Abstract
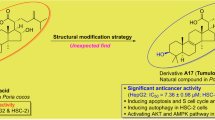
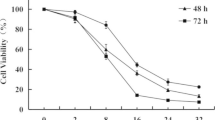
A series of new Parthenolide analogs (4a-4k) were designed and synthesized. It was known that most of the compounds showed antiproliferative activities against the six cancer cell lines including DU-145, Hela, HepG2, MCF-7, SGC-7901 and K562 by evaluating their biological activities. Among them, compound 4f exhibited better cell proliferative inhibition against the tested cell lines. In addition, anticancer mechanism studies illustrated that compound 4f could induce apoptosis in MCF-7/ADR cells via PI3K/Akt, MAPKs pathway and caspase-dependent mitochondrial pathway. More importantly, compound 4f induced an increase of autophagy levels in MCF-7/ADR cells. It was found that compared with the lead compound Parthenolide, compound 4f showed more significant anticancer effects. Our research provided an efficient strategy for targeting anticancer drug development.

Graphical abstract









Similar content being viewed by others
References
Hussain S, Singh A, Nazir SU, et al. Cancer drug resistance: afleet to conquer. J Cell Biochem 2019;120:14213–25. https://doi.org/10.1002/jcb.28782
Singh M, Tammam S, Boushehri MS, et al. MDR in cancer: addressing the underlying cellular alterations with the use of nanocarriers. Pharm Res 2017;126:2–30. https://doi.org/10.1016/j.phrs.2017.07.023
Vera AD, Gupta P, Lei ZN, et al. Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo. Cancer Lett 2019;442:91–103. https://doi.org/10.1016/j.canlet.2018.10.020
Ranjbar S, Khonkarn R, Moreno A, et al. 5-Oxo-hexahydroquinoline derivatives as modulators of P-gp, MRP1 and BCRP transporters to overcome multidrug resistance in cancer cells. Toxicol Appl Pharm 2019;362:136–49. https://doi.org/10.1016/j.taap.2018.10.025
Cao Y, Li ZY, Mao LZ, et al. The use of proteomic technologies to study molecular mechanisms of multidrug resistance in cancer. Eur J Med Chem 2019;162:423–34. https://doi.org/10.1016/j.ejmech.2018.10.001
Yang X, Ding Y, Xiao M, et al. Anti-tumor compound RY10-4 suppresses multidrug resistance in MCF-7/ADR cells by inhibiting PI3K/Akt/NF-κB signaling. Chem-Biol Interact 2017;278:22–31.
Wu YS, Chen XX, Wang SD, et al. Advances in the relationship between glycosyltransferases and multidrug resistance in cancer. Clin Chim Acta 2019;495:417–21. https://doi.org/10.1016/j.cca.2019.05.015
Yakirevich E, Sabo E, Naroditsky I, et al. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Dig World Core Med J 2006;100:152–9. https://doi.org/10.1016/j.ygyno.2005.08.050
Boysen M, Kityk R, Mayer M. Hsp70- and Hsp90-mediated regulation of the conformation of p53 DNA binding domain and p53 cancer variants. Mol Cell 2019;74:831–43. https://doi.org/10.1016/j.molcel.2019.03.032
Su YM, Zhang XP, Sinko P. Exploitation of drug-induced Bcl-2 overexpression for restoring normal apoptosis function: a promising new approach to the treatment of multidrug resistant cancer. Cancer Lett 2007;253:115–23. https://doi.org/10.1016/j.canlet.2007.01.018
Choi BH, Kim CG, Lim Y, et al. Curcumin down-regulates the multidrug-resistance mdr l b gene by inhibiting the P13K/Akt/NF-κB pathway. Cancer Lett 2008;259:111–8. https://doi.org/10.1016/j.canlet.2007.10.003
Jandial DD, Blair CA, Zhang S, et al. Molecular targeted approaches to cancer therapy and prevention using chalcones. Curr Cancer Drug Targets 2014;14:181–200. https://doi.org/10.2174/1568009614666140122160515
Ghantous A, Sinjab A, Herceg Z, et al. Parthenolide: from plant shoots to cancer roots. Drug Disco Today 2013;18:894–905. https://doi.org/10.1016/j.drudis.2013.05.005
Kalia M, Yadav VK, Singh PK, et al. Exploring the impact of parthenolide as anti-quorum sensing and anti-biofilm agent against Pseudomonas aeruginosa. Life Sci 2018;199:151–8. https://doi.org/10.1016/j.lfs.2018.03.013
Jafari N, Nazeri S, Enferadi ST. Parthenolide reduces metastasis by inhibition of vimentin expression and induces apoptosis by suppression Elongation factor α-1 expression. Phytomedicine 2018;41:67–73. https://doi.org/10.1016/j.phymed.2018.01.022
Kempema AM, Widen JC, Hexum JK, et al. Synthesis and antileukemic activities of C1-C10-modified Parthenolide analogues. Bioorg Med Chem 2015;23:4737–45. https://doi.org/10.1016/j.bmc.2015.05.037
Xu YZ, Gu XY, Peng SJ, et al. Design, synthesis and biological evaluation of novel sesquiterpene mustards as potential anticancer agents. Eur J Med Chem 2015;94:284–97. https://doi.org/10.1016/j.ejmech.2015.03.001
Schepetkin IA, Kirpotina LN, Mitchell PT, et al. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018;146:36–46. https://doi.org/10.1016/j.phytochem.2017.11.010
Diamanti P, Cox CV, Moppett JP, et al. Parthenolide eliminates leukemia-initiating cell populations and improves survival in xenografts of childhood acute lymphoblastic leukemia. Blood 2013;121:1384–93. https://doi.org/10.1182/blood-2012-08-448852
Kim CY, Kang B, Suh HJ, et al. Parthenolide, a feverfew-derived phytochemical, ameliorates obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 pathway. Pharm Res 2019;145:104259 https://doi.org/10.1016/j.phrs.2019.104259
Malgorzata C, Kamila K, Malgorzata SS, et al. Parthenolide reduces the frequency of ABCB5-positive cells and clonogenic capacity of melanoma cells from anchorage independent melanospheres. Cancer Biol Ther 2013;14:135–45. https://doi.org/10.4161/cbt.22952
Nakshatri H, Sweeney CJ. Use of Parthenolide to inhibit cancer, Indiana University Research and Technology Coporation Patent. US6890946. 2003.
Skalska J, Brookes PS, Nadtochiy SM, et al. Modulation of cell surface protein free thiols; a potential novel mechanism of action of the sesquiterpene lactone parthenolide in non-Hodgkin’s lymphoma. Blood 2009;114:3774 https://doi.org/10.1182/blood.V114.22.3774.3774
Talib WH, Al KLT. Parthenolide inhibits tumor-promoting effects of nicotine in lung cancer by inducing P53-dependent apoptosis and inhibiting VEGF expression. Biomedicine Pharmacother 2018;107:1488–95. https://doi.org/10.1016/j.biopha.2018.08.139.
Hewamana S, Alghazal S, Lin TT, et al. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood 2008;111:4681–9. https://doi.org/10.1182/blood-2007-11-125278
Ge WZ, Hao X, Han FZ, et al. Synthesis and structure-activity relationship studies of parthenolide derivatives as potential anti-triple negative breast cancer agents. Eur J Medicinal Chem 2019;166:445–69. https://doi.org/10.1016/j.ejmech.2019.01.058
Garcı́a-Piñeres AJ, Castro V, Mora G, et al. Cysteine 38 in p65/NF-κB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem 2001;276:39713–20. https://doi.org/10.1074/jbc.M101985200
Jun TF, De LW, Yong LW, et al. New antifungal scaffold derived from a natural pharmacophore: synthesis of α-methylene-γ-butyrolactone derivatives and their antifungal activity against Colletotrichum lagenarium. Bioorg Med Chem Lett 2013;23:4393–7. https://doi.org/10.1016/j.bmcl.2013.05.073
Han C, Barrios FJ, Mark VR, et al. Semisynthetic derivatives of sesquiterpene lactones by palladium-catalyzed arylation of theα-methylene-γ-lactone substructure. J Org Chem 2009;74:7176–9. https://doi.org/10.1021/jo901533e
Ravinder R, Laura J. Cross metathesis of α-methylene lactones II: γ- and δ-lactones. Cheminform 2007;38:1699–701. https://doi.org/10.1002/chin.200736092.
Yusuke M, Masaki T. Construction of spiro-fused 2-oxindole/α-methylene-γ-butyrolactone systems with extremely high enantioselectivity via indium-catalyzed amide allylation of N methyl isatin. Org Lett 2013;15:6182–5. https://doi.org/10.1002/chin.201421109
Irakusne L, Santiago R, Javier I, Florenci VG. Highly stereoselective epoxidation of α-methyl-γ-hydroxy-α, β-unsaturated esters: rationalization and synthetic applications. J Org Chem 2007;72:6614–7. https://doi.org/10.1021/jo0709955
Antonio G, Silva MH, Juan I, et al. Synthesis and antiproliferative activity of a new compound containing an α-methylene-γ-lactone group. J Med Chem 2002;45:2358–61. https://doi.org/10.1021/jm025518n
Baraldi PG, Nunez M, Tabrizi MA, et al. Design, synthesis, and biological evaluation of hybrid molecules containing alpha-methylene-gamma-butyrolactones and polypyrrole minor groove binders. J Medicinal Chem 2004;47:2877–86. https://doi.org/10.1021/jm031104y
Miyazawa M, Shimabayashi H, Hayashi S, et al. Synthesis and biological activity of alpha-methylene-gamma-lactones as new aroma chemicals. J Agric Food Chem 2000;48:5406–10. https://doi.org/10.1021/jf000346t
Tang Q, Peng T, Hu J, et al. Discovery of N-(3-bromo-1H-indol-5-yl)-quinazolin-4-amine as an effective molecular skeleton to develop reversible/irreversible pan-HER inhibitors. Eur J Med Chem 2022;233:114249 https://doi.org/10.1016/j.ejmech.2022.114249
Nakano H, Miyao T, Funatsu K. Exploring topological pharmacophore graphs for scaffold hopping. J Chem Inf Model 2020;60:2073–81. https://doi.org/10.1021/acs.jcim.0c00098.
Pathak D, Choudhary S, Singh PK, et al. Pharmacophore-based designing of putative ROS-1 targeting agents for NSCLC. Mol Divers 2021;25:1091–102. https://doi.org/10.1007/s11030-020-10036-y
Ghamari N, Kouhi HS, Zivkovic A, et al. Guided rational design with scaffold hopping leading to novel histamine H receptor ligands. Bioorg Chem 2021;117:105411 https://doi.org/10.1016/j.bioorg.2021.105411
Schneidera G, Schneiderband P, Renner S. Scaffold-hopping: how far can you jump. Qsar Combinatorial Sci. 2006;25:1162–71. https://doi.org/10.1002/qsar.200610091.
Langdon SR, Ertl P, Brown N. Bioisosteric replacement and scaffold hopping in lead generation and optimization. Qsar Combinatorial Sci 2010;29:366–85. https://doi.org/10.1002/minf.201000019
BHm HJ, Flohr A, Stahl M. Scaffold hopping. Drug Disco Today Technol 2004;1:217–24. https://doi.org/10.1016/j.ddtec.2004.10.009
Zhong CN, Mai YC, Gao HY, et al. Mitochondrial targeting of TR3 is involved in TPA induced apoptosis in breast cancer cells. Gene 2019;693:61–68. https://doi.org/10.1016/j.gene.2018.12.072
Mei H, Li J, Cai SS, et al. Mitochondria-acting carrier-free nanoplatform self-assembled by α-tocopheryl succinate carrying cisplatin for combinational tumor therapy. Regen Biomater 2021;8:rbab029 https://doi.org/10.1093/rb/rbab029
Vázquez CL, Colombo MI. Chapter 6 assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ‐BSA. Method Enzymol 2019;452:85–95. https://doi.org/10.1016/s0076-6879(08)03606-9
Li P, Li YW, Ma LT. Long noncoding RNA highly upregulated in liver cancer promotes the progression of hepatocellular carcinoma and attenuates the chemosensitivity of oxaliplatin by regulating miR-383-5p/vesicle-associated membrane protein-2 axis. Pharm Res Perspect 2021;9:e00815 https://doi.org/10.1002/prp2.815
Acknowledgements
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No. SJCX21_1294).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, J., Yu, P., Zhang, M. et al. Discovery of α-methylene-γ-lactone-δ-epoxy derivatives with anti-cancer activity: synthesis, SAR study, and biological activity. Med Chem Res 31, 1803–1817 (2022). https://doi.org/10.1007/s00044-022-02925-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02925-8