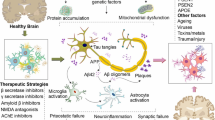
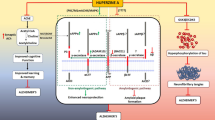
Abstract
Sulfur is widely existent in natural products and synthetic organic compounds as organosulfur, which are often associated with a multitude of biological activities. OBenzothiazole, in which benzene ring is fused to the 4,5-positions of the thiazolerganosulfur compounds continue to garner increasing amounts of attention in the field of medicinal chemistry, especially in the development of therapeutic agents for Alzheimer’s disease (AD). AD is a fatal neurodegenerative disease and the primary cause of age-related dementia posing severe societal and economic burdens. Unfortunately, there is no cure for AD. A lot of research has been conducted on sulfur-containing compounds in the context of AD due to their innate antioxidant potential and some are currently being evaluated in clinical trials. In this review, we have described emerging trends in the field, particularly the concept of multi-targeting and formulation of disease-modifying strategies. SAR, pharmacological targets, in vitro/vivo ADMET, efficacy in AD animal models, and applications in clinical trials of such sulfur compounds have also been discussed. This article provides a comprehensive review of organosulfur-based AD therapeutic agents and provides insights into their future development.



































Similar content being viewed by others
References
Meyer B. Elemental sulfur. Chem Rev. 1976;76:367–88.
Oae S. Organic chemistry of sulfur: Springer Science & Business Media; 2012. pp. 33–67.
Huxtable RJ. Biochemistry of sulfur: Springer Science & Business Media; 2013. pp. 121–257.
Maren TH. Relations between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol. 1976;16:309–27.
Wilke MS, Lovering AL, Strynadka NC. β-Lactam antibiotic resistance: a current structural perspective. Curr Opin Microbiol. 2005;8:525–33.
Association As. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018;14:367–429.
Lanctôt KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimer’s Dement Transl Res Clin Interv. 2017;3:440–9.
Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dement. 2019;15:17–24.
Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. The cost of Alzheimer’s disease in China and re‐estimation of costs worldwide. Alzheimer’s Dement. 2018;14:483–91.
Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24:974–82.e3.
Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:195–214.
Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med. 2017;68:413–30.
Paul BD, Sbodio JI, Snyder SH. Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci. 2018;39:513–24.
Rani P, Krishnan S, Rani, Cathrine C. Study on analysis of peripheral biomarkers for Alzheimer’s disease diagnosis. Front Neurol. 2017;8:328.
Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–60.
Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–42.
Brown N. Bioisosterism in medicinal chemistry. Wiley Online Library; 2012. pp. 15–29.
Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, et al. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 1985;97:1477–85.
Jones SV, Kounatidis I. Nuclear Factor-Kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front Immunol. 2017;8:1805.
Yan CY, Greene LA. Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway. J Neurosci. 1998;18:4042–9.
Qian HR, Yang Y. Neuron differentiation and neuritogenesis stimulated by N-acetylcysteine (NAC). Acta Pharmacol Sin. 2009;30:907–12.
Ates B, Abraham L, Ercal N. Antioxidant and free radical scavenging properties of N-acetylcysteine amide (NACA) and comparison with N-acetylcysteine (NAC). Free Radic Res. 2008;42:372–7.
Kahns AH, Bundgaard H. Prodrugs as drug delivery systems. 107. Synthesis and chemical and enzymatic hydrolysis kinetics of various mono-and diester prodrugs of N-acetylcysteine. Int J Pharm. 1990;62:193–205.
Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, et al. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007;39:756–68.
Ham Y-H, Jason Chan K, Chan W. Thioproline serves as an efficient antioxidant protecting human cells from oxidative stress and improves cell viability. Chem Res Toxicol. 2020;33:1815–21.
Liu J, Chan KJ, Chan W. Identification of protein thiazolidination as a novel molecular signature for oxidative stress and formaldehyde exposure. Chem Res Toxicol. 2016;29:1865–71.
Porta P, Aebi S, Summer K, Lauterburg BH. L-2-oxothiazolidine-4-carboxylic acid, a cysteine prodrug: pharmacokinetics and effects on thiols in plasma and lymphocytes in human. J Pharmacol Exp Ther. 1991;257:331–4.
Roberts JC, Nagasawa HT, Zera RT, Fricke RF, Goon DJ. Prodrugs of L-cysteine as protective agents against acetaminophen-induced hepatotoxicity. 2-(Polyhydroxyalkyl)-and 2-(polyacetoxyalkyl) thiazolidine-4 (R)-carboxylic acids. J Med Chem. 1987;30:1891–6.
Jones MG, Hughes J, Tregova A, Milne J, Tomsett AB, Collin HA. Biosynthesis of the flavour precursors of onion and garlic. J Exp Bot. 2004;55:1903–18.
Mandal PK, Saharan S, Tripathi M, Murari G. Brain glutathione levels-a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol Psychiatry. 2015;78:702–10.
Fernandez-Checa JC, Garcia-Ruiz C, Ookhtens M, Kaplowitz N. Impaired uptake of glutathione by hepatic mitochondria from chronic ethanol-fed rats. Tracer kinetic studies in vitro and in vivo and susceptibility to oxidant stress. J Clin Investig. 1991;87:397–405.
Puri RN, Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci USA. 1983;80:5258–60.
Tsan MF, White JE, Rosano CL. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J Appl Physiol. 1989;66:1029–34.
Wellner VP, Anderson ME, Puri RN, Jensen GL, Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci USA. 1984;81:4732–5.
Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson’s disease. Exp Neurol. 2007;203:512–20.
Berkeley LI, Cohen JF, Crankshaw DL, Shirota FN, Nagasawa HT. Hepatoprotection by L‐cysteine‐glutathione mixed disulfide, a sulfhydryl‐modified prodrug of glutathione. J Biochem Mol Toxicol. 2003;17:95–7.
More SS, Vince R. Potential of a γ-glutamyl-transpeptidase-stable glutathione analogue against amyloid-β toxicity. ACS Chem Neurosci. 2012;3:204–10.
Potter LT. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970;206:145–66.
Nachmansohn D, Machado A. THE FORMATION OF ACETYLCHOLINE. A NEW ENZYME:“CHOLINE ACETYLASE”. J Neurophysiol. 1943;6:397–403.
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14:101–15.
Cao Z, Tsang M, Zhao H, Li Y. Induction of endogenous antioxidants and phase 2 enzymes by alpha-lipoic acid in rat cardiac H9C2 cells: protection against oxidative injury. Biochem Biophys Res Commun. 2003;310:979–85.
ZHANG W-J, FREI B. α-Lipoic acid inhibits TNF-a-induced NF-κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–32.
Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, et al. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol Ther. 2007;113:154–64.
Rosini M, Andrisano V, Bartolini M, Bolognesi ML, Hrelia P, Minarini A, et al. Rational approach to discover multipotent anti-Alzheimer drugs. J Med Chem. 2005;48:360–3.
Rosini M, Simoni E, Bartolini M, Tarozzi A, Matera R, Milelli A, et al. Exploiting the lipoic acid structure in the search for novel multitarget ligands against Alzheimer’s disease. Eur J Med Chem. 2011;46:5435–42.
Benchekroun M, Romero A, Egea J, Leon R, Michalska P, Buendia I, et al. The antioxidant additive approach for Alzheimer’s disease therapy: new ferulic (lipoic) acid plus melatonin modified tacrines as cholinesterases inhibitors, direct antioxidants, and nuclear factor (erythroid-derived 2)-like 2 activators. J Med Chem. 2016;59:9967–73.
Bolognesi ML, Cavalli A, Bergamini C, Fato R, Lenaz G, Rosini M, et al. Toward a rational design of multitarget-directed antioxidants: merging memoquin and lipoic acid molecular frameworks. J Med Chem. 2009;52:7883–6.
Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol. 1989;37:501–6.
Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82.
Pendyala L, Creaven PJ. Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a phase I trial. Cancer Epidemiol Biomark Prev. 1995;4:245–51.
Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, et al. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005;45:402–8.
Santangelo F. Intracellular thiol concentration modulating inflammatory response: influence on the regulation of cell functions through cysteine prodrug approach. Curr Med Chem. 2003;10:2599–610.
Giustarini D, Milzani A, Dalle-Donne I, Tsikas D, Rossi R. N-Acetylcysteine ethyl ester (NACET): a novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem Pharmacol. 2012;84:1522–33.
Holdiness MR. Clinical Pharmacokinetics of N-Acetylcysteine. Clin Pharmacokinet. 1991;20:123–34.
Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, et al. Pharmacokinetics of glutathione and its metabolites in normal subjects. J Korean Med Sci. 2005;20:721–6.
Grattagliano I, Wieland P, Schranz C, Lauterburg BH. Effect of oral glutathione monoethyl ester and glutathione on circulating and hepatic sulfhydrils in the rat. Pharmacol Toxicol. 1994;75:343–7.
Takaishi N, Yoshida K, Satsu H, Shimizu M. Transepithelial transport of alpha-lipoic acid across human intestinal Caco-2 cell monolayers. J Agric Food Chem. 2007;55:5253–9.
Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Alter Med Rev. 2007;12:343–51.
Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–31.
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–60.
Tchantchou F, Graves M, Rogers E, Ortiz D, Shea TB. N-acteyl cysteine alleviates oxidative damage to central nervous system of ApoE-deficient mice following folate and vitamin E-deficiency. J Alzheimers Dis. 2005;7:135–8.
Huang Q, Aluise CD, Joshi G, Sultana R, St Clair DK, Markesbery WR, et al. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J Neurosci Res. 2010;88:2618–29.
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–83.
Joy T, Rao MS, Madhyastha S. N-Acetyl cysteine supplement minimize tau expression and neuronal loss in animal model of Alzheimer’s disease. Brain Sci. 2018;8:185–200.
Tucker S, Ahl M, Cho HH, Bandyopadhyay S, Cuny GD, Bush AI, et al. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Curr Alzheimer Res. 2006;3:221–7.
More SS, Vartak AP, Vince R. Restoration of glyoxalase enzyme activity precludes cognitive dysfunction in a mouse model of Alzheimer’s disease. ACS Chem Neurosci. 2013;4:330–8.
Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid—biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849–58.
Salehi B, Berkay Yılmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules. 2019;9:356–81.
Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, et al. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–25.
Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43:156–64.
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, et al. alpha-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535–48.
Remington R, Lortie JJ, Hoffmann H, Page R, Morrell C, Shea TB. A Nutritional formulation for cognitive performance in mild cognitive impairment: a placebo-controlled trial with an open-label extension. J Alzheimers Dis. 2015;48:591–5.
Remington R, Bechtel C, Larsen D, Samar A, Doshanjh L, Fishman P, et al. A Phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer’s disease. J Alzheimers Dis. 2015;45:395–405.
Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–9.
Allen J, Bradley RD. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Alter Complement Med. 2011;17:827–33.
Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–9.
Richie JP Jr., Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD, et al. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur J Nutr. 2015;54:251–63.
Schmitt B, Vicenzi M, Garrel C, Denis FM. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: a comparative crossover study. Redox Biol. 2015;6:198–205.
Hager K, Marahrens A, Kenklies M, Riederer P, Münch G. Alpha-lipoic acid as a new treatment option for Alzheimer [corrected] type dementia. Arch Gerontol Geriatr. 2001;32:275–82.
Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J Alzheimers Dis. 2014;38:111–20.
Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69:836–41.
Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71.
Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14:329–45.
Savage JC, Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1990;526:540–5.
Tang XQ, Shen XT, Huang YE, Chen RQ, Ren YK, Fang HR, et al. Inhibition of endogenous hydrogen sulfide generation is associated with homocysteine-induced neurotoxicity: role of ERK1/2 activation. J Mol Neurosci. 2011;45:60–7.
Liu XQ, Liu XQ, Jiang P, Huang H, Yan Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi. 2008;88:2246–9.
Wei H-J, Li X, Tang X-Q. Therapeutic benefits of H2S in Alzheimer’s disease. J Clin Neurosci. 2014;21:1665–9.
Whiteman M, Cheung NS, Zhu Y-Z, Chu SH, Siau JL, Wong BS, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–8.
Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, et al. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer’s disease. J Neuroinflammation. 2012;9:202.
Tang X-Q, Yang C-T, Chen J, Yin W-L, Tian S-W, Hu B, et al. Effect of hydrogen sulphide on beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol Physiol. 2008;35:180–6.
Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–8.
Zhao FL, Qiao PF, Yan N, Gao D, Liu MJ, Yan Y. Hydrogen sulfide selectively inhibits γ-secretase activity and decreases mitochondrial Aβ production in neurons from APP/PS1 transgenic mice. Neurochem Res. 2016;41:1145–59.
Cheng X-j, Gu J-x, Pang Y-p, Liu J, Xu T, Li X-r, et al. Tacrine–hydrogen sulfide donor hybrid ameliorates cognitive impairment in the aluminum chloride mouse model of Alzheimer’s disease. ACS Chem Neurosci. 2019;10:3500–9.
Tang XQ, Ren YK, Zhou CF, Yang CT, Gu HF, He JQ, et al. Hydrogen sulfide prevents formaldehyde-induced neurotoxicity to PC12 cells by attenuation of mitochondrial dysfunction and pro-apoptotic potential. Neurochem Int. 2012;61:16–24.
Zheng Y, Ji X, Ji K, Wang B. Hydrogen sulfide prodrugs—a review. Acta Pharm Sin B. 2015;5:367–77.
Powell CR, Dillon KM, Matson JB. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochemical Pharmacol. 2018;149:110–23.
Song ZJ, Ng MY, Lee Z-W, Dai W, Hagen T, Moore PK, et al. Hydrogen sulfide donors in research and drug development. MedChemComm. 2014;5:557–70.
Chiu S, Khan AS, Terpstra KJ, Elias H, Khazaeipool Z, Carriere A, et al. editors. Repurposing elemental sulfur for alzheimer dementia (AD) therapeutics: role of hydrogen sulfide and Î-Galactosidase. J Nerv Syst. 2017;1:6–7.
Guzmán R, Campos C, Yuguero R, Masegù C, Gil P, Moragón ÁC. Protective effect of sulfurous water in peripheral blood mononuclear cells of Alzheimer’s disease patients. Life Sci. 2015;132:61–7.
Kimura Y, Goto Y-I, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2009;12:1–13.
Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–7.
Liu Y, Deng Y, Liu H, Yin C, Li X, Gong Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: a novel mechanism mediated by the activation of Nrf2. Pharmacol Biochem Behav. 2016;150–151:207–16.
Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15:405–19.
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37.
Giggenbach W. Optical spectra of highly alkaline sulfide solutions and the second dissociation constant of hydrogen sulfide. Inorg Chem. 1971;10:1333–8.
DeLeon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem. 2012;421:203–7.
Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Medicinal Chem. 2010;53:6275–86.
Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–82.
Griffin B, Selassie M, Gwebu ET. Effect of aged garlic extract on the cytotoxicity of alzheimer β-amyloid peptide in neuronal PC12 Cells. Nutritional Neurosci. 2000;3:139–42.
Gupta VB, Rao KSJ. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Neurosci Lett. 2007;429:75–80.
Pan LL, Liu XH, Gong QH, Zhu YZ. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–15.
Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–8.
Ozturk T, Ertas E, Mert O. Use of Lawesson’s reagent in organic syntheses. Chem Rev. 2007;107:5210–78.
Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–19.
Nicolau LAD, Silva RO, Damasceno SRB, Carvalho NS, Costa NRD, Aragão KS, et al. The hydrogen sulfide donor, Lawesson’s reagent, prevents alendronate-induced gastric damage in rats. Braz J Med Biol Res. 2013;46:708–14.
Medeiros JV, Bezerra VH, Gomes AS, Barbosa AL, Lima-Júnior RC, Soares PM, et al. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Thera. 2009;330:764–70.
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–60.
Yurinskaya MM, Krasnov GS, Kulikova DA, Zatsepina OG, Vinokurov MG, Chuvakova LN, et al. H2S counteracts proinflammatory effects of LPS through modulation of multiple pathways in human cells. Inflamm Res. 2020;69:481–95.
Park C-M, Zhao Y, Zhu Z, Pacheco A, Peng B, Devarie-Baez NO, et al. Synthesis and evaluation of phosphorodithioate-based hydrogen sulfide donors. Mol Biosyst. 2013;9:2430–4.
Qandil AM. Prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs), more than meets the eye: a critical review. Int J Mol Sci. 2012;13:17244–74.
Sehajpal S, Prasad DN, Singh RK. Prodrugs of non-steroidal anti-inflammatory drugs (NSAIDs): a long march towards synthesis of safer NSAIDs. Mini Rev Med Chem. 2018;18:1199–219.
Sparatore A, Santus G, Giustarini D, Rossi R, Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev Clin Pharmacol. 2011;4:109–21.
Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–71.
Chattopadhyay M, Kodela R, Nath N, Dastagirzada YM, Velázquez-Martínez CA, Boring D, et al. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochem Pharmacol. 2012;83:715–22.
Liu YY, Sparatore A, Del Soldato P, Bian JS. H2S releasing aspirin protects amyloid beta induced cell toxicity in BV-2 microglial cells. Neuroscience. 2011;193:80–8.
Zhao F-L, Fang F, Qiao P-f, Yan N, Gao D, Yan Y. AP39, a mitochondria-targeted hydrogen sulfide donor, supports cellular bioenergetics and protects against Alzheimer’s disease by preserving mitochondrial function in APP/PS1 mice and neurons. Oxid Med Cell Longev. 2016;2016:8360738.
Nesi G, Sestito S, Digiacomo M, Rapposelli S. Oxidative stress, mitochondrial abnormalities and proteins deposition: multitarget approaches in Alzheimer’s disease. Curr Top Med Chem. 2017;17:3062–79.
Huang CS, Lin AH, Liu CT, Tsai CW, Chang IS, Chen HW, et al. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFκB activation. Mol Nutr Food Res. 2013;57:1918–30.
Shehatou GS, Suddek GM. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol Med. 2016;241:426–36.
Citi V, Martelli A, Testai L, Marino A, Breschi MC, Calderone V. Hydrogen sulfide releasing capacity of natural isothiocyanates: is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014;80:610–3.
Martelli A, Citi V, Testai L, Brogi S, Calderone V. Organic isothiocyanates as hydrogen sulfide donors. Antioxid Redox Signal. 2020;32:110–44.
Levinn CM, Cerda MM, Pluth MD. Activatable small-molecule hydrogen sulfide donors. Antioxid Redox Signal. 2020;32:96–109.
Lin Y, Yang X, Lu Y, Liang D, Huang D. Isothiocyanates as H(2)S donors triggered by cysteine: reaction mechanism and structure and activity relationship. Org Lett. 2019;21:5977–80.
Bricker GV, Riedl KM, Ralston RA, Tober KL, Oberyszyn TM, Schwartz SJ. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol Nutr food Res. 2014;58:1991–2000.
Rapposelli S, Gambari L, Digiacomo M, Citi V, Lisignoli G, Manferdini C, et al. A Novel H2S-releasing Amino-Bisphosphonate which combines bone anti-catabolic and anabolic functions. Sci Rep. 2017;7:11940.
Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2018;38:57–100.
Bolognesi ML, Matera R, Minarini A, Rosini M, Melchiorre C. Alzheimer’s disease: new approaches to drug discovery. Curr Opin Chem Biol. 2009;13:303–8.
Sestito S, Daniele S, Pietrobono D, Citi V, Bellusci L, Chiellini G, et al. Memantine prodrug as a new agent for Alzheimer’s Disease. Sci Rep. 2019;9:4612.
Alam S, Lingenfelter KS, Bender AM, Lindsley CW. Classics in chemical neuroscience: memantine. ACS Chem Neurosci. 2017;8:1823–9.
Giuliani D, Ottani A, Zaffe D, Galantucci M, Strinati F, Lodi R, et al. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol Learn Mem. 2013;104:82–91.
Sestito S, Pruccoli L, Runfola M, Citi V, Martelli A, Saccomanni G, et al. Design and synthesis of H(2)S-donor hybrids: a new treatment for Alzheimer’s disease? Eur J Med Chem. 2019;184:111745.
Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–8.
Furne J, Saeed A, Levitt M. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–85.
Ritter JM. Human pharmacology of hydrogen sulfide, putative gaseous mediator. Br J Clin Pharmacol. 2010;69:573–5.
Saeedi A, Najibi A, Mohammadi-Bardbori A. Effects of long-term exposure to hydrogen sulfide on human red blood cells. Int J Occup Environ Med. 2015;6:20–5.
Gong QH, Wang Q, Pan LL, Liu XH, Huang H, Zhu YZ. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a pro-inflammatory pathway in rats. Pharmacol Biochem Behav. 2010;96:52–8.
Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–19.
Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011;117:388–402.
Polhemus DJ, Li Z, Pattillo CB, Gojon G Sr., Gojon G Jr., Giordano T, et al. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther. 2015;33:216–26.
Cremlyn RJW, Cremlyn RJ. An introduction to organosulfur chemistry. New York: Wiley; 1996.
Bruno MA, Mufson EJ, Wuu J, Cuello AC. Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol. 2009;68:1309–18.
Whittaker M, Floyd CD, Brown P, Gearing AJ. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99:2735–76.
De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 2010;90:465–94.
Py NA, Bonnet AE, Bernard A, Marchalant Y, Charrat E, Checler F, et al. Differential spatio-temporal regulation of MMPs in the 5xFAD mouse model of Alzheimer’s disease: evidence for a pro-amyloidogenic role of MT1-MMP. Front Aging Neurosci. 2014;6:247.
Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–96.
Jourquin J, Tremblay E, Decanis N, Charton G, Hanessian S, Chollet AM, et al. Neuronal activity-dependent increase of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainate. Eur J Neurosci. 2003;18:1507–17.
Nubling G, Levin J, Bader B, Israel L, Botzel K, Lorenzl S, et al. Limited cleavage of tau with matrix-metalloproteinase MMP-9, but not MMP-3, enhances tau oligomer formation. Exp Neurol. 2012;237:470–6.
Terni B, Ferrer I. Abnormal expression and distribution of MMP2 at initial stages of Alzheimer’s disease-related pathology. J Alzheimers Dis. 2015;46:461–9.
Hanzel CE, Iulita MF, Eyjolfsdottir H, Hjorth E, Schultzberg M, Eriksdotter M, et al. Analysis of matrix metallo-proteases and the plasminogen system in mild cognitive impairment and Alzheimer’s disease cerebrospinal fluid. J Alzheimers Dis. 2014;40:667–78.
Bjerke M, Zetterberg H, Edman Å, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimer’s Dis. 2011;27:665–76.
Rasmussen HS, McCann PP. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther. 1997;75:69–75.
Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–93.
Rothenberg ML, Nelson AR, Hande KR. New drugs on the horizon: matrix metalloproteinase inhibitors. Oncologist. 1998;3:271–4.
Sorensen MD, Blaehr LK, Christensen MK, Hoyer T, Latini S, Hjarnaa PJ, et al. Cyclic phosphinamides and phosphonamides, novel series of potent matrix metalloproteinase inhibitors with antitumour activity. Bioorg Med Chem. 2003;11:5461–84.
Bursavich MG, Harrison BA. Blain J-Fo. Gamma secretase modulators: new Alzheimer’s drugs on the horizon? J Med. Chem. 2016;59:7389–409.
Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–6.
Prade E, Barucker C, Sarkar R, Althoff-Ospelt G, Lopez del Amo JM, Hossain S, et al. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry. 2016;55:1839–49.
Beattie GJ, Smyth JF. Phase I study of intraperitoneal metalloproteinase inhibitor BB94 in patients with malignant ascites. Clin Cancer Res. 1998;4:1899–902.
Macaulay VM, O’Byrne KJ, Saunders MP, Braybrooke JP, Long L, Gleeson F, et al. Phase I study of intrapleural batimastat (BB-94), a matrix metalloproteinase inhibitor, in the treatment of malignant pleural effusions. Clin Cancer Res. 1999;5:513–20.
Doherty AM, Bristol JA. Annual reports in medicinal chemistry. Academic press; 1998. pp.131–40.
Rowinsky EK, Humphrey R, Hammond LA, Aylesworth C, Smetzer L, Hidalgo M, et al. Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor BAY 12-9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J Clin Oncol. 2000;18:178–86.
Hande KR, Collier M, Paradiso L, Stuart-Smith J, Dixon M, Clendeninn N, et al. Phase I and pharmacokinetic study of prinomastat, a matrix metalloprotease inhibitor. Clin Cancer Res. 2004;10:909–15.
Weber GF. Molecular therapies of cancer. Springer; 2015. pp. 246–51.
Dubrovskaya NM, Nalivaeva NN, Turner AJ, Zhuravin IA. Effects of an inhibitor of alpha-secretase, which metabolizes the amyloid peptide precursor, on memory formation in rats. Neurosci Behav Physiol. 2006;36:911–3.
Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science. 2002;295:2387–92.
Talbot DC, Brown PD. Experimental and clinical studies on the use of matrix metalloproteinase inhibitors for the treatment of cancer. Eur J Cancer. 1996;32A:2528–33.
Hirte H, Goel R, Major P, Seymour L, Huan S, Stewart D, et al. A phase I dose escalation study of the matrix metalloproteinase inhibitor BAY 12-9566 administered orally in patients with advanced solid tumours. Ann Oncol. 2000;11:1579–84.
Szekely C, Green R, Breitner JC, Østbye T, Beiser A, Corrada M, et al. No advantage of Aβ42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–8.
Marcum ZA, Hanlon JT. Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care. 2010;18:24–7.
Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. Clin Pharmacokinet. 1997;32:437–59.
Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, et al. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–24.
Duggan DE. Sulindac: therapeutic implications of the prodrug/pharmacophore equilibrium. Drug Metab Rev. 1981;12:325–37.
Walters MJ, Blobaum AL, Kingsley PJ, Felts AS, Sulikowski GA, Marnett LJ. The influence of double bond geometry in the inhibition of cyclooxygenases by sulindac derivatives. Bioorg Med. Chem Lett. 2009;19:3271–4.
Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003;278:31831–7.
Prade E, Bittner HJ, Sarkar R, Lopez Del Amo JM, Althoff-Ospelt G, Multhaup G, et al. Structural mechanism of the interaction of Alzheimer disease Aβ fibrils with the non-steroidal anti-inflammatory drug (NSAID) sulindac sulfide. J Biol Chem. 2015;290:28737–45.
Fu Z, Aucoin D, Ahmed M, Ziliox M, Van Nostrand WE, Smith SO. Capping of aβ42 oligomers by small molecule inhibitors. Biochemistry. 2014;53:7893–903.
Prade E, Barucker C, Sarkar R, Althoff-Ospelt G, Lopez del Amo JM, Hossain S, et al. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry. 2016;55:1839–49.
Feng M, Tang B, Liang SH, Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr Top Med Chem. 2016;16:1200–16.
Benhamú B, Martín-Fontecha M, Vázquez-Villa H, Pardo L, López-Rodríguez ML. Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer’s disease. J Med Chem. 2014;57:7160–81.
Maher-Edwards G, Zvartau-Hind M, Hunter AJ, Gold M, Hopton G, Jacobs G, et al. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr Alzheimer Res. 2010;7:374–85.
Ivachtchenko AV, Ivanenkov YA, Veselov MS, Okun IM. AVN-322 is a safe orally bio-available potent and highly selective antagonist of 5-HT6R with demonstrated ability to improve impaired memory in animal models. Curr Alzheimer Res. 2017;14:268–94.
Liu KG, Robichaud AJ, Bernotas RC, Yan Y, Lo JR, Zhang MY, et al. 5-Piperazinyl-3-sulfonylindazoles as potent and selective 5-hydroxytryptamine-6 antagonists. J Med Chem. 2010;53:7639–46.
Ivachtchenko AV, Dmitriev DE, Golovina ES, Kadieva MG, Koryakova AG, Kysil VM, et al. (3-Phenylsulfonylcycloalkano[e and d]pyrazolo[1,5-a]pyrimidin-2-yl)amines: potent and selective antagonists of the serotonin 5-HT6 receptor. J Med Chem. 2010;53:5186–96.
Ivachtchenko AV, Golovina ES, Kadieva MG, Koryakova AG, Mitkin OD, Tkachenko SE, et al. 2-Substituted 5,6-dimethyl-3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidines: New series of highly potent and specific serotonin 5-HT6 receptor antagonists. Eur J Med Chem. 2011;46:1189–97.
Ivachtchenko AV, Golovina ES, Kadieva MG, Kysil VM, Mitkin OD, Tkachenko SE, et al. Synthesis and SAR of 3-arylsulfonyl-pyrazolo[1,5-a]pyrimidines as potent serotonin 5-HT6 receptor antagonists. Bioorg Med Chem. 2011;19:1482–91.
Ivachtchenko AV, Golovina ES, Kadieva MG, Kysil VM, Mitkin OD, Tkachenko SE, et al. Synthesis and structure–activity relationship (SAR) of (5,7-Disubstituted 3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidin-2-yl)-methylamines as potent serotonin 5-HT6 Receptor (5-HT6R) antagonists. J Med Chem. 2011;54:8161–73.
Rueeger H, Lueoend R, Rogel O, Rondeau J-M, Möbitz H, Machauer R, et al. Discovery of cyclic sulfone hydroxyethylamines as potent and selective β-Site APP-cleaving enzyme 1 (BACE1) inhibitors: structure-based design and in vivo reduction of amyloid β-peptides. J Med Chem. 2012;55:3364–86.
Jakaria M, Azam S, Haque ME, Jo S-H, Uddin MS, Kim I-S, et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223.
Chung MC, Malatesta P, Bosquesi PL, Yamasaki PR, Santos JLD, Vizioli EO. Advances in drug design based on the amino Acid approach: taurine analogues for the treatment of CNS diseases. Pharmaceuticals. 2012;5:1128–46.
Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noël F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004;18:511–8.
Manzano S, Agüera L, Aguilar M, Olazarán J. A review on tramiprosate (Homotaurine) in Alzheimer’s disease and other neurocognitive disorders. Front Neurol. 2020;11:614.
Oh SJ, Lee H-J, Jeong YJ, Nam KR, Kang KJ, Han SJ, et al. Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci Rep. 2020;10:15551.
Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, et al. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006;67:1757–63.
Tóth S, Csaba G. γ-l-Glutamyl-taurine (Litoralon®) prevents the micronucleus formation induced by mitomycin C. Mutat Res Lett. 1988;209:85–9.
Salimäki J, Scriba G, Piepponen TP, Rautolahti N, Ahtee L. The effects of systemically administered taurine and N-pivaloyltaurine on striatal extracellular dopamine and taurine in freely moving rats. Naunyn-Schmiedebergs Arch Pharmacol. 2003;368:134–41.
Ricci L, Frosini M, Gaggelli N, Valensin G, Machetti F, Sgaragli G, et al. Inhibition of rabbit brain 4-aminobutyrate transaminase by some taurine analogues: a kinetic analysis. Biochemical Pharmacol. 2006;71:1510–9.
Ward R, Cirkovic-Vellichovia T, Ledeque F, Tirizitis G, Dubars G, Datla K, et al. Neuroprotection by Taurine and Taurine Analogues. Springer; 2006. pp. 299–306.
Klusa V, Klimaviciusa L, Duburs G, Poikans J, Zharkovsky A. Anti-neurotoxic effects of tauropyrone, a taurine analogue. Adv Exp Med Biol. 2006;583:499–508.
Mancinelli A. Pharmacokinetics of 5-HT6 receptor ligands. Int Rev Neurobiol. 2010;94:153–72.
O’Flaherty L, Stapleton PP, Redmond HP, Bouchier-Hayes DJ. Intestinal taurine transport: a review. Eur J Clin Investig. 1997;27:873–80.
Cosar M, Kaner T, Sahin O, Topaloglu N, Guven M, Aras AB, et al. The neuroprotective effect of Sulindac after ischemia-reperfusion injury in rats. Acta Cir Bras. 2014;29:268–73.
Jang H, Lee S, Choi SL, Kim HY, Baek S, Kim Y. Taurine directly binds to oligomeric amyloid-β and recovers cognitive deficits in Alzheimer model mice. Adv Exp Med Biol. 2017;975:233–41.
Murakami S, Kondo Y, Nagate T. Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp Med Biol. 2000;483:177–86.
Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28:537–47.
Javed H, Khan A, Vaibhav K, Moshahid Khan M, Ahmad A, Ejaz Ahmad M, et al. Taurine ameliorates neurobehavioral, neurochemical and immunohistochemical changes in sporadic dementia of Alzheimer’s type (SDAT) caused by intracerebroventricular streptozotocin in rats. Neurological Sci. 2013;34:2181–92.
El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett. 2008;436:19–22.
Benedetti R, Marchegiani A, Tambella AM, Fruganti A, Serri E, Malfatti A, et al. Effects of chronic supplementation of homotaurine on cognitive processes and spatial cognition in aged dogs: preliminary results. J Vet Behav. 2019;33:90–5.
Goluboff ET. Exisulind, a selective apoptotic antineoplastic drug. Expert Opin Investig Drugs. 2001;10:1875–82.
Maher-Edwards G, Watson C, Ascher J, Barnett C, Boswell D, Davies J, et al. Two randomized controlled trials of SB742457 in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2015;1:23–36.
Andrews M, Tousi B, Sabbagh MN. 5HT6 antagonists in the treatment of Alzheimer’s dementia: current progress. Neurol Ther. 2018;7:51–8.
Hey JA, Yu JY, Versavel M, Abushakra S, Kocis P, Power A, et al. Clinical pharmacokinetics and safety of ALZ-801, a novel prodrug of tramiprosate in development for the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2018;57:315–33.
Bossù P, Salani F, Ciaramella A, Sacchinelli E, Mosca A, Banaj N, et al. Anti-inflammatory effects of homotaurine in patients with amnestic mild cognitive impairment. Front Aging Neurosci. 2018;10:285.
Zhao C, Rakesh KP, Ravidar L, Fang W-Y, Qin H-L. Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery: a critical review. Eur J Med Chem. 2019;162:679–734.
Grygorenko OO, Biitseva AV, Zhersh S. Amino sulfonic acids, peptidosulfonamides and other related compounds. Tetrahedron. 2018;74:1355–421.
Bag S, Tulsan R, Sood A, Cho H, Redjeb H, Zhou W, et al. Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg Med Chem Lett. 2015;25:626–30.
Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH. BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev. 2020;40:339–84.
Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci. 2001;4:231–2.
Yuan J, Venkatraman S, Zheng Y, McKeever BM, Dillard LW, Singh SB. Structure-based design of β-site APP cleaving enzyme 1 (BACE1) inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2013;56:4156–80.
Specker E, Böttcher J, Heine A, Sotriffer CA, Lilie H, Schoop A, et al. Hydroxyethylene sulfones as a new scaffold to address aspartic proteases: design, synthesis, and structural characterization. J Med Chem. 2005;48:6607–19.
Stachel SJ, Coburn CA, Steele TG, Crouthamel MC, Pietrak BL, Lai MT, et al. Conformationally biased P3 amide replacements of beta-secretase inhibitors. Bioorg Med Chem Lett. 2006;16:641–4.
Ghosh AK, Kumaragurubaran N, Hong L, Kulkarni SS, Xu X, Chang W, et al. Design, synthesis, and X-ray structure of potent memapsin 2 (β-Secretase) inhibitors with isophthalamide derivatives as the P2-P3-ligands. J Med Chem. 2007;50:2399–407.
Sandgren V, Bäck M, Kvarnström I, Dahlgren A. Design and synthesis of hydroxyethylene-based BACE-1 inhibitors incorporating extended P1 substituents. Open Med Chem J. 2013;7:1–15.
Rajapakse HA, Nantermet PG, Selnick HG, Munshi S, McGaughey GB, Lindsley SR, et al. Discovery of oxadiazoyl tertiary carbinamine inhibitors of β-secretase (BACE-1). J Med Chem. 2006;49:7270–3.
Lindsley SR, Moore KP, Rajapakse HA, Selnick HG, Young MB, Zhu H, et al. Design, synthesis, and SAR of macrocyclic tertiary carbinamine BACE-1 inhibitors. Bioorg Med Chem Lett. 2007;17:4057–61.
Moore KP, Zhu H, Rajapakse HA, McGaughey GB, Colussi D, Price EA, et al. Strategies toward improving the brain penetration of macrocyclic tertiary carbinamine BACE-1 inhibitors. Bioorg Med Chem Lett. 2007;17:5831–5.
Beswick P, Charrier N, Clarke B, Demont E, Dingwall C, Dunsdon R, et al. BACE-1 inhibitors part 3: identification of hydroxy ethylamines (HEAs) with nanomolar potency in cells. Bioorg Med Chem Lett. 2008;18:1022–6.
Charrier N, Clarke B, Cutler L, Demont E, Dingwall C, Dunsdon R, et al. Second generation of hydroxyethylamine BACE-1 inhibitors: optimizing potency and oral bioavailability. J Med Chem. 2008;51:3313–7.
Charrier N, Clarke B, Demont E, Dingwall C, Dunsdon R, Hawkins J, et al. Second generation of BACE-1 inhibitors part 2: optimisation of the non-prime side substituent. Bioorg Med Chem Lett. 2009;19:3669–73.
Charrier N, Clarke B, Cutler L, Demont E, Dingwall C, Dunsdon R, et al. Second generation of BACE-1 inhibitors. Part 1: the need for improved pharmacokinetics. Bioorg Med Chem Lett. 2009;19:3664–8.
Scott JD, Li SW, Brunskill AP, Chen X, Cox K, Cumming JN, et al. Discovery of the 3-Imino-1,2,4-thiadiazinane 1,1-dioxide derivative verubecestat (MK-8931)-A β-site amyloid precursor protein cleaving enzyme 1 inhibitor for the treatment of Alzheimer’s disease. J Med Chem. 2016;59:10435–50.
Zhao SH, Berger J, Clark RD, Sethofer SG, Krauss NE, Brothers JM, et al. 3,4-Dihydro-2H-benzo[1,4]oxazine derivatives as 5-HT6 receptor antagonists. Bioorg Med Chem Lett. 2007;17:3504–7.
Nirogi R, Abraham R, Benade V, Medapati RB, Jayarajan P, Bhyrapuneni G, et al. SUVN-502, a novel, potent, pure, and orally active 5-HT6 receptor antagonist: pharmacological, behavioral, and neurochemical characterization. Behav Pharmacol. 2019;30:16–35.
Grychowska K, Satała G, Kos T, Partyka A, Colacino E, Chaumont-Dubel S, et al. Novel 1H-Pyrrolo[3,2-c]quinoline based 5-HT6 receptor antagonists with potential application for the treatment of cognitive disorders associated with Alzheimer’s disease. ACS Chem Neurosci. 2016;7:972–83.
Canale V, Grychowska K, Kurczab R, Ryng M, Keeri AR, Satała G, et al. A dual-acting 5-HT6 receptor inverse agonist/MAO-B inhibitor displays glioprotective and pro-cognitive properties. Eur J Med Chem. 2020;208:112765.
Więckowska A, Kołaczkowski M, Bucki A, Godyń J, Marcinkowska M, Więckowski K, et al. Novel multi-target-directed ligands for Alzheimer’s disease: combining cholinesterase inhibitors and 5-HT(6) receptor antagonists. Design, synthesis and biological evaluation. Eur J Med Chem. 2016;124:63–81.
Kreft AF, Martone R, Porte A. Recent advances in the identification of γ-secretase inhibitors to clinically test the Aβ oligomer hypothesis of Alzheimer’s disease. J Med Chem. 2009;52:6169–88.
Strooper BD, Gutiérrez LC. Learning by failing: ideas and concepts to tackle γ-secretases in Alzheimer’s disease and beyond. Annu Rev Pharmacol Toxicol. 2015;55:419–37.
Zhao Z, Pissarnitski DA, Josien HB, Bara TA, Clader JW, Li H, et al. Substituted 4-morpholine N-arylsulfonamides as γ-secretase inhibitors. Eur J Med Chem. 2016;124:36–48.
Gillman KW, Starrett JE, Parker MF, Xie K, Bronson JJ, Marcin LR, et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med Chem Lett. 2010;1:120–4.
Stepan AF, Karki K, McDonald WS, Dorff PH, Dutra JK, DiRico KJ, et al. Metabolism-directed design of oxetane-containing arylsulfonamide derivatives as γ-secretase inhibitors. J Med Chem. 2011;54:7772–83.
Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2005;25:8898–902.
Zhang M, Porte A, Diamantidis G, Sogi K, Kubrak D, Resnick L, et al. Asymmetric synthesis of novel α-amino acids with β-branched side chains. Bioorg Med Chem Lett. 2007;17:2401–3.
Probst G, Aubele DL, Bowers S, Dressen D, Garofalo AW, Hom RK, et al. Discovery of (R)-4-Cyclopropyl-7,8-difluoro-5-(4-(trifluoromethyl)phenylsulfonyl)-4,5-dihydro-1H-pyrazolo[4,3-c]quinoline (ELND006) and (R)-4-Cyclopropyl-8-fluoro-5-(6-(trifluoromethyl)pyridin-3-ylsulfonyl)-4,5-dihydro-2H-pyrazolo[4,3-c]quinoline (ELND007): metabolically stable γ-secretase inhibitors that selectively inhibit the production of amyloid-β over Notch. J Med Chem. 2013;56:5261–74.
Hyde LA, Zhang Q, Del Vecchio RA, Leach PT, Cohen-Williams ME, Chen L, et al. In vivo characterization of a novel γ-secretase inhibitor SCH 697466 in Rodents and investigation of strategies for managing notch-related side effects. Int J Alzheimer’s Dis. 2013;2013:823528.
DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64.
Diamant S, Podoly E, Friedler A, Ligumsky H, Livnah O, Soreq H. Butyrylcholinesterase attenuates amyloid fibril formation in vitro. Proc Natl Acad Sci USA. 2006;103:8628.
Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep. 2019;20:1479–87.
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–97.
Apaydın S, Török M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg Med Chem Lett. 2019;29:2042–50.
Girisha HR, Narendra Sharath Chandra JN, Boppana S, Malviya M, Sadashiva CT, Rangappa KS. Active site directed docking studies: Synthesis and pharmacological evaluation of cis-2,6-dimethyl piperidine sulfonamides as inhibitors of acetylcholinesterase. Eur J Med Chem. 2009;44:4057–62.
Hassan M, Abbasi MA, Aziz ur R, Siddiqui SZ, Shahzadi S, Raza H, et al. Designing of promising medicinal scaffolds for Alzheimer’s disease through enzyme inhibition, lead optimization, molecular docking and dynamic simulation approaches. Bioorg Chem. 2019;91:103138.
Mutahir S, Jończyk J, Bajda M, Khan IU, Khan MA, Ullah N, et al. Novel biphenyl bis-sulfonamides as acetyl and butyrylcholinesterase inhibitors: Synthesis, biological evaluation and molecular modeling studies. Bioorg Chem. 2016;64:13–20.
Riaz S, Khan IU, Bajda M, Ashraf M, Qurat ul A, Shaukat A, et al. Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and Alzheimer’s disease: in vitro studies against yeast α-glucosidase, acetylcholinesterase and butyrylcholinesterase. Bioorg Chem. 2015;63:64–71.
Ulus R, Zengin Kurt B, Gazioğlu I, Kaya M. Microwave assisted synthesis of novel hybrid tacrine-sulfonamide derivatives and investigation of their antioxidant and anticholinesterase activities. Bioorg Chem. 2017;70:245–55.
Košak U, Knez D, Coquelle N, Brus B, Pišlar A, Nachon F, et al. N-Propargylpiperidines with naphthalene-2-carboxamide or naphthalene-2-sulfonamide moieties: potential multifunctional anti-Alzheimer’s agents. Bioorg Med Chem. 2017;25:633–45.
Baldwin AG, Brough D, Freeman S. Inhibiting the inflammasome: a chemical perspective. J Med Chem. 2016;59:1691–710.
Fulp J, He L, Toldo S, Jiang Y, Boice A, Guo C, et al. Structural insights of benzenesulfonamide analogues as NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization. J Med Chem. 2018;61:5412–23.
Zhang Y, Zhao Y, Zhang J, Yang G. Mechanisms of NLRP3 inflammasome activation: its role in the treatment of Alzheimer’s disease. Neurochem Res. 2020;45:2560–72.
Cui W, Sun C, Ma Y, Wang S, Wang X, Zhang Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s disease. Front Neurosci. 2020;14:444.
Rotella DP. Phosphodiesterase type 5 inhibitors: discovery and therapeutic utility. Drugs Future. 2001;26:153–62.
Zuccarello E, Acquarone E, Calcagno E, Argyrousi EK, Deng SX, Landry DW, et al. Development of novel phosphodiesterase 5 inhibitors for the therapy of Alzheimer’s disease. Biochem Pharmacol. 2020;176:113818.
Prickaerts J, Heckman PRA, Blokland A. Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin Investig Drugs. 2017;26:1033–48.
García-Osta A, Cuadrado-Tejedor M, García-Barroso C, Oyarzábal J, Franco R. Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem Neurosci. 2012;3:832–44.
Sanders O. Sildenafil for the treatment of Alzheimer’s disease: a systematic review. J Alzheimers Dis Rep. 2020;4:91–106.
Eros D, Cs S-K, Kiss R, Gy K, Hegymegi-Barakonyi B, Kovesdi I, et al. Structure—activity relationships of PDE5 inhibitors (supporting material). Curr Med Chem. 2008;15:1570–85.
Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;16:S11–4.
Bischoff E. Vardenafil preclinical trial data: potency, pharmacodynamics, pharmacokinetics, and adverse events. Int J Impot Res. 2004;16:S34–7.
Cumming J, Babu S, Huang Y, Carrol C, Chen X, Favreau L, et al. Piperazine sulfonamide BACE1 inhibitors: design, synthesis, and in vivo characterization. Bioorg Med Chem Lett. 2010;20:2837–42.
Popugaeva E, Cherniuk D, Zhang H, Postnikova T, Pac K, Fedorova E, et al. Derivatives of piperazines as potential therapeutic agents for Alzheimers disease. Mol Pharmacol. 2019. https://doi.org/10.1124/mol.118.114348.
Blume T, Filser S, Jaworska A, Blain JF, Koenig G, Moschke K, et al. BACE1 inhibitor MK-8931 alters formation but not stability of dendritic spines. Front Aging Neurosci. 2018;10:229.
Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 2016;8:363ra150.
Fullerton T, Binneman B, David W, Delnomdedieu M, Kupiec J, Lockwood P, et al. A Phase 2 clinical trial of PF-05212377 (SAM-760) in subjects with mild to moderate Alzheimer’s disease with existing neuropsychiatric symptoms on a stable daily dose of donepezil. Alzheimer’s Res Ther. 2018;10:38.
Truong AP, Aubele DL, Probst GD, Neitzel ML, Semko CM, Bowers S, et al. Design, synthesis, and structure-activity relationship of novel orally efficacious pyrazole/sulfonamide based dihydroquinoline gamma-secretase inhibitors. Bioorg Med Chem Lett. 2009;19:4920–3.
Ye XM, Konradi AW, Sun M, Yuan S, Aubele DL, Dappen M, et al. Discovery of a novel [3.2.1] benzo fused bicyclic sulfonamide-pyrazoles as potent, selective and efficacious γ-secretase inhibitors. Bioorg Med Chem Lett. 2013;23:996–1000.
Wolfe MS. γ-Secretase inhibitors and modulators for Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):89–98.
Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017;61:306–16.
Areosa Sastre A, Vernooij RW, González-Colaço Harmand M, Martínez G. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804-CD.
Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53 Suppl 1:5S–12S.
Foley AG, Murphy KJ, Hirst WD, Gallagher HC, Hagan JJ, Upton N, et al. The 5-HT6 receptor antagonist SB-271046 reverses scopolamine-disrupted consolidation of a passive avoidance task and ameliorates spatial task deficits in aged rats. Neuropsychopharmacology. 2004;29:93–100.
Da Silva Costa-Aze V, Quiedeville A, Boulouard M, Dauphin F. 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology. 2012;222:99–115.
Barten DM, Meredith JE Jr., Zaczek R, Houston JG, Albright CF. Gamma-secretase inhibitors for Alzheimer’s disease: balancing efficacy and toxicity. Drugs R D 2006;7:87–97.
Mayer SC, Kreft AF, Harrison B, Abou-Gharbia M, Antane M, Aschmies S, et al. Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer’s disease. J Med Chem. 2008;51:7348–51.
Martone RL, Zhou H, Atchison K, Comery T, Xu JZ, Huang X, et al. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther. 2009;331:598–608.
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8.
Yin J, Zhao F, Chojnacki JE, Fulp J, Klein WL, Zhang S, et al. NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2018;55:1977–87.
Perregaux DG, McNiff P, Laliberte R, Hawryluk N, Peurano H, Stam E, et al. Identification and Characterization of a Novel Class of Interleukin-1 Post-Translational Processing Inhibitors. J Pharmacol Exp Ther. 2001;299:187.
Cuadrado-Tejedor M, Hervias I, Ricobaraza A, Puerta E, Pérez-Roldán JM, García-Barroso C, et al. Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Br J Pharmacol. 2011;164:2029–41.
Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci. 2009;29:8075–86.
Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med. 2019;380:1408–20.
Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1430–40.
Schneider LS. A critical review of cholinesterase inhibitors as a treatment modality in Alzheimer’s disease. Dialogues Clin Neurosci. 2000;2:111–28.
Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med. 2013;16:277–86.
Sheng M, Lu H, Liu P, Li Y, Ravi H, Peng S-L, et al. Sildenafil improves vascular and metabolic function in patients with Alzheimer’s disease. J Alzheimers Dis. 2017;60:1351–64.
Theunissen EL, Heckman P, de Sousa Fernandes Perna EB, Kuypers KP, Sambeth A, Blokland A, et al. Rivastigmine but not vardenafil reverses cannabis-induced impairment of verbal memory in healthy humans. Psychopharmacology. 2015;232:343–53.
Shim YS, Pae CU, Kim SW, Kim HW, Kim JC, Koh JS. Effects of repeated dosing with Udenafil (Zydena) on cognition, somatization and erection in patients with erectile dysfunction: a pilot study. Int J Impot Res. 2011;23:109–14.
Rouf A, Tanyeli C. Bioactive thiazole and benzothiazole derivatives. Eur J Med Chem. 2015;97:911–27.
Ayati A, Emami S, Asadipour A, Shafiee A, Foroumadi A. Recent applications of 1, 3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur J Med Chem. 2015;97:699–718.
Mishra CB, Kumari S, Tiwari M. Thiazole: a promising heterocycle for the development of potent CNS active agents. Eur J Med Chem. 2015;92:1–34.
Nagahama K, Matsunaga Y, Kawachi M, Ito K, Tanaka T, Hori Y, et al. Acotiamide, a new orally active acetylcholinesterase inhibitor, stimulates gastrointestinal motor activity in conscious dogs. Neurogastroenterol Motil. 2012;24:566–74.e256.
Sun Z-Q, Tu L-X, Zhuo F-J, Liu S-X. Design and discovery of Novel Thiazole acetamide derivatives as anticholinesterase agent for possible role in the management of Alzheimer’s. Bioorg Med Chem Lett. 2016;26:747–50.
Nayak S, Gaonkar SL. A review on recent synthetic strategies and pharmacological importance of 1, 3-thiazole derivatives. Mini Rev Med Chem. 2019;19:215–38.
Shi DH, Tang ZM, Liu YW, Harjani JR, Zhu HL, Ma XD, et al. Design, synthesis and biological evaluation of novel 2‐phenylthiazole derivatives for the treatment of Alzheimer’s disease. ChemistrySelect. 2017;2:10572–9.
Sahin Z, Ertas M, Bender C, Bülbül EF, Berk B, Biltekin SN, et al. Thiazole‐substituted benzoylpiperazine derivatives as acetylcholinesterase inhibitors. Drug Dev Res. 2018;79:406–25.
Rahim F, Javed MT, Ullah H, Wadood A, Taha M, Ashraf M, et al. Synthesis, molecular docking, acetylcholinesterase and butyrylcholinesterase inhibitory potential of thiazole analogs as new inhibitors for Alzheimer disease. Bioorg Chem. 2015;62:106–16.
Sağlık BN, Levent S, Osmaniye D, Acar Çevik U, Kaya Çavuşoğlu B, Özkay Y, et al. Design, synthesis, and biological activity evaluation of new donepezil-like compounds bearing thiazole ring for the treatment of Alzheimer’s disease. Crystals. 2020;10:637.
Mumtaz A, Shoaib M, Zaib S, Shah MS, Bhatti HA, Saeed A, et al. Synthesis, molecular modelling and biological evaluation of tetrasubstituted thiazoles towards cholinesterase enzymes and cytotoxicity studies. Bioorg Chem. 2018;78:141–8.
Shidore M, Machhi J, Shingala K, Murumkar P, Sharma MK, Agrawal N, et al. Benzylpiperidine-linked diarylthiazoles as potential anti-Alzheimer’s agents: synthesis and biological evaluation. J Med Chem. 2016;59:5823–46.
Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H-P, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. Bmj. 2005;331:321–7.
Bhounsule AS, Bhatt LK, Prabhavalkar KS, Oza M. Cyclin dependent kinase 5: a novel avenue for Alzheimer’s disease. Brain Res Bull. 2017;132:28–38.
Nugiel DA, Vidwans A, Etzkorn A-M, Rossi KA, Benfield PA, Burton CR, et al. Synthesis and evaluation of indenopyrazoles as cyclin-dependent kinase inhibitors. 2. Probing the indeno ring substituent pattern. J Med Chem. 2002;45:5224–32.
Shiradkar MR, Akula KC, Dasari V, Baru V, Chiningiri B, Gandhi S, et al. Clubbed thiazoles by MAOS: a novel approach to cyclin-dependent kinase 5/p25 inhibitors as a potential treatment for Alzheimer’s disease. Bioorg Med Chem. 2007;15:2601–10.
Helal CJ, Sanner MA, Cooper CB, Gant T, Adam M, Lucas JC, et al. Discovery and SAR of 2-aminothiazole inhibitors of cyclin-dependent kinase 5/p25 as a potential treatment for Alzheimer’s disease. Bioorg Med Chem Lett. 2004;14:5521–5.
Keri RS, Patil MR, Patil SA, Budagumpi S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur J Med Chem. 2015;89:207–51.
Klunk WE, Wang Y, Huang G-f, Debnath ML, Holt DP, Mathis CA. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 2001;69:1471–84.
Al Mamun A, Uddin MS, Mathew B, Ashraf GM. Toxic tau: structural origins of tau aggregation in Alzheimer’s disease. Neural Regen Res. 2020;15:1417.
Chang E, Congdon EE, Honson NS, Duff KE, Kuret J. Structure− activity relationship of cyanine tau aggregation inhibitors. J Med Chem. 2009;52:3539–47.
Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–37.
Schafer KN, Murale DP, Kim K, Cisek K, Kuret J, Churchill DG. Structure–activity relationship of cyclic thiacarbocyanine tau aggregation inhibitors. Bioorg Med Chem Lett. 2011;21:3273–6.
Van Kan H, Groeneveld G, Kalmijn S, Spieksma M, Van Den Berg L, Guchelaar H. Association between CYP1A2 activity and riluzole clearance in patients with amyotrophic lateral sclerosis. Br J Clin Pharmacol. 2005;59:310–3.
Whitcomb DJ, Molnár E. Is riluzole a new drug for Alzheimer’s disease? J Neurochem. 2015;135:207–9.
Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. 2019;5:272–93.
Mokhtari Z, Baluchnejadmojarad T, Nikbakht F, Mansouri M, Roghani M. Riluzole ameliorates learning and memory deficits in Aβ25-35-induced rat model of Alzheimer’s disease and is independent of cholinoceptor activation. Biomed Pharmacother. 2017;87:135–44.
Pereira AC, Gray JD, Kogan JF, Davidson RL, Rubin TG, Okamoto M, et al. Age and Alzheimer’s disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry. 2017;22:296–305.
Jarhad DB, Mashelkar KK, Kim H-R, Noh M, Jeong LS. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors as potential therapeutics. J Med Chem. 2018;61:9791–810.
Soppa U, Becker W. DYRK protein kinases. Curr Biol. 2015;25:R488–9.
Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98.
Kimura R, Kamino K, Yamamoto M, Nuripa A, Kida T, Kazui H, et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between β-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet. 2007;16:15–23.
Ryoo SR, Cho HJ, Lee HW, Jeong HK, Radnaabazar C, Kim YS, et al. Dual‐specificity tyrosine (Y)‐phosphorylation regulated kinase 1A‐mediated phosphorylation of amyloid precursor protein: evidence for a functional link between Down syndrome and Alzheimer’s disease. J Neurochem. 2008;104:1333–44.
Coutadeur S, Benyamine H, Delalonde L, de Oliveira C, Leblond B, Foucourt A, et al. A novel DYRK1A (dual specificity tyrosine phosphorylation‐regulated kinase 1A) inhibitor for the treatment of Alzheimer’s disease: effect on Tau and amyloid pathologies in vitro. J Neurochemistr. 2015;133:440–51.
Ogawa Y, Nonaka Y, Goto T, Ohnishi E, Hiramatsu T, Kii I, et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat Commun. 2010;1:1–9.
Chaikuad A, Diharce J, Schröder M, Foucourt A, Leblond B, Casagrande A-S, et al. An unusual binding model of the methyl 9-anilinothiazolo [5, 4-f] quinazoline-2-carbimidates (EHT 1610 and EHT 5372) confers high selectivity for dual-specificity tyrosine phosphorylation-regulated kinases. J Med Chem. 2016;59:10315–21.
Rosse G. Tricyclic pyrimidines as inhibitors of DYRK1A/DYRK1B as potential treatment for Down’s syndrome or Alzheimer’s disease. ACS Med Chem Lett. 2013;4:502–503.
Masaki S, Kii I, Sumida Y, Kato-Sumida T, Ogawa Y, Ito N, et al. Design and synthesis of a potent inhibitor of class 1 DYRK kinases as a suppressor of adipogenesis. Bioorg Med Chem. 2015;23:4434–41.
Morsy A, Trippier PC. Amyloid-binding alcohol dehydrogenase (ABAD) inhibitors for the treatment of Alzheimer’s disease: miniperspective. J Med Chem. 2018;62:4252–64.
Grimm A, Lim Y-A, Mensah-Nyagan AG, Götz J, Eckert A. Alzheimer’s disease, oestrogen and mitochondria: an ambiguous relationship. Mol Neurobiol. 2012;46:151–60.
Xu H, Wang R, ZHANG YW, Zhang X. Estrogen, β‐amyloid metabolism/trafficking, and Alzheimer’s disease. Ann NY Acad Sci. 2006;1089:324–42.
ALVAREZ‐DE‐LA‐ROSA M, Silva I, Nilsen J, Perez M, GARCÍA‐SEGURA LM, Ávila J, et al. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann NY Acad Sci. 2005;1052:210–24.
Goodenough S, Schleusner D, Pietrzik C, Skutella T, Behl C. Glycogen synthase kinase 3β links neuroprotection by 17β-estradiol to key Alzheimer processes. Neuroscience. 2005;132:581–9.
Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–58.
Xie Y, Deng S, Chen Z, Yan S, Landry DW. Identification of small-molecule inhibitors of the Aβ–ABAD interaction. Bioorg Med Chem Lett. 2006;16:4657–60.
Aitken L, Benek O, McKelvie BE, Hughes RE, Hroch L, Schmidt M, et al. Novel benzothiazole-based ureas as 17β-HSD10 inhibitors, a potential alzheimer’s disease treatment. Molecules. 2019;24:2757.
Valasani KR, Hu G, Chaney MO, Yan SS. Structure‐based design and synthesis of benzothiazole phosphonate analogues with inhibitors of human ABAD‐Aβ for treatment of Alzheimer’s disease. Chem Biol drug Des. 2013;81:238–49.
Benek O, Hroch L, Aitken L, Dolezal R, Guest P, Benkova M, et al. 6-Benzothiazolyl ureas, thioureas and guanidines are potent inhibitors of ABAD/17β-HSD10 and potential drugs for Alzheimer. Med Chem. 2017;13:345–58.
Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009;78:927–32.
Akoury E, Pickhardt M, Gajda M, Biernat J, Mandelkow E, Zweckstetter M. Mechanistic basis of phenothiazine‐driven inhibition of Tau aggregation. Angew Chem Int Ed. 2013;52:3511–5.
Yuksel M, Biberoglu K, Onder S, Akbulut KG, Tacal O. Effects of phenothiazine-structured compounds on APP processing in Alzheimer’s disease cellular model. Biochimie. 2017;138:82–9.
Bruchey AK, Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am J Pharmacol Toxicol. 2008;3:72.
Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–12.
Tin G, Mohamed T, Gondora N, Beazely MA, Rao PP. Tricyclic phenothiazine and phenoselenazine derivatives as potential multi-targeting agents to treat Alzheimer’s disease. MedChemComm. 2015;6:1930–41.
Makhaeva GF, Shevtsova EF, Boltneva NP, Lushchekina SV, Kovaleva NV, Rudakova EV, et al. Overview of novel multifunctional agents based on conjugates of γ-carbolines, carbazoles, tetrahydrocarbazoles, phenothiazines, and aminoadamantanes for treatment of Alzheimer’s disease. Chem-Biol Interact. 2019;308:224–34.
Makhaeva GF, Lushchekina SV, Boltneva NP, Sokolov VB, Grigoriev VV, Serebryakova OG, et al. Conjugates of γ-Carbolines and Phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer Disease. Sci Rep. 2015;5:13164.
Jain AK, Vaidya A, Ravichandran V, Kashaw SK, Agrawal RK. Recent developments and biological activities of thiazolidinone derivatives: a review. Bioorg Med Chem. 2012;20:3378–95.
Tripathi AC, Gupta SJ, Fatima GN, Sonar PK, Verma A, Saraf SK. 4-Thiazolidinones: the advances continue…. Eur J Med Chem. 2014;72:52–77.
Verma A, Saraf SK. 4-Thiazolidinone—a biologically active scaffold. Eur J Med Chem. 2008;43:897–905.
Bulic B, Pickhardt M, Khlistunova I, Biernat J, Mandelkow EM, Mandelkow E, et al. Rhodanine‐based tau aggregation inhibitors in cell models of tauopathy. Angew Chem Int Ed. 2007;46:9215–9.
Bulic B, Pickhardt M, Mandelkow E. Progress and developments in tau aggregation inhibitors for Alzheimer disease. J Med Chem. 2013;56:4135–55.
Maqbool M, Mobashir M, Hoda N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur J Med Chem. 2016;107:63–81.
Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–9.
Castro A, Encinas A, Gil C, Bräse S, Porcal W, Pérez C, et al. Non-ATP competitive glycogen synthase kinase 3β (GSK-3β) inhibitors: study of structural requirements for thiadiazolidinone derivatives. Bioorg Med Chem. 2008;16:495–510.
Martinez A, Alonso M, Castro A, Dorronsoro I, Gelpí JL, Luque FJ, et al. SAR and 3D-QSAR studies on thiadiazolidinone derivatives: exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J Med Chem. 2005;48:7103–12.
Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45:1292–9.
Xu M, Wang S, Zhu L, Wu P, Dai W, Rakesh K. Structure-activity relationship (SAR) studies of synthetic glycogen synthase kinase-3β inhibitors: a critical review. Eur J Med Chem. 2019;164:448–70.
Palomo V, Perez DI, Perez C, Morales-Garcia JA, Soteras I, Alonso-Gil S, et al. 5-imino-1, 2, 4-thiadiazoles: first small molecules as substrate competitive inhibitors of glycogen synthase kinase 3. J Med Chem. 2012;55:1645–61.
Gandini A, Bartolini M, Tedesco D, Martinez-Gonzalez L, Roca C, Campillo NE, et al. Tau-centric multitarget approach for Alzheimer’s disease: development of first-in-class dual glycogen synthase kinase 3β and tau-aggregation inhibitors. J Med Chem. 2018;61:7640–56.
Dixit VA, Bharatam PV. SAR and computer-aided drug design approaches in the discovery of peroxisome proliferator-activated receptor γ activators: a perspective. J Comput Med. 2013;2013:1–38.
Galimberti D, Scarpini E. Pioglitazone for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs. 2017;26:97–101.
Rosen CJ. The rosiglitazone story—lessons from an FDA Advisory Committee meeting. N Engl J Med. 2007;357:844–6.
Smith MT. Mechanisms of troglitazone hepatotoxicity. Chem Res Toxicol. 2003;16:679–87.
Garcia-Vallvé S, Guasch L, Tomas-Hernández S, del Bas JM, Ollendorff V, Arola L, et al. Peroxisome proliferator-activated receptor γ (PPARγ) and ligand choreography: Newcomers Take the Stage: Miniperspective. J Med Chem. 2015;58:5381–94.
Ji C, Zhang J. Protein polarization is critical to stabilizing AF-2 and helix-2′ domains in ligand binding to PPAR-γ. J Am Chem Soc. 2008;130:17129–33.
Yamagishi K, Yamamoto K, Mochizuki Y, Nakano T, Yamada S, Tokiwa H. Flexible ligand recognition of peroxisome proliferator-activated receptor-γ (PPARγ). Bioorg Med Chem Lett. 2010;20:3344–7.
Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–59.
Deeks ED, Keam SJ. Rosiglitazone : a review of its use in type 2 diabetes mellitus. Drugs. 2007;67:2747–79.
Gray E, Ginty M, Kemp K, Scolding N, Wilkins A. The PPAR-gamma agonist pioglitazone protects cortical neurons from inflammatory mediators via improvement in peroxisomal function. J Neuroinflammation. 2012;9:63.
Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci USA. 2006;103:443–8.
Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19:129–37.
Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–6.
Toyota Y, Nomura S, Makishima M, Hashimoto Y, Ishikawa M. Structure-activity relationships of rosiglitazone for peroxisome proliferator-activated receptor gamma transrepression. Bioorg Med Chem Lett. 2017;27:2776–80.
Patel PN, Kalariya PD, Swamy CV, Gananadhamu S, Srinivas R. Quantitation of acotiamide in rat plasma by UHPLC‐Q‐TOF‐MS: method development, validation and application to pharmacokinetics. Biomed Chromatogr. 2016;30:363–8.
Nolan ML, Scott LJ. Acotiamide: first global approval. Drugs. 2013;73:1377–83.
Furuta S, Kamada E, Omata T, Sugimoto T, Kawabata Y, Yonezawa K, et al. Drug–drug interactions of Z-338, a novel gastroprokinetic agent, with terfenadine, comparison with cisapride, and involvement of UGT1A9 and 1A8 in the human metabolism of Z-338. Eur J Pharmacol. 2004;497:223–31.
Tack J, Pokrotnieks J, Urbonas G, Banciu C, Yakusevich V, Bunganic I, et al. Long‐term safety and efficacy of acotiamide in functional dyspepsia (postprandial distress syndrome)—results from the European phase 3 open‐label safety trial. Neurogastroenterol Motil. 2018;30:e13284.
Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R. Minocycline and riluzole brain disposition: interactions with p‐glycoprotein at the blood–brain barrier. J Neurochem. 2007;103:164–73.
Toklu HZ, Uysal MK, Kabasakal L, Sirvanci S, Ercan F, Kaya M. The effects of riluzole on neurological, brain biochemical, and histological changes in early and late term of sepsis in rats. J Surg Res. 2009;152:238–48.
Bryson HM, Fulton B, Benfield P. Riluzole. Drugs. 1996;52:549–63.
Vangavaragu JR, Valasani KR, Fang D, Williams TD, Yan SS. Determination of small molecule ABAD inhibitors crossing blood-brain barrier and pharmacokinetics. J Alzheimer’s Dis. 2014;42:333–44.
Disanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs II: methylene blue—absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972;61:1086–90.
Walter-Sack I, Rengelshausen J, Oberwittler H, Burhenne J, Mueller O, Meissner P, et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol. 2009;65:179–89.
Peter C, Hongwan D, Küpfer A, Lauterburg B. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56:247–50.
Saini NK, Suresh PS, Lella M, Bhamidipati RK, Rajagopal S, Mullangi R. LC-MS/MS determination of tideglusib, a novel GSK-3β inhibitor in mice plasma and its application to a pharmacokinetic study in mice. J Pharm Biomed Anal. 2018;148:100–7.
Bharathy N, Svalina MN, Settelmeyer TP, Cleary MM, Berlow NE, Airhart SD, et al. Preclinical testing of the glycogen synthase kinase-3β inhibitor tideglusib for rhabdomyosarcoma. Oncotarget. 2017;8:62976–83.
Al-Majed A, Bakheit AH, Abdel Aziz HA, Alharbi H, Al-Jenoobi FI. Pioglitazone. Profiles Drug Subst Excip Relat Methodol. 2016;41:379–438.
Eckland D, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes. 2000;108:234–42.
Baldwin SJ, Clarke SE, Chenery RJ. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br J Clin Pharmacol. 1999;48:424–32.
Chinnam P, Mohsin M, Shafee LM. Evaluation of acute toxicity of pioglitazone in mice. Toxicol Int. 2012;19:250–4.
Wallach JD, Wang K, Zhang AD, Cheng D, Grossetta Nardini HK, Lin H, et al. Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ. 2020;368:l7078.
Stamford AW, Scott JD, Li SW, Babu S, Tadesse D, Hunter R, et al. Discovery of an orally available, brain penetrant BACE1 inhibitor that affords robust CNS Aβ reduction. ACS Med Chem Lett. 2012;3:897–902.
Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of Tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–108.
Hunsberger HC, Weitzner DS, Rudy CC, Hickman JE, Libell EM, Speer RR, et al. Riluzole rescues glutamate alterations, cognitive deficits, and tau pathology associated with P301L tau expression. J Neurochem. 2015;135:381–94.
Okamoto M, Gray JD, Larson CS, Kazim SF, Soya H, McEwen BS, et al. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl Psychiatry. 2018;8:1–13.
Kerr F, Sofola-Adesakin O, Ivanov DK, Gatliff J, Gomez Perez-Nievas B, Bertrand HC, et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017;13:e1006593.
Koehler D, Shah ZA, Williams FE. The GSK3beta inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochemistry Int. 2019;122:31–7.
Sereno L, Coma M, Rodriguez M, Sanchez-Ferrer P, Sanchez MB, Gich I, et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–67.
Bolos M, Fernandez S, Torres-Aleman I. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J Biol Chem. 2010;285:17693–700.
Morales-Garcia JA, Luna-Medina R, Alonso-Gil S, Sanz-Sancristobal M, Palomo V, Gil C, et al. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci. 2012;3:963–71.
Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S, Morales-Garcia JA, Martinez A, Santos A, et al. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J Neurosci. 2007;27:5766–76.
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–53.
Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2004;17:500–6.
Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–73.
Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, et al. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35:1593–604.
Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–8.
Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–46.
Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45–50.
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298:1180–8.
Edwards PD, Albert JS, Sylvester M, Aharony D, Andisik D, Callaghan O, et al. Application of fragment-based lead generation to the discovery of novel, cyclic amidine beta-secretase inhibitors with nanomolar potency, cellular activity, and high ligand efficiency. J Med Chem. 2007;50:5912–25.
Zhu Z, Sun Z-Y, Ye Y, Voigt J, Strickland C, Smith EM, et al. Discovery of cyclic acylguanidines as highly potent and selective β-site amyloid cleaving enzyme (BACE) inhibitors: part I—inhibitor design and validation. J Med Chem. 2010;53:951–65.
May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, et al. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16.
Stamford AW, Scott JD, Li SW, Babu S, Tadesse D, Hunter R, et al. Discovery of an orally available, brain penetrant BACE1 inhibitor that affords robust CNS Aβ reduction. ACS Med Chem Lett. 2012;3:897–902.
Nowak P, Cole DC, Aulabaugh A, Bard J, Chopra R, Cowling R, et al. Discovery and initial optimization of 5,5′-disubstituted aminohydantoins as potent β-secretase (BACE1) inhibitors. Bioorg Med Chem Lett. 2010;20:632–5.
Prati F, Bottegoni G, Bolognesi ML, Cavalli A. BACE-1 inhibitors: from recent single-target molecules to multitarget compounds for Alzheimer’s disease. J Med Chem. 2018;61:619–37.
Stamford A, Strickland C. Inhibitors of BACE for treating Alzheimer’s disease: a fragment-based drug discovery story. Curr Opin Chem Biol. 2013;17:320–8.
Tadano G, Komano K, Yoshida S, Suzuki S, Nakahara K, Fuchino K, et al. Discovery of an extremely potent thiazine-based β-secretase inhibitor with reduced cardiovascular and liver toxicity at a low projected human dose. J Med Chem. 2019;62:9331–7.
Hsiao C-C, Rombouts F, Gijsen HJM. New evolutions in the BACE1 inhibitor field from 2014 to 2018. Bioorg Med Chem Lett. 2019;29:761–77.
O’Neill BT, Beck EM, Butler CR, Nolan CE, Gonzales C, Zhang L, et al. Design and synthesis of clinical candidate PF-06751979: a potent, brain penetrant, β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor lacking hypopigmentation. J Med Chem. 2018;61:4476–504.
May PC, Willis BA, Lowe SL, Dean RA, Monk SA, Cocke PJ, et al. The potent BACE1 inhibitor LY2886721 elicits robust central Aβ pharmacodynamic responses in mice, dogs, and humans. J Neurosci. 2015;35:1199–210.
McKinzie DL, May PC, Boggs LN, Yang Z, Brier RA, Monk SA, et al. P1-080: nonclinical pharmacological characterization of the Bace1 inhibitor LY3202626. Alzheimer’s Dement. 2016;12:P432–3.
Johansson P, Kaspersson K, Gurrell IK, Bäck E, Eketjäll S, Scott CW, et al. Toward β-secretase-1 inhibitors with improved isoform selectivity. J Med Chem. 2018;61:3491–502.
Brodney MA, Beck EM, Butler CR, Barreiro G, Johnson EF, Riddell D, et al. Utilizing structures of CYP2D6 and BACE1 complexes to reduce risk of drug-drug interactions with a novel series of centrally efficacious BACE1 inhibitors. J Med Chem. 2015;58:3223–52.
Wu Y-J, Guernon J, Yang F, Snyder L, Shi J, McClure A, et al. Targeting the BACE1 active site flap leads to a potent inhibitor that elicits robust brain Aβ reduction in rodents. ACS Med Chem Lett. 2016;7:271–6.
Butler CR, Brodney MA, Beck EM, Barreiro G, Nolan CE, Pan F, et al. Discovery of a series of efficient, centrally efficacious BACE1 inhibitors through structure-based drug design. J Med Chem. 2015;58:2678–702.
Fujimoto K, Matsuoka E, Asada N, Tadano G, Yamamoto T, Nakahara K, et al. Structure-based design of selective β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitors: targeting the flap to gain selectivity over BACE2. J Med Chem. 2019;62:5080–95.
Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N Engl J Med. 2019;380:1483–5.
Wu YJ, Guernon J, Rajamani R, Toyn JH, Ahlijanian MK, Albright CF, et al. Discovery of furo[2,3-d][1,3]thiazinamines as beta amyloid cleaving enzyme-1 (BACE1) inhibitors. Bioorg Med Chem Lett. 2016;26:5729–31.
Pettus LH, Bourbeau MP, Bradley J, Bartberger MD, Chen K, Hickman D, et al. Discovery of AM-6494: a potent and orally efficacious β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor with in vivo selectivity over BACE2. J Med Chem. 2020;63:2263–81.
Morsy A, Trippier PC. Amyloid-binding alcohol dehydrogenase (ABAD) inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2019;62:4252–64.
Kissinger CR, Rejto PA, Pelletier LA, Thomson JA, Showalter RE, Abreo MA, et al. Crystal structure of human ABAD/HSD10 with a bound inhibitor: implications for design of Alzheimer’s disease therapeutics. J Mol Biol. 2004;342:943–52.
Li X, Wang H, Lu Z, Zheng X, Ni W, Zhu J, et al. Development of multifunctional pyrimidinylthiourea derivatives as potential anti-Alzheimer agents. J Med Chem. 2016;59:8326–44.
Xu Y-X, Wang H, Li X-K, Dong S-N, Liu W-W, Gong Q, et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;143:33–47.
Park J-E, Elkamhawy A, Hassan AHE, Pae AN, Lee J, Paik S, et al. Synthesis and evaluation of new pyridyl/pyrazinyl thiourea derivatives: neuroprotection against amyloid-β-induced toxicity. Eur J Med Chem. 2017;141:322–34.
Gazova Z, Soukup O, Sepsova V, Siposova K, Drtinova L, Jost P, et al. Multi-target-directed therapeutic potential of 7-methoxytacrine-adamantylamine heterodimers in the Alzheimer’s disease treatment. Biochim Biophys Acta Mol Basis Dis. 2017;1863:607–19.
Hroch L, Guest P, Benek O, Soukup O, Janockova J, Dolezal R, et al. Synthesis and evaluation of frentizole-based indolyl thiourea analogues as MAO/ABAD inhibitors for Alzheimer’s disease treatment. Bioorg Med Chem. 2017;25:1143–52.
Crider AM, Witt KA. Somatostatin sst4 ligands: chemistry and pharmacology. Mini Rev Med Chem. 2007;7:213–20.
Ankersen M, Crider M, Liu S, Ho B, Andersen HS, Stidsen C. Discovery of a novel non-peptide somatostatin agonist with SST4 selectivity. J Am Chem Soc. 1998;120:1368–73.
Crider AM, Liu S, Li T, Mahajan S, Ankersen M, Stidsen CE. Somatostatin receptor subtype 4 (sst4) ligands: synthesis and evaluation of Indol-3-yl- and 2-Pyridyl-Thioureas. Lett Drug Des Discov. 2004;1:84–7.
Sandoval KE, Farr SA, Banks WA, Crider AM, Morley JE, Witt KA. Somatostatin receptor subtype-4 agonist NNC 26-9100 decreases extracellular and intracellular Aβ1-42 trimers. Eur J Pharmacol. 2012;683:116–24.
Buchholz M, Hamann A, Aust S, Brandt W, Böhme L, Hoffmann T, et al. Inhibitors for human glutaminyl cyclase by structure based design and bioisosteric replacement. J Med Chem. 2009;52:7069–80.
Buchholz M, Heiser U, Schilling S, Niestroj AJ, Zunkel K, Demuth H-U. The first potent inhibitors for human glutaminyl cyclase: synthesis and structure–activity relationship. J Med Chem. 2006;49:664–77.
Hoang VH, Tran PT, Cui M, Ngo VT, Ann J, Park J, et al. Discovery of potent human glutaminyl cyclase inhibitors as anti-Alzheimer’s agents based on rational design. J Med Chem. 2017;60:2573–90.
Katyayan K, Yi P, Monk S, Cassidy K. Excretion, mass balance, and metabolism of [(14)C]LY3202626 in humans: an interplay of microbial reduction, reabsorption, and aldehyde oxidase oxidation that leads to an extended excretion profile. Drug Metab Dispos. 2020;48:698–707.
Qiu R, Ahn JE, Alexander R, Brodney MA, He P, Leurent C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamic effects of PF-06751979, a potent and selective oral BACE1 inhibitor: results from phase I studies in healthy adults and healthy older subjects. J Alzheimers Dis. 2019;71:581–95.
Matijevic M, Watanabe H, Sato Y, Bernier F, McGrath S, Burns L, et al. P4-163: a single dose of the beta-secretase inhibitor, e2609, decreases CSF bace1 enzymatic activity in cynomolgus monkeys. Alzheimers Dement. 2015;11:P841.
Timmers M, Van Broeck B, Ramael S, Slemmon J, De Waepenaert K, Russu A, et al. Profiling the dynamics of CSF and plasma Aβ reduction after treatment with JNJ-54861911, a potent oral BACE inhibitor. Alzheimers Dement. 2016;2:202–12.
Brooks AF, Jackson IM, Shao X, Kropog GW, Sherman P, Quesada CA, et al. Synthesis and evaluation of [11C]PBD150, a radiolabeled glutaminyl cyclase inhibitor for the potential detection of Alzheimer’s disease prior to amyloid β aggregation. MedChemComm. 2015;6:1065–8.
Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM, et al. Pyroglutamate-3 amyloid-β deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am J Pathol. 2013;183:369–81.
Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer’s disease–like pathology. Nat Med. 2008;14:1106–11.
Morawski M, Schilling S, Kreuzberger M, Waniek A, Jäger C, Koch B, et al. Glutaminyl cyclase in human cortex: correlation with (pGlu)-amyloid-β load and cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2014;39:385–400.
Timmers M, Streffer JR, Russu A, Tominaga Y, Shimizu H, Shiraishi A, et al. Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer’s disease: randomized, double-blind, placebo-controlled study. Alzheimers Res Ther. 2018;10:85.
Novak G, Streffer JR, Timmers M, Henley D, Brashear HR, Bogert J, et al. Long-term safety and tolerability of atabecestat (JNJ-54861911), an oral BACE1 inhibitor, in early Alzheimer’s disease spectrum patients: a randomized, double-blind, placebo-controlled study and a two-period extension study. Alzheimers Res Ther. 2020;12:58.
Benek O, Korabecny J, Soukup O. A perspective on multi-target drugs for Alzheimer’s disease. Trends Pharmacol Sci. 2020; 41:434–45.
Cummings J, Ritter A, Zhong K. Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. J Alzheimers Dis. 2018;64:S3–22.
Savelieff MG, Nam G, Kang J, Lee HJ, Lee M, Lim MH. Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis in the last decade. Chem Rev. 2018;119:1221–322.
Cummings JL, Tong G, Ballard C. Treatment combinations for Alzheimer’s disease: current and future pharmacotherapy options. J Alzheimers Dis. 2019;67:779–94.
Bachurin SO, Bovina EV, Ustyugov AA. Drugs in clinical trials for Alzheimer’s disease: the major trends. Med Res Rev. 2017;37:1186–225.
Acknowledgements
This work was supported in part by the Research Endowment funds from the Center for Drug Design (CDD) at the University of Minnesota, Minneapolis, and by the NIH grant # R01AG062469.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, H., Dronamraju, V., Xie, W. et al. Sulfur-containing therapeutics in the treatment of Alzheimer’s disease. Med Chem Res 30, 305–352 (2021). https://doi.org/10.1007/s00044-020-02687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02687-1