Abstract
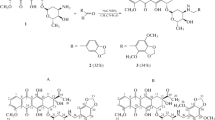
Modification of daunorubicin on its NH2 moiety is a well-established synthetic approach to obtain novel derivatives of this compound. Moreover, the daunosamine moiety of this antibiotic is considered to be the most sensitive part of a molecule in terms of biological response to chemical transformations. Using simple and effective synthetic techniques, namely, alkylation under phase-transfer catalysis and an amine addition across an activated multiple bond, a series of daunorubicin derivatives retaining the amine functionality have been obtained, which could also be used as potential precursors for further transformations.




Similar content being viewed by others
References
Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 20;333–53. https://doi.org/10.1002/1097-0142(1967)20:33.0.CO;2-K
Cortes-Funes H, Coronado C. Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol. 2007;7:56–60. https://doi.org/ 10.1007/s12012-007-0015-3
Artyushin OI, Brel VK, Moiseeva AA. Anthracycline derivatives and their anticancer activity. INEOS OPEN. 2019;2:9–18. Moscow University Chemistry Bulletin. https://doi.org/10.32931/io1902r.
Zhang YS, Zhang G, Zhang W, Luo H, Qiu L, Liu Q, et al. Synthesis and biological activities of a 3′-azido analogue of doxorubicin against drug-resistant cancer cells. Int J Mol Sci. 2012;13:3671–84. https://doi.org/10.3390/ijms13033671
Zunino F, Pratesi G, Perego P. Role of the sugar moiety in the pharmacological activity of anthracyclines: development of a novel series of disaccharide analogs. Biochem Pharm. 2001;61:933–8. https://doi.org/10.1016/s0006-2952(01)00522-6
Burke TG, Morin MJ, Sartorelli AC, Lane PE, Tritton TR. Function of the anthracycline amino group in cellular transport and cytotoxicity. Mol Pharm. 1987;31:552–6. PMID: 3472065.
Burke TG, Sartorelli AC, Tritton TR. Selectivity of the anthracyclines for negatively charged model membranes: role of the amino group. Cancer Chemother Pharm. 1988;21:274–80. https://doi.org/10.1007/BF00264191
Zunino F, Gambetta R, Di Marco A, Zaccara A. Interaction of daunomycin and its derivatives with DNA. Biochim Biophys Acta. 1972;277:489–98. https://doi.org/10.1016/0005-2787(72)90092-5
Marzin D, Jasmin C, Maral R, Mathe G. Mutagenicity of eight anthracycline derivatives in five strains of Salmonella typhimurium. Eur J Cancer Clin Oncol. 1983;19:641–7. https://doi.org/10.1016/0277-5379(83)90180-3
Umezawa K, Haresaku M, Muramatsu M, Matsushima T. Mutagenicity of anthracycline glycosides and bleomycins in Salmonella assay system. Biomed Pharmacother. 1987;41:214–8. https://doi.org/10.1002/tcm.1770060307
Moiseeva AA, Artyushin OI, Anikina LV, Brel VK. Synthesis and antitumor activity of daunorubicin conjugates with of 3,4-methylendioxybenzaldehyde. Bioorg Med Chem Lett. 2019;29:126617. https://doi.org/10.1016/j.bmcl.2019.08.021
Tong GL, Wu HY, Smith TH, Henry DW. Adriamycin analogs. 3. Synthesis of N-alkylated anthracyclines with enhanced efficacy and reduced cardiotoxicity. J Med Chem. 1979;22:912–8. https://doi.org/10.1021/jm00194a005
Masquelier M, Tirzitis G, Peterson CO, Pålsson M, Amolins A, Plotniece M, et al. Plasma stability and cytotoxicity of lipophilic daunorubicin derivatives incorporated into low density lipoproteins. Eur J Med Chem. 2000;35:429–38. https://doi.org/10.1016/s0223-5234(00)00139-2
Chaires JB, Leng F, Przewloka T, Fokt I, Ling Y-H, Perez–Solarand R, et al. Structure-based design of a new bisintercalating anthracycline antibiotic. J Med Chem. 1997;40:261–6. https://doi.org/10.1021/jm9607414
Hu GG, Shui X, Leng F, Priebe W, Chaires JB, Williams LD. Structure of a DNA–bisdaunomycin complex. Biochem. 1997;36:5940–6. https://doi.org/10.1021/bi9705218
De K, Legros J, Crousse B, Bonnet-Delpon D. Solvent-promoted and -controlled Aza-Michael reaction with aromatic amines. J Org Chem. 2009;74:6260–5. https://doi.org/10.1021/jo9012699
Semakov AV, Anikina LV, Afanasyeva SV, Pukhov SA, Klochkov SG. Synthesis and antiproliferative activity of conjugates of anthracycline antibiotics with sesquiterpene lactones. Russ J Bioorg Chem. 2018;44:538–46. https://doi.org/10.1134/S1068162018040167
Gruber BM, Anuszewska EL, Bubko I, Goździk A, Fokt I, Priebe W. Effect of structural modification at the 4, 3’, and 2’ positions of doxorubicin on topoisomerase II poisoning, apoptosis, and cytotoxicity in human melanoma cells. Arch Immunol Ther Exp. 2007;55:193–8. https://doi.org/10.1007/s00005-007-0018-6
Tsedilin AM, Fakhrutdinov AN, Eremin DB, Zalesskiy SS, Chizhov AO, Kolotyrkina NG, et al. How sensitive and accurate are routine NMR and MS measurements? Mendeleev Comm. 2015;25:454–6. https://doi.org/10.1016/j.mencom.2015.11.019
Acknowledgements
This work was supported by RFBR (№ 18-03-00073) and the scholarship of the President of the Russian Federation for young scientists and postgraduates (Competition «SP-2019», No SP-2717.2019.4). NMR studies, spectral characterization, and elemental analysis were performed with the financial support from Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brel, V.K., Moiseeva, A.A., Artyushin, O.I. et al. Simple methods of modification of daunorubicin on the daunosamine nitrogen atom. Med Chem Res 30, 564–573 (2021). https://doi.org/10.1007/s00044-020-02664-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02664-8