Attallah NGM, Altwaijry N, Alkahtani MM, Henidi HA. Design and synthesis of new thiophene/ thieno[2,3-d]pyrimidines along with their cytotoxic biological evaluation as tyrosine kinase inhibitors in addition to their apoptotic and autophagic induction. Molecules. 2022;27:123.
Elrazaz EZ, Serya RAT, Ismail NSM, Albohy A, Abou El Ella DA, Abouzid KAM. Discovery of potent thieno[2,3-d]pyrimidine VEGFR-2 inhibitors: Design, synthesis and enzyme inhibitory evaluation supported by molecular dynamics simulations. Bioorg Chem. 2021;113:105019. https://doi.org/10.1016/j.bioorg.2021.105019.
Wang X, Chen D, Yu S, Zhang Z, Wang Y, Qi X, et al. Synthesis and evaluation of biological and antitumor activities of tetrahydrobenzothieno[2,3-d]pyrimidine derivatives as novel inhibitors of FGFR1. Chem Biol Drug Des. 2016;87:499–507.
Nedergaard MK, Hedegaard CJ, Poulsen HS. Targeting the epidermal growth factor receptor in solid tumor malignancies. BioDrugs 2012;26:83–99.
Warnault P, Yasri A, Coisy-Quivy M, Cheve G, Bories C, Fauvel B, et al. Recent advances in drug design of epidermal growth factor receptor inhibitors. Curr Med Chem. 2013;20:2043–67.
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N. Engl J Med. 2008;358:1160–74.
Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010.
Yang XL, Wang TC, Lin S, Fan HX. Irreversible inhibitors of the epidermal growth factor receptor: Thienopyrimidine core with α,β-unsaturated amide side chain. Arch Pharm (Weinh). 2014;347:552–8.
Toolabi M, Moghimi S, Bakhshaiesh TO, Salarinejad S, Aghcheli A, Hasanvand Z, et al. 6-Cinnamoyl-4-arylaminothienopyrimidines as highly potent cytotoxic agents: Design, synthesis and structure-activity relationship studies. Eur J Med Chem. 2020;185:111786. https://doi.org/10.1016/j.ejmech.2019.111786.
Jeltsch M, Leppänen VM, Saharinen P, Alitalo K. Receptor tyrosine Kinase-Mediated angiogenesis. Cold Spring Harb Perspect Med. 2013;3:a009183.
Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–105.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64.
Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4.
Musumeci F, Radi M, Brullo C, Schenone S. Vascular endothelial growth factor (VEGF) receptors: Drugs and new inhibitors. J Med Chem. 2012;55:10797–822.
Kopparapu PK, Boorjian SA, Robinson BD, Downes M, Gudas LJ, Mongan NP, et al. Expression of VEGF and Its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res. 2013;33:2381–90.
El-Adl K, Ibrahim MK, Khedr F, Abulkhair HS, Eissa IH. N-Substituted-4-phenylphthalazin-1-amine-derived VEGFR-2 inhibitors: Design, synthesis, molecular docking, and anticancer evaluation studies. Arch Pharm (Weinh). 2021;354:2000219.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–8.
Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–75.
Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: Results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–9.
Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K, et al. Safety and efficacy profile of lenvatinib in cancer therapy: A systematic review and meta-analysis. Oncotarget. 2016;7:44545–57.
Aversa C, Leone F, Zucchini G, Serini G, Geuna E, Milani A, et al. Linifanib: Current status and future potential in cancer therapy. Expert Rev Anticancer Ther. 2015;15:677–87.
Abdel Aziz YM, Said MM, El Shihawy HA, Abouzid KAM. Discovery of novel tricyclic pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4-amine derivatives as VEGFR-2 inhibitors. Bioorg Chem. 2015;60:1–12. https://doi.org/10.1016/j.bioorg.2015.03.004.
El-Dash Y, Elzayat E, Abdou AM, Hassan RA. Novel thienopyrimidine-aminothiazole hybrids: Design, synthesis, antimicrobial screening, anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis and VEGFR-2 inhibition. Bioorg Chem. 2021;114:105137. https://doi.org/10.1016/j.bioorg.2021.105137.
El-Metwally SA, Abou-El-Regal MM, Eissa IH, Mehany ABM, Mahdy HA, Elkady H, et al. Discovery of thieno[2,3-d]pyrimidine-based derivatives as potent VEGFR-2 kinase inhibitors and anti-cancer agents. Bioorg Chem. 2021;112:104947. https://doi.org/10.1016/j.bioorg.2021.104947.
Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19.
Garces AE, Stocks MJ. Class 1 PI3K clinical candidates and recent inhibitor design strategies: a medicinal chemistry perspective. J Med Chem. 2019;62:4815–50.
Perry MWD, Abdulai R, Mogemark M, Petersen J, Thomas MJ, Valastro B, et al. Evolution of PI3Kγand δinhibitors for inflammatory and autoimmune diseases. J Med Chem. 2019;62:4783–814.
Sun Y, Fu R, Lin S, Zhang J, Ji M, Zhang Y, et al. Discovery of new thieno[2,3-d]pyrimidine and thiazolo[5,4-d]pyrimidine derivatives as orally active phosphoinositide 3-kinase inhibitors. Bioorg Med Chem. 2021;29:115890. https://doi.org/10.1016/j.bmc.2020.115890.
Elmenier FM, Lasheen DS, Abouzid KAM. Design, synthesis, and biological evaluation of new thieno[2,3-d]pyrimidine derivatives as targeted therapy for PI3K with molecular modelling study. J Enzym Inhib Med Chem. 2022;37:315–32. https://doi.org/10.1080/14756366.2021.2010729.
Han Y, Tian Y, Wang R, Fu S, Jiang J, Dong J, et al. Design, synthesis and biological evaluation of thieno[3,2-d]pyrimidine derivatives containing aroyl hydrazone or aryl hydrazide moieties for PI3K and mTOR dual inhibition. Bioorg Chem. 2020;104:104197. https://doi.org/10.1016/j.bioorg.2020.104197.
Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: Couples therapy. Nat Rev Cancer. 2013;13:663–73. https://doi.org/10.1038/nrc3559.
Mayor-López L, Tristante E, Carballo-Santana M, Carrasco-García E, Grasso S, García-Morales P, et al. Comparative study of 17-AAG and NVP-AUY922 in pancreatic and colorectal cancer cells: Are there common determinants of sensitivity. Transl Oncol. 2014;7:590–604. https://doi.org/10.1016/j.tranon.2014.08.001.
Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995;35:115–32.
Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1:e000060.
Milik SN, Lasheen DS, Serya RAT, Abouzid KAM. How to train your inhibitor: Design strategies to overcome resistance to Epidermal Growth Factor Receptor inhibitors. Eur J Med Chem. 2017;142:131–51. https://doi.org/10.1016/j.ejmech.2017.07.023.
Padfield E, Ellis HP, Kurian KM. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol. 2015;5:5.
Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689–708.
Hsu JL, Hung MC. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35:575–88. https://doi.org/10.1007/s10555-016-9649-6.
Abd El Hadi SR, Lasheen DS, Hassan MA, Abouzid KAM. Design and synthesis of 4-anilinothieno[2,3-d]pyrimidine-based compounds as dual EGFR/HER-2 inhibitors. Arch Pharm (Weinh). 2016;349:827–47.
Milik SN, Abdel-Aziz AK, Lasheen DS, Serya RAT, Minucci S, Abouzid KAM. Surmounting the resistance against EGFR inhibitors through the development of thieno[2,3-d]pyrimidine-based dual EGFR/HER2 inhibitors. Eur J Med Chem. 2018;155:316–36. https://doi.org/10.1016/j.ejmech.2018.06.011.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Shepherd C, Puzanov I, Sosman JA. B-RAF inhibitors: An evolving role in the therapy of malignant melanoma. Curr Oncol Rep. 2010;12:146–52.
Fiskus W, Mitsiades N. B-Raf inhibition in the clinic: Present and future. Annu Rev Med. 2016;67:29–43.
Herr R, Köhler M, Andrlová H, Weinberg F, Möller Y, Halbach S, et al. B-Raf inhibitors induce epithelial differentiation in BRAF-mutant colorectal cancer cells. Cancer Res. 2015;75:216–29.
Wang Y, Wan S, Li Z, Fu Y, Wang G, Zhang J, et al. Design, synthesis, biological evaluation and molecular modeling of novel 1H-pyrazolo[3,4-d]pyrimidine derivatives as BRAFV600E and VEGFR-2 dual inhibitors. Eur J Med Chem. 2018;155:210–28.
Abdel-Mohsen HT, Omar MA, El Kerdawy AM, Mahmoud AEE, Ali MM, El Diwani HI. Novel potent substituted 4-amino-2-thiopyrimidines as dual VEGFR-2 and BRAF kinase inhibitors. Eur J Med Chem. 2019;179:707–22. https://doi.org/10.1016/j.ejmech.2019.06.063.
Hassan RA, Hamed MIA, Abdou AM, El-Dash Y. Novel antiproliferative agents bearing substituted thieno[2,3-d]pyrimidine scaffold as dual VEGFR-2 and BRAF kinases inhibitors and apoptosis inducers; design, synthesis and molecular docking. Bioorg Chem. 2022;125:105861. https://doi.org/10.1016/j.bioorg.2022.105861.
Rosnet O, Schiff C, Pebusque MJ, Marchetto S, Tonnelle C, Toiron Y, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–9.
Parcells BW, Ikeda AK, Simms-Waldrip T, Moore TB, Sakamoto KM. FMS-like tyrosine kinase 3 in normal hematopoiesis and acute myeloid leukemia. Stem Cells. 2006;24:1174–84.
Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, Ehninger G, et al. Acute myeloid leukaemia. Nat Rev Dis Prim 2016;2:1–22.
Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–52.
Park CH, Lee C, Yang JS, Joe BY, Chun K, Kim H, et al. Discovery of thienopyrimidine-based FLT3 inhibitors from the structural modification of known IKKβ inhibitors. Bioorg Med Chem Lett. 2014;24:2655–60. https://doi.org/10.1016/j.bmcl.2014.04.058.
Kim H, Lee C, Yang JS, Choi S, Park CH, Kang JS. .et al. Structural modifications at the 6-position of thieno[2,3- d]pyrimidines and their effects on potency at FLT3 for treatment of acute myeloid leukemia. Eur J Med Chem. 2016;120:74–85. https://doi.org/10.1016/j.ejmech.2016.05.022.
Elmongy EI, Altwaijry N, Attallah NGM, Alkahtani MM, Henidi HA. In-silico screening of novel synthesized thienopyrimidines targeting fms related receptor tyrosine kinase-3 and their in-vitro biological evaluation. Pharmaceuticals. 2022;15:170.
Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–33. https://doi.org/10.1016/j.chembiol.2010.04.012.
Hevener KE, Verstak TA, Lutat KE, Riggsbee DL, Mooney JW. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm Sin B. 2018;8:844–61.
Hande KR. Topoisomerase II inhibitors. Update Cancer Ther. 2008;3:13–26.
Abdelhaleem EF, Abdelhameid MK, Kassab AE, Kandeel MM. Design and synthesis of thienopyrimidine urea derivatives with potential cytotoxic and pro-apoptotic activity against breast cancer cell line MCF-7. Eur J Med Chem. 2018;143:1807–25. https://doi.org/10.1016/j.ejmech.2017.10.075.
El-Metwally SA, Khalil AK, El-Sayed WM. Design, molecular modeling and anticancer evaluation of thieno[2,3-d]pyrimidine derivatives as inhibitors of topoisomerase II. Bioorg Chem. 2020;94:103492. https://doi.org/10.1016/j.bioorg.2019.103492.
Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65.
McIntosh JR, Grishchuk EL, West RR. Chromosome-microtubule interactions during mitosis. Annu Rev Cell Dev Biol. 2002;18:193–219.
Sorger PK, Dobles M, Tournebize R, Hyman AA. Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9:807–14.
Lu Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 2012;29:2943–71.
Tian C, Chen X, Zhang Z, Wang X, Liu J. Design and synthesis of (2-(phenylamino)thieno[3,2-d]pyrimidin-4-yl)(3,4,5-trimethoxyphenyl)methanone analogues as potent anti-tubulin polymerization agents. Eur J Med Chem. 2019;183:111679 https://doi.org/10.1016/j.ejmech.2019.111679.
Yang CR, Peng B, Cao SL, Ren TT, Jiang W, Wang FC, et al. Synthesis, cytotoxic evaluation and target identification of thieno[2,3-d]pyrimidine derivatives with a dithiocarbamate side chain at C2 position. Eur J Med Chem. 2018;154:324–40. https://doi.org/10.1016/j.ejmech.2018.05.028.
Abbass S, Hassan H, Mohamed M, Moustafa G, Abuo-Rahma G. Recent prospectives of anticancer histone deacetylase inhibitors. J Adv Biomed Pharm Sci. 2019;2:135–51.
Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24.
Manal M, Manish K, Sanal D, Selvaraj A, Devadasan V, Chandrasekar MJN. Novel HDAC8 inhibitors: A multi-computational approach. SAR QSAR Environ Res. 2017;28:707–33. https://doi.org/10.1080/1062936X.2017.1375978.
Balch C, Montgomery JS, Paik HI, Kim S, Huang THNK. New anti-cancer strategies: epigenetic therapies and biomarkers. Front Biosci. 2005;10:1897–931.
Wang J, Su M, Li T, Gao A, Yang W, Sheng L, et al. Design, synthesis and biological evaluation of thienopyrimidine hydroxamic acid based derivatives as structurally novel histone deacetylase (HDAC) inhibitors. Eur J Med Chem. 2017;128:293–9. https://doi.org/10.1016/j.ejmech.2017.01.035.
Mohamed MFA, Youssif BGM, Shaykoon MSA, Abdelrahman MH, Elsadek BEM, Aboraia AS, et al. Utilization of tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidinone as a cap moiety in design of novel histone deacetylase inhibitors. Bioorg Chem. 2019;91:103127. https://doi.org/10.1016/j.bioorg.2019.103127.
Acknowledgements
The authors are thankful to the Faculty of Pharmacy, Cairo University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayed, M.T.M., Hassan, R.A., Halim, P.A. et al. Recent updates on thienopyrimidine derivatives as anticancer agents. Med Chem Res 32, 659–681 (2023). https://doi.org/10.1007/s00044-023-03040-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03040-y