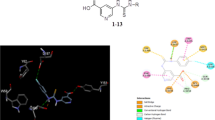
Abstract
Series of the 5-methyl-, 5-bromo- and 5-chloro substituted 2-hydroxy-3-nitrochalcones (2a–d), (2e–h) and (2i–l), respectively, have been synthesized and characterized using a combination of spectroscopic and single crystal X-ray diffraction techniques. The compounds were, in turn, evaluated through enzymatic assays in vitro for inhibitory effect against α-glucosidase and α-amylase activities. Most of the test compounds exhibited increased inhibitory activity against α-glucosidase compared to the anti-diabetic drug, acarbose. Chalcones 2a, 2c, 2d, and 2g–j exhibited dual inhibitory effect against both enzymes with minimal cytotoxicity against the Raw-264,7 macrophage (Murine) cells compared to the anticancer drug, curcumin. Derivatives 2e and 2k exhibited increased inhibitory activity and selectivity against α-amylase with significantly reduced cytotoxicity against the Raw-264,7 cells. Chalcones 2a, 2i, and 2j showed the capability to increase the phagocytic ability of Raw-264,7 cells in the presence of lipopolysaccharide (LPS) stimuli. Molecular docking has been performed on the most active derivatives to predict the hypothetical protein–ligand binding modes into the α-glucosidase and α-amylase binding sites. Selected compounds were also docked into the Toll-like receptor-myeloid differentiation factor 2 (TLR4-MD2) active sites to determine their binding energies at least at theoretical level. The key aspects of the pharmacokinetics of these compounds, namely, absorption, distribution, metabolism, and excretion have also been simulated.






Similar content being viewed by others
Data availability
The CIF file containing complete information on the studied structure of 2k was deposited with the Cambridge Crystallographic Data Center, CCDC 2192594, and is freely available upon request from the following website: www.ccdc.cam.ac.uk/datarequest/cif or by contacting the Cambridge Crystallographic Data Center, 12, Union Road, Cambridge CB2 1EZ, UK; fax: + 44-1223-336033; email: deposit@ccdc.cam.ac.uk.
References
WHO, World Health Organization. Global Report on Diabetes. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf, 2016 (Accessed 2021).
Vieira R, Souto SB, Sánchez-López E, Machado AL, Severino P, Jose S. et al. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome- Review of classical and new compounds: Part-I. Pharmaceuticals. 2019;12:152. https://doi.org/10.3390/ph12040152.
Chaudhury A, Duvoor C, Dendi VSR, Kraleti S, Chada A, Ravilla R. et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus, management. Front Endocrinol. 2017;8:1–12. 0.3389/fendo.2017.0000.
Grosick R, Alvarado-Vazquez PA, Messersmith AR, Romero-Sandoval EA. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J Pain Res. 2018;11:1769–78. https://doi.org/10.2147/JPR.S164493.
Meshkani R, Vakili S. Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta. 2016;462:77–89. https://doi.org/10.1016/j.cca.2016.08.015.
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis G-E, Vogiatzi G, Spyridon P. et al. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol. 2019;14:50–59. https://doi.org/10.15420/ecr.2018.33.1.
Imran S, Taha M, Selvaraj M, Ismail NH, Chigurupati S, Mohammad JI. Synthesis and biological evaluation of indole derivatives as α-amylase inhibitor. Bioorg Chem. 2017;73:121–7. https://doi.org/10.1016/j.bioorg.2017.06.007.
Alqahtani AS, Hidayathulla S, Rehman MT, ElGamal AA, Al-Massarani S, Razmovski-Naumovski V. et al. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules. 2020;10:61. https://doi.org/10.3390/biom10010061.
Ghabi A, Brahmi J, Alminderej F, Messaoudi S, Vidald S, Kadrie A. et al. Multifunctional isoxazolidine derivatives as α-amylase and α-glucosidase inhibitors. Bioorg Chem. 2020;98:103713. https://doi.org/10.1016/j.bioorg.2020.103713.
Aispuro-Pérez A, López-Ávalos J, García-Páez F, Montes-Avila J, Picos Corrales LA, Ochoa-Terán A. et al. Synthesis and molecular docking studies of imines as α-glucosidase and α-amylase inhibitors. Bioorg Chem. 2020;94:103491. https://doi.org/10.1016/j.bioorg.2019.103491.
Ghani U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur J Med Chem. 2015;103:133–62. https://doi.org/10.1016/j.ejmech.2015.08.043.
Mahapatra DK, Asati V, Bharti SJ. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur J Med Chem. 2015;92:839–65. https://doi.org/10.1016/j.ejmech.2015.01.051.
Alberton AH, Damazio RG, Cazarolli LH, Chiaradia LD, Leal PC, Nunes RJ. et al. Influence of chalcone analogues on serum glucose levels in hyperglycemic rats. Chem Biol Interact. 2008;171:355–62. https://doi.org/10.1016/j.cbi.2007.11.001.
Gómez-Rivera A, Aguilar-Mariscal H, Romero-Ceronio N, Roa-de la Fuente LF, Lobato-García CE. Synthesis and anti-inflammatory activity of three nitro chalcones. Bioorg Med Chem Lett. 2013;23:5519–522. https://doi.org/10.1002/chin.201409094.
Olender J, Zwawiak L, Zaprutko L. Multidirectional efficacy of biologically active nitro compounds included in medicines. Pharmaceuticals. 2018;11:54 https://doi.org/10.3390/ph11020054.
Rahman A, Ali MT, Shawan MMAK, Sarwar MG, Khan MAK, Halim MA. Halogen-directed drug design for Alzheimer’s disease: A combined density functional and molecular docking study. SpringerPlus. 2016;5:1346–59. https://doi.org/10.1186/s40064-016-2996-5.
Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem. 2012;56:1363–88. https://doi.org/10.1021/jm3012068.
Kolář M, Hobza P, Bronowska A. Plugging the explicit σ-holes in molecular docking. Chem Comm. 2013;49:981–3. https://doi.org/10.1039/c2cc37584b.
Filarowski A, Kochel A, Hansen PE, Urbanowicz A, Szymborska K. The role of ring substituents on hydrogen bonding of 5-cyano-2-hydroxyacetophenone and 2-hydroxy-4-methoxy-5-nitroacetophenone in the ground and excited states. J Mol Struct. 2007;844–845:77–88. https://doi.org/10.1016/j.molstruc.2007.04.007.
Macrae CF, Bruno JJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E. et al. Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J Appl Crystallogr. 2008;41:466–70. https://doi.org/10.1107/S0021889807067908.
Rezai T, Bock JA, Zhou MV, Kalyanraman C, Loky R, Jacobson M. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: Successful in silico prediction of the relative permeabilities of cyclic peptides. J Am Chem Soc. 2006;128:14073–80. https://doi.org/10.1021/ja063076p.
Chen H, Yan T, Song Z, Ying S, Wu B, Ju X. et al. MD2 blockade prevents modified LDL-induced retinal injury in diabetes by suppressing NADPH oxidase-4 interaction with Toll-like receptor-4. Exp Mol Med. 2021;53:681–94. https://doi.org/10.1038/s12276-021-00607-w.
Aksöz BE, Ertan R. Chemical and structural properties of chalcones I. FABAD. J Pharm Sci. 2011;36:223–42.
West-Nielsen M, Dominiak PM, Wozniak K, Hansen PE.Strong intramolecular hydrogen bonding involving nitro- and acetyl groups. Deuterium isotope effects on chemical shifts. J Mol Struct. 2006;78:81–91. https://doi.org/10.1016/j.molstruc.2005.12.03.
Pajak J, Maes G, De Borggraeve WM, Boens N, Filarowski A. Matrix-isolation FT-IR and theoretical investigation of the competitive intramolecular hydrogen bonding in 5-methyl-3-nitro-2-hydroxyacetophenone. J Mol Struct. 2008;880:86–96. https://doi.org/10.1016/j.molstruc.2007.12.019.
Hansen E, Spanget-Larsen J. NMR and IR investigations of strong intramolecular hydrogen bonds. Molecules. 2017;22:552. https://doi.org/10.3390/molecules22040552.
Sobczyk L, Grabowski S, Krygowski TM. Interrelation between H-bond and Pi-electron delocalization. Chem Rev. 2005;105:3513–60. https://doi.org/10.1021/cr030083c.
Kumar R, Karthick T, Tandon P, Agarwal P, Menezes AP, Jayarama A. Structural and vibrational characteristics of a non-linear optical material 3-(4-nitrophenyl)-1-(pyridine-3-yl) prop-2-en-1-one probed by quantum chemical computation and spectroscopic techniques. J Mol Struct. 2018;1164:180–90. https://doi.org/10.1016/j.molstruc.2018.03.06.
Desiraju GR, Steiner T. In the Weak Hydrogen Bond. International Union of Crystallography. New York: Oxford University Press; 2006. p. 15–16.
Rocha S, Sousa A, Ribeiro D, Correia CM, Silva VLM, Santos CMM. et al. A study towards drug discovery for the management of type 2 diabetes mellitus through inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by chalcone derivatives. Food Funct. 2019;10:5510–20. https://doi.org/10.1039/c9fo01298b.
Brayer GD, Luo Y, Withers SG. The structure of human pancreatic α-amylase at 1.8 Å resolution and comparisons with related enzymes. Protein Sci. 1995;4:1730–42. https://doi.org/10.1002/pro.5560040908.
Rosak C, Mertes G. Critical evaluation of the role of acarbose in the treatment of diabetes: patient considerations. Diabetes Metab Syndr Obes. 2012;5:357–67. https://doi.org/10.2147/DMSO.S28340.
Simone MI, Wood A, Campkin D, Kiefel MJ, Houston TA. Recent results from non-basic glycosidase inhibitors: How structural diversity can inform general strategies for improving inhibition potency. Eur J Med Chem. 2022;235:114282. https://doi.org/10.1016/j.ejmech.2022.114282.
Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995;18:303–11.
Pili R, Chang J, Partis RA, Mueller RA, Chrest FJ, Passaniti A. The α-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth. Cancer Res. 1995;55:2920–6.
Atsumi S, Nosaka C, Ochi Y, Linuma H, Umezawa K. Inhibition of experimental metastasis by an α-glucosidase inhibitor, 1,6-epi-cyclophellitol. Cancer Res. 1993;53:4896–4899.
Gamblin DP, Scanlan EM, Davis BG. Glycoprotein synthesis: An update. Chem Rev. 2009;109:131–163. https://doi.org/10.1002/chin.200919254.
Rawlings AJ, Lomas H, Pilling AW, Lee MJ-R, Alonzi DS, Rountree JSS, et al. Synthesis and biological characterisation of novel N-alkyl-deoxynojirimycin α-glucosidase inhibitors. ChemBioChem. 2009;10:1101–1105.
Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharm Sci. 2005;26:178–82. https://doi.org/10.1016/j.tips.2005.02.007.
Tomeh MA, Hadianamrei R, Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci. 2019;20:1033. https://doi.org/10.3390/ijms20051033.
Fang H, Ruma A, Pengal RA, Cao X, Ganesan LP, Wewers MD. et al. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP1. J Immunol. 2004;173:360–6. https://doi.org/10.4049/jimmunol.173.1.360.
Bahgat MM, Ibrahim DR. Proinflammatory cytokine polarization in type 2 diabetes. Cent Eur J Immunol. 2020;45:170–5. https://doi.org/10.5114/ceji.2020.97904.
Liu L, Guo H, Song A, Huang J, Zhang Y, Jin S. et al. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol. 2020;21:32 https://doi.org/10.1186/s12865-020-00355-y.
Mitsuzawa H, Nishitani C, Hyakushima N, Shimizu T, Sano H, Matsushima N. et al. Recombinant soluble forms of extracellular TLR4 domain and MD-2 inhibit lipopolysaccharide binding on cell surface and dampen lipopolysaccharide-induced pulmonary inflammation in mice. J Immunol. 2006;177:8133–9. https://doi.org/10.4049/jimmunol.177.11.8133.
Park C, HwangBo H, Lee H, Kim, G-Y, Cha H-J, Choi SH, et al., The immunostimulatory effect of indole-6-carboxaldehyde isolated from Sargassum thunbergii (Mertens) Kuntze in RAW 264.7 macrophages. Anim. Cells Syst. 2020. https://doi.org/10.1080/19768354.2020.1808529.
Roig-Zamboni V, Cobucci-Ponzano B, Iacono R, Ferrara MC, Germany S, Bourne Y. et al. Structure of human lysosomal acid α-glucosidase– a guide for the treatment of Pompe disease. Nat Commun. 2017;8:1111. https://doi.org/10.1038/s41467-017-01263-3.
Kandra L, Zajacz A, Remenyik J, Gyemant G. Kinetics investigation of a new inhibitor for human salivary alph-amylase. Biochem Biophyd Res Commun. 2005;334:824–8. https://doi.org/10.1016/J.BBRC.2005.06.165.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. https://doi.org/10.1016/S0169-409X(00)00129-0.
Dighade AS. Synthesis of substituted nitro-chalcones and 3, 5-diaryl-nitro-Δ2-pyrazolines. Int J Chem Sci. 2010;8:851–6.
Mphahlele MJ, Choong YS, Maluleka MM, Gildenhuys S. Synthesis, in vitro evaluation and molecular docking of the 5-acetyl-2-aryl-6-hydroxybenzo[b]furans against multiple targets linked to type 2 diabetes. Biomolecules. 2020;10:418. https://doi.org/10.3390/biom10030418.
Sileshi G, Wubshet S, Schmidt JS, Wiese S, Staerk D. High-resolution screening combined with HPLC-HRMS-SPE-NMR for identification of potential health-promoting constituents in sea aster and searocket–new Nordic food ingredients. J Agric Food Chem. 2013;61:8616–23.
Morris GM, Huey P, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;40:2785–91.
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4:17.
Adasme MF, Linnemann KL, Bolz SN, Kaiser F, Salentin S, Haupt VJ, et al. PLIP 2021: expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021;49:W530–W534.
Acknowledgements
We thank University of the Witwatersrand for X-ray diffraction data using the single-crystal diffractometer purchased through the NRF Equipment Program (UID:78572). HRMS analysis was conducted on Sciex X500R TOF-MS of the University of Limpopo, funded by the South African National Research Foundation (NRF), grant number: 120326.
Funding
This project received financial support from the University of South Africa, University of Limpopo and the National Research Foundation (NRF) in South Africa (NRF GUN’s: 118554 & 138285), South African Medical Research Council (SAMRC) (Division of Research Capacity Development) (SAMRC Project Code Number: 57009 & 57039) as well as Malaysia Ministry of Higher Education Fundamental Research Grant Scheme (203/CIPPM/6711680; FRGS/1/2018/STG05/USM/02/1).
Author information
Authors and Affiliations
Contributions
M.J.M.: Conceptualization, Formal analysis, Writing–original draft, Methodology, Writing– review & editing; M.M.M.: Conceptualization, Methodology, Investigation, Writing– original draft. Y.S.C.: Formal analysis, Methodology, Writing– review & editing. B.A.M.: Formal analysis, Methodology, Writing– original draft; V.G.M.: Writing– review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mphahlele, M.J., Maluleka, M.M., Choong, Y.S. et al. An in vitro study of the 5-methyl- and 5-bromo/chloro substituted 2-hydroxy-3-nitrochalcones as α-glucosidase and/or α-amylase inhibitors with potential anti-inflammatory activity. Med Chem Res 31, 2243–2259 (2022). https://doi.org/10.1007/s00044-022-02980-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02980-1