Abstract
8-Nitro-1,3-benzothiazin-4-ones (BTZs), with BTZ043 and PBTZ169 as the most advanced compounds, represent a new class of potent antitubercular agents, which irreversibly inhibit decaprenylphosphoryl-β-d-ribose-2′-epimerase (DprE1), an enzyme crucial for cell wall synthesis in the pathogen Mycobacterium tuberculosis. Synthesis, structural characterization and in vitro testing against Mycobacterium aurum DSM 43999 and M. tuberculosis H37Rv of halogenated 2-(4-ethoxycarbonylpiperazin-1-yl)-1,3-benzothiazin-4-ones lacking a nitro group are reported. X-ray crystallography reveals that the structure of the BTZ scaffold can significantly deviate from planarity. In contrast to recent reports, the results of the present study indicate that further investigation of halogenated non-nitro BTZs for antitubercular activity is less than a promising approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is an infectious disease, which is among the top ten causes of death worldwide. An estimated 1.2 million HIV-negative and 208,000 HIV-positive patients died of TB in 2019 [1]. TB is caused by Mycobacterium tuberculosis and typically manifests in the lungs (pulmonary TB) but can also affect other organs (extrapulmonary TB). Although about 85% of patients with TB can be cured with a drug regimen of 6 months [1], drug-resistant TB has become a threat to public health [2,3,4]. Moreover, disruptions of routine services for the management of TB due to lockdowns against SARS-CoV2 may cause a long-lasting increase in TB burdens in certain regions [5]. Novel anti-TB drugs are therefore needed to improve cure rates and to decrease the duration of TB treatments.
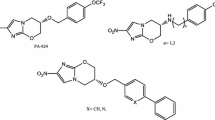
Both progression of late stage anti-TB drug candidates into the clinics and early drug discovery are vital to maintain a TB drug pipeline [6]. 1,3-Benzothiazin-4-ones (BTZs) are a promising class of new anti-TB drug candidates, which were first reported by Makarov et al. [7]. The as yet most advanced compounds BTZ043 and PBTZ169 (Scheme 1) exhibit nanomolar in vitro activity against M. tuberculosis and have reached clinical trials [6, 8]. It has been shown that 8-nitro-BTZs are suicide inhibitors of decaprenylphosphoryl-β-d-ribose 2′-epimerase (DprE1) [9], a mycobacterial enzyme that is crucial for cell wall synthesis and a known drug target [10, 11]. The drawbacks of nitro-aromatic anti-infective agents [12] such as BTZ043 and PBTZ169 are potential hepatotoxicity [13] and mutagenicity [14, 15]. Thus, efforts have been made to develop antitubercular BTZs in which the nitro group at C-8 of the BTZ scaffold has been replaced by e.g. a pyrrole [16], an azide [17] or a cyano group [18,19,20].
Recently, Nosova et al. published a series of fluorinated BTZs lacking a nitro group, for one of which, namely 5-fluoro-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazin-4-one, a promising in vitro activity against M. tuberculosis H37Rv of 0.7 µg mL−1 (2 µM) was reported [21, 22]. Motivated by these findings, we synthesized and structurally characterized the aforementioned most active compound described by Nosova et al. and four related halogenated non-nitro BTZs and tested their in vitro activities against Mycobacterium aurum DSM 43999 and M. tuberculosis H37Rv. M. aurum, a fast-growing mycobacterial species with low pathogenicity [23], is considered a good model for M. tuberculosis [24, 25]. Herein, we discuss the molecular structures and results of antimycobacterial evaluation of five halogenated non-nitro BTZs.
Results and discussion
Synthesis
A variety of methods for the synthesis of BTZs have been described in the literature [26]. The halogenated non-nitro BTZs studied in this work were synthesized from ortho-fluorobenzoic acids (1) as starting materials (Scheme 2), in analogy to the previously reported synthesis of 2a [21, 22]. The synthesis essentially followed Makarov’s original route to BTZs [27]. Treatment of 1 with thionyl chloride in toluene afforded the corresponding substituted benzoyl chlorides, which were reacted with ammonium thiocyanate in acetonitrile to yield the halogenated benzoyl isothiocyanates. Reaction with ethyl piperazine-1-carboxylate in acetonitrile gives the corresponding substituted thiourea intermediate products, which in general can be isolated in good yields [22]. In the work described here, the respective isothiocyanate and ethyl piperazine-1-carboxylate were treated in situ with triethylamine to obtain the halogenated BTZs 2 directly through an intramolecular heterocyclization reaction in a one-pot synthesis. Compounds 2a-e were characterized by 1H and 13C NMR spectroscopy and high-resolution mass spectrometry (see Supplementary Material).
Structural characterization
The molecular structures of 2a-e were revealed by X-ray crystallography. 2a · H2O, 2d and 2e crystallize with one BTZ molecule in the asymmetric unit (Z′ = 1). Figure 1 depicts the molecular structures. It should be noted that the space groups of these crystal structures are all centrosymmetric and thus the crystals contain the enantiomeric conformers of the molecular structures shown in Fig. 1. In 2a · H2O and 2e, the BTZ scaffold is virtually planar, whereas the 1,3-thiazin-4-one moiety deviates significantly from planarity in 2d. O1 in 2d is displaced from the mean plane through the condensed benzene ring by 0.515(3) Å. The piperazine six-membered ring adopts a low-energy chair conformation in the three structures. The structure at the piperazine nitrogen atoms N1 and N2 is virtually planar in each case, which can be ascribed to conjugation of the nitrogen lone pair with the carboxylate group and the BTZ π electron system, respectively. The carbamate group is virtually planar, as expected, and the ethyl group adopts an anti conformation about the O2−C14 bond. In 2e, the methoxy group lies almost in the benzene ring plane, as expected [28], with a C6−C7−O4−C16 torsion angle of 2.1(3)°. The water molecule in 2a · H2O acts as a hydrogen donor towards the carbonyl oxygen atom O3 of the carbamate group and O1 of the BTZ scaffold of an adjacent molecule (Fig. 2). Geometric parameters of the hydrogen bonds lie within the expected range for O−H···O=C hydrogen bonds [29]. Aside from the hydrogen-bonded water molecule in 2a · H2O, the crystal structures appear to be governed by close packing. The packing index is 72.1% for 2a · H2O, 70.5% for 2d and 72.2% for 2e, indicating a dense crystal packing [30]. Short interhalogen contacts are not encountered.
Hydrogen-bonded association between the solvate water molecule and two molecules of 2a in the crystal structure of 2a · H2O. Carbon-bound hydrogen atoms are omitted for clarity. d(O4···O3) = 2.8472(9) Å, <(O4−H4A···O3) = 169(1)°; d(O4···O1i) = 3.001(1) Å, <(O4−H4B···O1i) = 172(1)°. Symmetry code: (i) −x + 1, y + 1/2, −z + 3/2
In contrast to 2a · H2O, 2d and 2e, 2b with fluorine substituents in the 5 and 7 positions of the BTZ scaffold crystallizes with two molecules in the asymmetric unit (Z′ = 2) [31]. Figure 3 depicts the two crystallographically unique molecules. Since the crystal structure is centrosymmetric, the enantiomeric conformers of both molecules are also present in the unit cell. In molecule 1, the BTZ scaffold is virtually planar as for 2a · H2O and 2e. In molecule 2, however, the 1,3-thiazin-4-one six-membered ring adopts a significant boat form with C22 and N32 located 0.46(2) and 0.41(2) Å, respectively, above the mean plane through the condensed benzene ring, and O12 lying 0.38(1) Å below this plane. A structure overlay diagram, shown in Fig. 4, illustrates the structural differences between the two crystallographically unique molecules. The ethyl piperazine-1-carboxylate moiety adopts the same conformation as in 2a · H2O, 2d and 2e, as described above. In the crystal, the molecules are densely packed with a packing index of 72.3%.
Compound 2c crystallizes with three molecules in the asymmetric unit (Z′ = 3) [31]. This crystal phase (space group Cc) was encountered at 100 K, 150 K and room temperature. Figure 5 depicts the structures of the three crystallographically unique molecules. With regard to the space group symmetry, the enantiomeric conformers are also present in the unit cell. Molecule 3 exhibits disorder of the ethyl piperazine-1-carboxylate moiety. In molecule 1, the benzothiazinone scaffold shows significant deviations from planarity with O1 displaced from the mean plane through the benzene ring by 0.488(6) Å. In molecules 2 and 3 the benzothiazinone scaffold is virtually planar. Figure 6 shows a structure overlay plot of the three unique molecules. As in 2a · H2O, 2b, 2d and 2e, the piperazine ring adopts a low-energy chair conformation in the three unique molecules in 2c, but in contrast the ethyl group is tilted out of plane of the carbamate moiety [C−O−C−C torsion angles: −88.1(4), −90.5(5) and −85.3(8)°], with exception of the minor disorder component of molecule 3 where the C−O−C−C torsion angle is 173.0(7)°. Intermolecular short F···F contacts, with respect to the van der Waals radii [32], are observed between molecule 2 and molecule 3. It is worth noting that in the solid of 2c there are short N−C−H···O=C intermolecular contacts (D···A distances < 3.3 Å) between the methylene groups of the piperazine moieties and carbonyl oxygen atoms of both the benzothiazone and the ester parts of all independent molecules, with the exception of the disordered ester in molecule, indicating that these contacts may be the result of an attractive N−C−H···O=C interaction.
Structural information on BTZs is still limited in the literature. Structures of the nitroso forms of BTZ043 (PDB code: 4F4Q) [33], related BTZs [34] and PBTZ169 (4NCR) [35] covalently bound to DprE1 can be found in the Protein Data Bank (PDB) [36]. In the crystal structure of the M. tuberculosis DprE1-PBTZ169 complex (4NCR; resolution: 1.9 Å), the covalently bound nitroso form of the piperazinyl-BTZ PBTZ169 (Scheme 1) exhibits a virtually planar BTZ scaffold, and the piperazine ring adopts chair conformation, similar to the structures of 2a-e. A water molecule bridges the BTZ carbonyl oxygen atom and the backbone carbonyl oxygen atom of a leucine moiety via hydrogen bonding in 4NCR, which has some similarity to the hydrogen-bonding pattern in the crystal structure of 2a · H2O (Fig. 2). In March 2021, only four small molecule structures of BTZs were available in the Cambridge Structural Database (CSD) [37], as revealed by a WebCSD search [38]. The crystal structure of PBTZ169 (CSD refcode: LOPXAS) was reported by Zhang and Aldrich [39] and that of its 8-CN analogue (WALHAW) very recently by Zhang et al. [18]. A virtually planar BTZ scaffold and a chair conformation of the piperazine ring are encountered in both structures. This is comparable with the conformations observed in 2a · H2O, 2e and molecule 1 in 2b. To date, the crystal structures of two fluorinated non-nitro BTZs have been reported, namely 6,7,8-trifluoro-2-(thiomorpholin-4-yl)-1,3-benzothiazin-4-one (DOGXUV) [22] and 5-fluoro-2-(1-methyl-1H-pyrrol-2-yl)-1,3-benzothiazin-4-one (SOJGOQ) [40]. DOGXUV likewise exhibits a planar BTZ scaffold with the tethered thiomorpholin ring in a chair conformation. In SOJGOQ, the 1,3-thiazin-4-one moiety adopts a slight boat shape similar to 2d and molecule 2 in 2b but with the sulfur atom disordered over two positions. In contrast to 2a · H2O, 2b, 2d, 2e and SOJGOQ, intermolecular F···F contacts that are closer than the sum of the van der Waals radii [32] are found in DOGXUV, which may be classified as type I C−F···F−C interactions [41].
Antimycobacterial evaluation
The antimycobacterial activity of halogenated non-nitro BTZs 2a-e was tested in vitro against M. tuberculosis H37Rv and M. aurum DSM 43999 (Table 1). BTZ043 was included as a reference compound, and the observed MIC90 value against M. tuberculosis H37Rv is consistent with the literature [7, 33]. Against M. tuberculosis H37Rv, 2d inhibited growth at a concentration of 60 µM, whereas the other non-nitro BTZs studied did not show any antimycobacterial effect at the concentrations tested. In contrast to our results, Nosova et al. reported a MIC of 0.7 µg mL−1 (2 µM) for 2a against M. tuberculosis H37Rv [21, 22]. We assume that major differences in the assay protocols used are responsible for this observation. Nosova et al. cultured M. tuberculosis H37Rv in Löwenstein-Jensen medium and evaluated the antimycobacterial effect of the test compounds by measuring the zone of growth retardation in test tubes, whereas our assay was carried out in 7H9 medium supplemented with 10% OADC and 0.05% polysorbate 80 using 96-well plates. For the assay system used in the present study, a M. tuberculosis H37Rv strain transformed with the pTEC27 plasmid [42, 43] for RFP expression was used and growth was analyzed after 7 days of incubation by fluorescence measurement [44, 45]. For MIC determination against M. aurum DSM 43999, a broth microdilution method with iodonitrotetrazolium chloride (INT) as indicator reagent was used. 2c, 2d and 2e showed modest activity with MICs between 36 and 71 µM against M. aurum DSM 43999, whereas 2a and 2b do not exhibit activity against this mycobacterial strain.
In summary, antimycobacterial evaluation of 2a-e did not reveal potent effects in vitro. Compared with 8-nitro-BTZs with nanomolar in vitro activity against M. tuberculosis [7, 46], the halogenated non-nitro congeners studied in the present work clearly display loss of activity. The presence of a nitro group at C-8 of the BTZ scaffold seems to be essential for the efficient, mechanism-based inhibition of DprE1 with high in vitro potency. Our findings thus are in line with previous studies that showed that 8-nitro BTZs bind covalently to DprE1 through linkage of their nitroso forms to the Cys387 residue in the FAD-binding domain [9, 33, 34, 47, 48]. It should be noted, however, that BTZs where the 8-nitro group was replaced by a pyrrole ring, and which are thought to act as non-covalent DprE1 inhibitors, showed similar in vitro activity but no efficacy in animal models [16].
Conclusions
We synthesized and structurally characterized halogenated BTZs 2a-e lacking a nitro group at C-8 of the BTZ scaffold, and investigated their in vitro activities against M. aurum DSM 43999 and M. tuberculosis H37Rv. Structural characterization by X-ray crystallography revealed that the crystal packing can cause significant deviations from planarity in the BTZ moiety. The orientation of the ethyl piperazine-1-carboxylate moiety with respect to the BTZ plane gives rise to two enantiomeric conformers in the solid state in every case. The crystal structure of 2a · H2O furthermore gives structural insight into hydrogen bonding acceptor properties of the BTZ molecule. In the absence of water of crystallization, the methylene groups of the piperazine ring appear to act as weak hydrogen bond donors to BTZ and carbamate carbonyl oxygen atoms. In vitro tests against M. aurum and M. tuberculosis did not reveal potent inhibitory effects, which lends support to the view that the 8-nitro group is essential for an efficient, mechanism-based inhibition of DprE1. In contrast to recent reports literature, our results thus indicate that further investigation of halogenated non-nitro BTZs for antitubercular activity is less than a promising approach.
Experimental
General
Starting materials were purchased and used as received. Solvents were distilled prior to use and stored over 4 Å molecular sieves. Column chromatography was carried out using Merck silica gel 60 (63–200 µm). Flash chromatography was performed on a puriFlash® 430 instrument (Interchim, Montluçon, France). Prepacked columns with silica gel (30 μm) were used. The maximum compound load per column was 5% (m/m) of the silica gel quantity. The synthesis of BTZ043 is described elsewhere [27]. Melting points (uncorrected) were determined on a Boëtius hot-stage apparatus (VEB Kombinat, NAGEMA, Dresden, GDR). NMR spectra were recorded on an Agilent Technologies VNMRS 400 MHz and a Varian INOVA 500 NMR spectrometer. Chemical shifts are reported relative to the residual solvent signal (chloroform-d: δH = 7.26 ppm, δC = 77.16 ppm; methanol-d4: δH = 3.31 ppm, δC = 49.00 ppm; DMSO-d6 δH = 2.50 ppm, δC = 39.52 ppm). Abbreviations: s = singlet, bs = broad singlet, d = doublet, dd = doublet of doublets, m = multiplet. ESI high-resolution mass spectra (HRMS) were recorded on a Thermo Fisher Scientific LTQ Orbitrap XL mass spectrometer for 2b, 2c and 2e, and on a Thermo Scientific Q ExactiveTM Plus Orbitrap mass spectrometer for 2a and 2d.
Synthesis
General method for the preparation of the substituted benzoyl chloride precursors
The respective substituted benzoic acid was dissolved in toluene (ca. 20 mL per mmol) and two equivalents of thionyl chloride were added. After heating to reflux for 2 h, the solvent and excess thionyl chloride were removed in vacuum and the substituted benzoyl chloride thus obtained was used in the next synthetic step without purification.
5-Fluoro-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazin-4-one (2a)
The synthesis of 2a can be found in the literature [22]. 1H NMR (400 MHz, DMSO-d6) δ 7.65 (td, J = 8.1, 5.0 Hz, 1H, aromatic CH), 7.46 (d, J = 8.0 Hz, 1H, aromatic CH), 7.29 (ddd, J = 11.2, 8.2, 0.9 Hz, 1H, aromatic CH), 4.08 (q, J = 7.1 Hz, 2H, ethyl CH2), 3.93–3.67 (m, 4H, piperazinyl CH2), 3.59–3.46 (m, 4H, piperazinyl CH2), 1.21 (t, J = 7.1 Hz, 3H, ethyl CH3). 13C NMR (126 MHz, DMSO-d6) δ 165.0 (d, JC,F = 5.1 Hz), 161.8 (d, JC,F = 262 Hz), 160.2, 154.5, 134.4, 133.6 (d, JC,F = 10 Hz), 122.3 (d, JC,F = 4 Hz), 116.3 (d, JC,F = 23 Hz), 112.4 (d, JC,F = 9 Hz), 61.0, 45.2, 42.6, 14.5 ppm. m/z [M + Na]+ calcd. for C15H16FN3NaO3S+ 360.07886, found 360.07878.
5,7-Difluoro-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazin-4-one (2b)
2,4,6-Trifluorobenzoyl chloride (5.14 mmol) was prepared by the general method and taken up in 2 mL of acetonitrile and a solution of ammonium thiocyanate (0.39 g, 5.14 mmol) in 15 mL of acetonitrile was added with stirring. The reaction mixture was stirred for 5 min at 40 °C and the ammonium chloride precipitate so formed was removed by filtration and ethyl piperazine-1-carboxylate (0.75 mL, 5.14 mmol) was added with stirring. After stirring for 3 h at room temperature, triethylamine (1.4 mL, 10.28 mmol) was added dropwise and the mixture was stirred overnight. Subsequently, the solvent was removed under reduced pressure and the crude product was purified by flash chromatography (ethyl acetate/heptane gradient). It was obtained as an off-white solid; mp 152–157 °C; yield: 0.56 mg (1.58 mmol, 31 %). 1H NMR (400 MHz, MeOH-d4) δ 7.28 (ddd, J = 8.3, 2.5 Hz, 1.5 Hz, 1H, aromatic CH), 7.13 (ddd, J = 11.3, 9.0, 2.5 Hz, 1H, aromatic CH), 4.17 (q, J = 7.1 Hz, 2H, ethyl CH2), 4.06–3.74 (m, 4H, piperazinyl CH2), 3.71–3.56 (m, 4H, piperazinyl CH2), 1.28 (t, J = 7.1 Hz, 3H, ethyl CH3) ppm. 13C NMR (101 MHz, MeOH-d4) δ 168.3 (d, JC,F = 5 Hz), 167.0–166.6 (m), 164.1 (dd, JC,F = 23, 13 Hz), 162.9, 157.0, 139.0 (dd, JC,F = 12 Hz, 2 Hz), 110.2 (dd, JC,F = 26 Hz), 110.2–110.1 (m), 106.4 (dd, JC,F = 27, 26 Hz), 63.1, 46.9, 44.0, 14.9 ppm. HRMS(ESI+): m/z [M + Na]+ calcd. for C15H15F2N3NaO3S+ 378.0695, found 378.0692.
5,6,8-Trifluoro-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazin-4-one (2c)
Compound 2c was synthesized analogously to 2b from 2,3,5,6-tetrafluorobenzoyl chloride (4.71 mmol). In addition to the target compound 2c, the thiourea intermediate product (see Scheme 2) was isolated by flash chromatography. It was taken up with 5 mL of dimethylformamide and treated with 2.3 mL of triethylamine. After stirring overnight and heating to 70 °C for 2 h, additional 2c was isolated after removal of the solvent and recrystallization from isopropanol. It was obtained as an off-white solid; mp 192–197 °C; yield: 0.62 g (1.66 mmol, 35 %). 1H NMR (500 MHz, chloroform-d) δ 7.22 (td, J = 9.1, 5.9 Hz, 1H, aromatic CH), 4.18 (q, J = 7.1 Hz, 2H, ethyl CH2), 3.89 (bs, 4H, piperazinyl CH2), 3.64–3.52 (m, 4H, piperazinyl CH2), 1.28 (t, J = 7.1 Hz, 3H, ethyl CH3). 13C NMR (126 MHz, chloroform-d) δ 165.1, 160.1, 155.3, 152.0 (ddd, JC,F = 246, 10, 5 Hz), 149.7 (ddd, JC,F = 254, 15, 12 Hz), 148.1 (ddd, JC,F = 265, 14, 4 Hz), 116.8 (dd, JC,F = 18, 4 Hz), 114.6 (dd, JC,F = 7, 2 Hz), 108.6 (dd, JC,F = 25, 23 Hz), 62.2, 46.1, 43.2, 14.7. HRMS(ESI+): m/z [M + H]+ calcd. for C15H15F3N3O3S+ 374.0781, found 374.0780, [M + Na]+ calcd. for C15H14F3O3N3NaS+ 396.0601, found 396.0603.
7-Bromo-5-fluoro-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazin-4-one (2d)
Compound 2d was synthesized analogously to 2b from 4-bromo-2,6-difluorobenzoyl chloride but heated to reflux overnight. It was obtained as an off-white solid; mp 225–227°; yield: 0.51 g (1.23 mmol, 29 %). 1H NMR (400 MHz, chloroform-d) δ 7.34–7.27 (m, 2H, aromatic CH), 4.18 (q, J = 7.1 Hz, 2H, ethyl CH2), 4.00–3.73 (m, 4H, piperazinyl CH2), 3.66–3.54 (m, 4H, piperazinyl CH2), 1.28 (t, J = 7.1 Hz, 3H, ethyl CH3). 13C NMR (126 MHz, chloroform-d) δ 165.8 (d, JC,F = 5 Hz), 162.4 (d, JC,F = 270.4 Hz), 160.0, 155.1, 136.1, 126.1 (d, JC,F = 11 Hz), 124.1 (d, JC,F = 5 Hz), 120.1 (d, JC,F = 26 Hz), 62.0, 45.8, 43.1, 14.6. HRMS(ESI+): m/z [M + Na]+ calcd. for C15H15BrFN3NaO3S+: 437.98939, found 437.98965.
5-Fluoro-7-methoxy-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazin-4-one (2e)
Compound 2e was synthesized analogously to 2b from 2,6-difluoro-4-methoxybenzoyl chloride (5.32 mmol) but heated to reflux overnight. It was obtained as a pale brown solid; mp 218–221 °C; yield: 0.32 g (0.87 mmol, 16 %). 1H NMR (400 MHz, chloroform-d) δ 6.67 (dd, J = 12.2, 2.5 Hz, 1H, aromatic CH), 6.63 (dd, J = 2.5, 1.1 Hz, 1H, aromatic CH), 4.18 (q, J = 7.1 Hz, 2H, ethyl CH2), 4.03–3.69 (m, 7H, piperazinyl CH2 and methoxy CH3), 3.64–3.55 (m, 4H, piperazinyl CH2), 1.29 (t, J = 7.1 Hz, 3H, ethyl CH3). 13C NMR (101 MHz, chloroform-d) δ 165.7 (d, JC,F = 5 Hz), 163.5 (d, JC,F = 266 Hz), 161.7 (d, JC,F = 13 Hz), 159.5, 154.4, 135.1 (d, JC,F = 3 Hz), 105.5 (d, JC,F = 4 Hz), 105.3 (d, JC,F = 10 Hz), 102.5 (d, JC,F = 27 Hz), 61.1, 55.1, 44.8, 42.3, 13.8. HRMS(ESI+): m/z [M + H] calcd. for C16H19FN3O4S+ 368.1075, found 368.1079, [M + Na]+ calcd. for C16H18FN3NaO4S+ 390.0895, found 390.0893.
X-ray crystallography
Crystals suitable for single-crystal X-ray diffraction were grown from dimethylformamide for 2a · H2O, toluene for 2b, acetone for 2c and methanol for 2d and 2e. Diffraction data were measured at the P11 beamline at PETRA III at DESY [49, 50] with a Pilatus 6 M detector [51] for 2a · H2O, 2b and 2e, on a Bruker AXS Kappa Mach3 APEXII diffractometer equipped with Incoatec microfocus source for 2c, and on a Bruker AXS Kappa Mach3 APEXII diffractometer equipped with a FR591 Cu rotating anode X-ray source for 2d. The data were processed with XDS [52] for 2a · H2O, 2b and 2e and SAINT [53] for 2c and 2d. For 2c and 2d, an absorption correction by face-indexed Gaussian integration was applied with SADABS [54]. The crystal structures were solved with SHELXT [55] and refined with SHELXL-2018/3 [56]. 2a · H2O, 2b and 2d were refined using aspherical scattering factors [57], except for the water oxygen atom O4 in 2a · H2O and Br1 and C7 in 2d. Disorder of an ethyl piperazine-1-carboxylate moiety in 2c was described by a split atom model using EADP constraints for respective atoms in the parts. Refinement of the ratio of occupancies by means of a free variable yielded 0.507(3):0.493(3). A Flack x parameter of 0.016(18) for 2c was determined using 6035 quotients [(I+) − (I−)]/[(I+) + (I−)] (Parsons’ method) [58]. The crystal of 2c studied was partially non-merohedrally twinned [−1 0 0 0 −1 0 0.75 0 1] with a refined minor component of 0.0279(6). In 2a · H2O and 2b, hydrogen atom positions and Uiso(H) values were refined freely. In 2d, hydrogen atom positions were refined and Uiso(H) values were set 1.2 Ueq(C) (1.5 for methyl groups). In 2c and 2e, hydrogen atoms were placed in geometrically calculated positions and refined using an appropriate riding model and Uiso(H) = 1.2 Ueq(C) (1.5 for methyl groups). Torsion angles of methyl groups in 2c and 2e were initially determined via circular Fourier syntheses and subsequently refined while maintaining tetrahedral angles at the carbon atom. Structure pictures were drawn with Diamond [59] and Mercury [60].
Crystal data for 2a · H2O
C15H18FN3O4S, Mr = 355.38, T = 100(2) K, λ = 0.6199 Å, monoclinic, space group P21/c, a = 10.659(2), b = 21.099(4), c = 7.4526(15) Å, β = 107.77(3)°, V = 1596.2(6) Å3, Z = 4, ρcalc = 1.479 mg m−3, μ = 0.167 mm−1, F(000) = 744, crystal size 0.136 × 0.035 × 0.013 mm, θ range 1.68–27.00°, reflections collected/unique 33425/5016 (Rint = 0.0293), 292 parameters, S = 1.071, R1 [I > 2σ(I)] = 0.0249, wR2 = 0.0704, Δρmax/Δρmin = 0.29/−0.34 eÅ–3.
Crystal data for 2b
C15H15F2N3O3S, Mr = 355.36, T = 100(2) K, λ = 0.6199 Å, triclinic, space group P-1, a = 10.305(2), b = 12.616(3), c = 13.567(3) Å, α = 112.19(3), β = 102.29(3), γ = 100.93(3)°, V = 1523.1(6) Å3, Z = 4, ρcalc = 1.550 mg m−3, μ = 0.178 mm−1, F(000) = 736, crystal size 0.087 × 0.052 × 0.043 mm, θ range 1.49–27.00°, reflections collected/unique 65202/10012 (Rint = 0.0349), 556 parameters, S = 1.034, R1 [I > 2σ(I)] = 0.0248, wR2 = 0.0682, Δρmax/Δρmin = 0.40/−0.36 eÅ–3.
Crystal data for 2c
C15H14F3N3O3S, Mr = 373.35, T = 150(2) K, λ = 0.71073 Å, monoclinic, space group Cc, a = 8.0599(3), b = 27.0465(11), c = 21.2617(8) Å, β = 98.159(2)°, V = 4588.0(3) Å3, Z = 12, ρcalc = 1.622 mg m−3, μ = 0.268 mm−1, F(000) = 2304, crystal size 0.094 × 0.035 × 0.024 mm, θ range 0.97–33.20°, reflections collected/unique 85259/17444 (Rint = 0.0369), 712 parameters, 2 restraints, S = 1.052, R1 [I > 2σ(I)] = 0.0484, wR2 = 0.1319, Δρmax/Δρmin = 0.78/−0.47 eÅ–3.
Crystal data for 2d
C15H15BrFN3O3S, Mr = 416.27, T = 100(2) K λ = 1.54178 Å, monoclinic, space group P21/c, a = 8.3506(2), b = 22.7598(5), c = 9.2142(2) Å, β = 110.481(1)°, V = 1640.53(6) Å3, Z = 4, ρcalc = 1.685 mg m−3, μ = 4.890 mm−1, F(000) = 840, crystal size 0.150 × 0.133 × 0.051 mm, θ range = 5.48–72.14°, reflections collected/unique = 25434/2970 (Rint = 0.0406), 265 parameters, S = 1.084, R1 [I > 2σ(I)] = 0.0219, wR2 = 0.0527, Δρmax/Δρmin = 0.42/−0.50 eÅ–3.
Crystal data for 2e
C16H18FN3O4S, Mr = 367.39, T = 100(2) K, λ = 0.6199 Å, monoclinic, space group P21/c, a = 10.525(2), b = 21.450(4), c = 7.6170(15) Å, β = 109.53(3)°, V = 1620.7(6) Å3, Z = 4, ρcalc = 1.506 mg m−3, μ = 0.166 mm−1, F(000) = 768, crystal size 0.084 × 0.011 × 0.005 mm, θ range 1.66–26.94°, reflections collected/unique 32985/5208 (Rint = 0.1033), 228 parameters, S = 1.029, R1 [I > 2σ(I)] = 0.0649, wR2 = 0.1878, Δρmax/Δρmin = 0.87/−0.38 eÅ–3.
Microbiological assays
The mycobacterial strains were routinely grown in 7H9 broth (Difco Middlebrook) supplemented with 10% (v/v) OADC (5 % bovine albumin fraction, 2 % dextrose, 0.004 % catalase, 0.05 % oleic acid and 0.8 % sodium chloride solution) and 0.05% (v/v) polysorbate 80 at 37 °C in standing cultures. Hygromycin B was added to the medium at a final concentration of 50 µg mL−1 for M. tuberculosis H37Rv pTEC27.
For the antimycobacterial activity assay against M. tuberculosis H37Rv (harbouring RFP expressing pTEC27 plasmid; the plasmid confers resistance to hygromycin), MIC90 values were determined by the broth microdilution method using flat-bottom 96-well Corning Costar plates. The highest concentration tested for each compound was 200 µg mL−1. Each well with the test compound and 7H9 medium supplemented with 10% OADC, 0.05% polysorbate 80 and hygromycin (50 µg mL−1) was then diluted twofold in a ten-point serial dilution. The concentration of the inoculum of 5 × 105 cells mL−1 (OD600, 0.1 = 0.33 × 108 CFU mL−1) was prepared from a starting inoculum that was diluted from a preculture at the mid-log phase (OD600, 0.3 to 0.7). In each plate a negative control (1 % DMSO) and a positive control (100 µg mL−1 gentamicin) were included. Subsequently, 100 µL of the bacterial inoculum were added to each well to give a final volume of 200 μL. The plates were then sealed with parafilm, placed in a container with moist tissue and incubated for six days at 37 °C. After incubation the fluorescence intensity of each well was measured with a BioTek Synergy H4 plate reader (λex = 530 nm, λem = 590 nm) and the MIC90 value was calculated as the concentration of the compound that caused more than 90% growth reduction. The determination was done in duplicate. The percentage of growth inhibition was calculated using the equation:
Controls were used to monitor the assay quality through determination of the Z′ score. The Z′ factor was calculated as follows:
(SD = standard deviation, M = mean)
Against M. aurum DSM 43999, MIC values were determined using the broth microdilution method in 96-well flat-bottom tissue culture plates (Sarstedt, 83.3924.500), following a slightly modified literature procedure [61]. The test samples were dissolved in 10% DMSO at a concentration of 1 mg mL−1. In all the wells of a 96-well microtiter plate, 100 μL of OADC supplemented Middlebrook broth were added followed by addition of 100 μL of each sample in the first three wells. Broth and sample were mixed with the pipette and serially diluted down the wells. The highest concentration of the compounds tested was 250 μg mL−1.
The positive controls used in this study were ciprofloxacin, isoniazid, rifampicin and triclosan at a concentration of 5 mg mL−1. Broth only, 10% DMSO and M. aurum DSM 43999 only wells were used as the negative controls. M. aurum DSM 43999 suspension (100 μL; adjusted to match McFarland standard 0.5 [62]) was added to all the wells, except the broth only wells and incubated for 72 h at 37 °C. INT from Sigma-Aldrich, Germany (40 μL, 0.2 mg mL−1) was added to one column with the M. aurum DSM 43999 control and incubated for 30 min to one hour. When there was a colour change from clear to pink, the INT was added to all the wells and incubated for 30 min to 1 h. The changing of the clear INT solution to a pink colour means that there is a bacterial growth in the well. Thus, the MIC is defined as the lowest concentration of sample where the colour of the INT remains still clear.
Data availability
NMR and MS spectra are depicted in the Supplementary Material. CCDC 2074617-2074621 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
References
Global Tuberculosis Report 2020. Geneva: World Health Organization; 2020
Lange C, Chesov D, Heyckendorf J, Leung CC, Udwadia Z, Dheda K. Drug-resistant tuberculosis: An update on disease burden, diagnosis and treatment. Respirology. 2018;23:656–73. https://doi.org/10.1111/resp.13304
Koch A, Cox H, Mizrahi V. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Curr Opin Pharmacol. 2018;42:7–15. https://doi.org/10.1016/j.coph.2018.05.013
Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR. Management of drug-resistant tuberculosis. Lancet. 2019;394:953–66. https://doi.org/10.1016/S0140-6736(19)31882-3
Cilloni L, Fu H, Vesga JF, Dowdy D, Pretorius C, Ahmedov S, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28:100603 https://doi.org/10.1016/j.eclinm.2020.100603
Shetye GS, Franzblau SG, Cho S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl Res. 2020;220:68–97. https://doi.org/10.1016/j.trsl.2020.03.007
Makarov V, Manina G, Mikusova K, Möllmann U, Ryabova O, Saint-Joanis B, et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science. 2009;324:801–4. https://doi.org/10.1126/science.1171583
Makarov V, Mikušová K. Development of Macozinone for TB treatment: an update. Appl Sci. 2020;10:2269 https://doi.org/10.3390/app10072269
Trefzer C, Škovierová H, Buroni S, Bobovská A, Nenci S, Molteni E, et al. Benzothiazinones are suicide inhibitors of mycobacterial decaprenylphosphoryl-β-d-ribofuranose 2′-oxidase DprE1. J Am Chem Soc. 2012;134:912–5. https://doi.org/10.1021/ja211042r
Chikhale RV, Barmade MA, Murumkar PR, Yadav MR. Overview of the development of DprE1 inhibitors for combating the menace of tuberculosis. J Med Chem. 2018;61:8563–93. https://doi.org/10.1021/acs.jmedchem.8b00281
Mikusova K, Makarov V, Neres J. DprE1 - from the discovery to the promising tuberculosis drug target. Curr Pharm Des. 2014;20:4379–403. https://doi.org/10.2174/138161282027140630122724
Kannigadu C, N’Da DD. Recent advances in the synthesis and development of nitroaromatics as anti-infective drugs. Curr Pharm Des. 2020;26:4658–74. https://doi.org/10.2174/1381612826666200331091853
Boelsterli UA, Ho HK, Zhou S, Leow KY. Bioactivation and hepatotoxicity of nitroaromatic drugs. Curr Drug Metab. 2006;7:715–27. https://doi.org/10.2174/138920006778520606
Purohit V, Basu AK. Mutagenicity of nitroaromatic compounds. Chem Res Toxicol. 2000;13:673–92. https://doi.org/10.1021/tx000002x
Nepali K, Lee HY, Liou JP. Nitro-group-containing drugs. J Med Chem. 2019;62:2851–93. https://doi.org/10.1021/acs.jmedchem.8b00147
Makarov V, Neres J, Hartkoorn RC, Ryabova OB, Kazakova E, Šarkan M, et al. The 8-pyrrole-benzothiazinones are noncovalent inhibitors of DprE1 from Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59:4446–52. https://doi.org/10.1128/AAC.00778-15
Tiwari R, Miller PA, Chiarelli LR, Mori G, Šarkan M, Centárová I, et al. Design, syntheses, and anti-TB activity of 1,3-benzothiazinone azide and click chemistry products inspired by BTZ043. ACS Med Chem Lett. 2016;7:266–70. https://doi.org/10.1021/acsmedchemlett.5b00424
Zhang G, Chen D, Wang S, Chen H, Wei N, Chen G. Molecular insight into the discrepancy of antitubercular activity between 8‐nitro and 8‐cyano benzothiazinones. ChemistrySelect. 2020;5:13775–9. https://doi.org/10.1002/slct.202004156
Zhang G, Sheng L, Hegde P, Li Y, Aldrich CC. 8-cyanobenzothiazinone analogs with potent antitubercular activity. Med Chem Res. 2021;30:449–58. https://doi.org/10.1007/s00044-020-02676-4
Liu L, Kong C, Fumagalli M, Savková K, Xu Y, Huszár S, et al. Design, synthesis and evaluation of covalent inhibitors of DprE1 as antitubercular agents. Eur J Med Chem. 2020;208:112773 https://doi.org/10.1016/j.ejmech.2020.112773
Charushin VN, Nosova EV, Poteeva AD, Kotovskaya SK, Lipunova GN, Kravchenko MA, et al., inventors; FGBUN Institut Organicheskogo Sinteza im. I. Ya. Postovskogo Uralskogo Otdeleniya RAN, Russia; FGBU “Natsionalnyi Meditsinskii Issledovatelskii Tsentr Ftiziopulmonologii i Infektsionnykh Zabolevanii” Ministerstva Zdravookhr. Ross. Federatsii. assignee. Preparation of 5-fluoro-2-(4-ethoxycarbonylpiperazine-1-yl)-1,3-benzothiazine-4-one with anti-tuberculosis activity patent RU2663848C1. 2018
Nosova EV, Batanova OA, Lipunova GN, Kotovskaya SK, Slepukhin PA, Kravchenko MA, et al. Synthesis and antitubercular evaluation of fluorinated 2-cycloalkylimino substituted 1,3-benzothiazin-4-ones. J Fluor Chem. 2019;220:69–77. https://doi.org/10.1016/j.jfluchem.2019.02.009
Phelan J, Maitra A, McNerney R, Nair M, Gupta A, Coll F, et al. The draft genome of Mycobacterium aurum, a potential model organism for investigating drugs against Mycobacterium tuberculosis and Mycobacterium leprae. Int J Mycobacteriology. 2015;4:207–16. https://doi.org/10.1016/j.ijmyco.2015.05.001
Madikizela B, McGaw LJ. Scientific rationale for traditional use of plants to treat tuberculosis in the eastern region of the OR Tambo district, South Africa. J Ethnopharmacol. 2018;224:250–60. https://doi.org/10.1016/j.jep.2018.06.002
Namouchi A, Cimino M, Favre-Rochex S, Charles P, Gicquel B. Phenotypic and genomic comparison of Mycobacterium aurum and surrogate model species to Mycobacterium tuberculosis: implications for drug discovery. BMC Genomics. 2017;18:530. https://doi.org/10.1186/s12864-017-3924-y
Nosova EV, Lipunova GN, Charushin VN, Chupakhin ON. Synthesis and biological activity of 2-amino- and 2-aryl (Heteryl) substituted 1,3-benzothiazin-4-ones. Mini Rev Med Chem. 2019;19:999–1014. https://doi.org/10.2174/1389557518666181015151801
Makarov VA, Cole ST, Moellmann U, inventors; Leibniz Institute for Natural Product Research and Infection Biology e.V. Hans-Knoell-Institut HKI, Germany. assignee. Preparation of piperidinylbenzothiazinones as antibacterials patent WO2007134625A1. 2007
Krygowski TM, Anulewicz R, Jarmula A, Ba̧k T, Rasala D, Howard S. The effect of the methoxy group on the geometry of the benzene ring supported by crystal structure studies and Ab Initio Calculations. Crystal and molecular structure of 4-(4-methoxyphenyl)-2,6-diphenylpyridine and 1-methyl-4-(4-methoxy-phenyl)-2,6-diphenylpyridinium perchlorate. Tetrahedron. 1994;50:13155–64. https://doi.org/10.1016/S0040-4020(01)89325-X
Thakuria R, Sarma B, Nangia A. Hydrogen bonding in molecular crystals. In: Atwood JL, editor. Comprehensive Supramolecular Chemistry II. Oxford: Elsevier; 2017. p. 25–48
Kitajgorodskij AI. Molecular crystals and molecules. New York, NY: Academic Press; 1973
Steed KM, Steed JW. Packing problems: high Z′ crystal structures and their relationship to cocrystals, inclusion compounds, and polymorphism. Chem Rev. 2015;115:2895–933. https://doi.org/10.1021/cr500564z
Bondi A. van der Waals volumes and radii. J Phys Chem. 1964;68:441–51. https://doi.org/10.1021/j100785a001
Neres J, Pojer F, Molteni E, Chiarelli LR, Dhar N, Boy-Röttger S, et al. Structural basis for benzothiazinone-mediated killing of Mycobacterium tuberculosis. Sci Transl Med. 2012;4:150ra21 https://doi.org/10.1126/scitranslmed.3004395
Richter A, Rudolph I, Möllmann U, Voigt K, Chung C-w, Singh OMP, et al. Novel insight into the reaction of nitro, nitroso and hydroxylamino benzothiazinones and of benzoxacinones with Mycobacterium tuberculosis DprE1. Sci Rep. 2018;8:13473. https://doi.org/10.1038/s41598-018-31316-6
Makarov V, Lechartier B, Zhang M, Neres J, van der Sar AM, Raadsen SA, et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med. 2014;6:372–83. https://doi.org/10.1002/emmm.201303575
Burley SK, Berman HM, Bhikadiya C, Bi C, Chen L, Di Costanzo L, et al. RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019;47:D464–D74. https://doi.org/10.1093/nar/gky1004
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The Cambridge Structural Database. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2016;72:171–9. https://doi.org/10.1107/S2052520616003954
Thomas IR, Bruno IJ, Cole JC, Macrae CF, Pidcock E, Wood PA. WebCSD: the online portal to the Cambridge Structural Database. J Appl Crystallogr. 2010;43:362–6. https://doi.org/10.1107/s0021889810000452
Zhang G, Aldrich CC. Macozinone: revised synthesis and crystal structure of a promising new drug for treating drug-sensitive and drug-resistant tuberculosis. Acta Crystallogr Sect C. 2019;75:1031–5. https://doi.org/10.1107/S2053229619009185
Nosova EV, Poteeva AD, Lipunova GN, Slepukhin PA, Charushin VN. Synthesis of fluorine-containing 2-pyrrolyl- and 2-indolyl-substituted 1,3-benzothiazin-4-ones. Russian J Org Chem. 2019;55:384–7. https://doi.org/10.1134/S1070428019030205
Baker RJ, Colavita PE, Murphy DM, Platts JA, Wallis JD. Fluorine–fluorine interactions in the solid state: an experimental and theoretical study. J Phys Chem A. 2012;116:1435–44. https://doi.org/10.1021/jp2099976
Takaki K, Davis JM, Winglee K, Ramakrishnan L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat Protoc. 2013;8:1114–24. https://doi.org/10.1038/nprot.2013.068
Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–22. https://doi.org/10.1038/nature12799
Richter A, Strauch A, Chao J, Ko M, Av-Gay Y. Screening of preselected libraries targeting Mycobacterium abscessus for drug discovery. Antimicrob Agents Chemother. 2018;62:e00828-18. https://doi.org/10.1128/aac.00828-18
Ollinger J, Bailey MA, Moraski GC, Casey A, Florio S, Alling T, et al. A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLOS ONE. 2013;8:e60531. https://doi.org/10.1371/journal.pone.0060531
Makarov V, Lechartier B, Zhang M, Neres J, van der Sar AM, Raadsen SA, et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med. 2014;6:372–83. https://doi.org/10.1002/emmm.201303575
Trefzer C, Rengifo-Gonzalez M, Hinner MJ, Schneider P, Makarov V, Cole ST, et al. Benzothiazinones: prodrugs that covalently modify the decaprenylphosphoryl-β-d-ribose 2′-epimerase DprE1 of Mycobacterium tuberculosis. J Am Chem Soc. 2010;132:13663–5. https://doi.org/10.1021/ja106357w
Piton J, Foo CSY, Cole ST. Structural studies of Mycobacterium tuberculosis DprE1 interacting with its inhibitors. Drug Discov Today. 2017;22:526–33. https://doi.org/10.1016/j.drudis.2016.09.014
Meents A, Reime B, Stuebe N, Fischer P, Warmer M, Goeries D et al. Development of an in-vacuum x-ray microscope with cryogenic sample cooling for beamline P11 at PETRA III. Proc SPIE 2013;8851;88510K. https://doi.org/10.1117/12.2027303
Burkhardt A, Pakendorf T, Reime B, Meyer J, Fischer P, Stübe N, et al. Status of the crystallography beamlines at PETRA III. Eur Phys J. Plus 2016;131:1–9. https://doi.org/10.1140/epjp/i2016-16056-0
Kraft P, Bergamaschi A, Broennimann C, Dinapoli R, Eikenberry EF, Henrich B, et al. Performance of single-photon-counting PILATUS detector modules. J Synchrot Radiat. 2009;16:368–75. https://doi.org/10.1107/s0909049509009911
Kabsch W. XDS. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:125–32. https://doi.org/10.1107/s0907444909047337
SAINT. Madison, Wisconsin, USA: Bruker AXS Inc.; 2012
SADABS. Madison, Wisconsin, USA: Bruker AXS Inc.; 2012
Sheldrick GM. SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr A Found Adv. 2015;71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallogr C Struct Chem. 2015;71:3–8. https://doi.org/10.1107/S2053229614024218
Lübben J, Wandtke CM, Hübschle CB, Ruf M, Sheldrick GM, Dittrich B. Aspherical scattering factors for SHELXL - model, implementation and application. Acta Crystallogr A Found Adv. 2019;75:50–62. https://doi.org/10.1107/S2053273318013840
Parsons S, Flack HD, Wagner T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2013;69:249–59. https://doi.org/10.1107/S2052519213010014
Brandenburg K. DIAMOND. 3.2k3 ed. Bonn, Germany: Crystal Impact GbR; 2018.
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, et al. Mercury 4.0: from visualization to analysis, design and prediction. J Appl Crystallogr. 2020;53:226–35. https://doi.org/10.1107/S1600576719014092
Mohammed MJ, Al-Bayati FA. Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine. 2009;16:632–7. https://doi.org/10.1016/j.phymed.2008.12.026
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. CLSI standard M07. Wayne, Pennsylvania: Clinical and laboratory standards institute (CLSI); 2018
Acknowledgements
Professor Christian W. Lehmann is gratefully acknowledged for providing access to the X-ray diffraction facilities. Thanks are due to Elke Dreher and Heike Schucht for technical assistance with the X-ray intensity data collections and Dr Christian Ihling, Antje Herbrich-Peters as well as Dirk Kampen for measuring the HRMS spectra. We would like to thank Professor Yossef Av-Gay, Sharlene Eivenmark and Stephanie Hughes for their support in planning and conducting the antimycobacterial testing. We acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association (HGF), for the provision of experimental facilities and we would like to thank Dr Sofiane Saouane for assistance in using the P11 beamline.
Funding
BM received a grant within the TWAS-DFG Coorperation Visits Programme from the Deutsche Forschungsgemeinschaft and The World Academy of Sciences. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
All authors have seen the manuscript and agree to its publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Madikizela, B., Eckhardt, T., Goddard, R. et al. Synthesis, structural characterization and antimycobacterial evaluation of several halogenated non-nitro benzothiazinones. Med Chem Res 30, 1523–1533 (2021). https://doi.org/10.1007/s00044-021-02735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02735-4