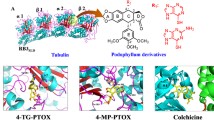
Abstract
A series of podophyllotoxin derivatives (M1–M16) that were selectively acylated by various phenoxy acids at the C-4 position of podophyllotoxin were synthesized, and their biological activities were also evaluated. Among them, compound M4 showed the most potent anti-cancer activity against HeLa cells with an IC50 value of 1.64 ± 0.41 μM. Additionally, flow cytometry analysis results indicated that it could cause a remarkable cell cycle arrest at G2/M phase, but the effect on apoptosis inducing was not significant. Moreover, the expression of cell cycle relative protein CDK1 was up regulated while cyclin B1 and Cdc25C, two proteins required for mitotic initiation were down regulated. Furthermore, the confocal assay and extracellular polymerized tubulin expression analysis also demonstrated that M4 was a potent tubulin polymerization inhibitor and the effect was comparable to that of colchicine. Finally, docking simulation results showed that M4 could nicely bind to the colchicine binding site of tubulin which further comfirmed the tubulin inhibiton activity of M4.









Similar content being viewed by others
References
Abad A, Lópezpérez JL, Olmo ED, Garcíafernández LF, Francesch A, Trigili C, Barasoain I, Andreu JM, Díaz JF, Feliciano AS (2012) Synthesis and antimitotic and tubulin interaction profiles of novel pinacol derivatives of podophyllotoxins. J Med Chem 55:6724–6737
Amos LA (2004) Microtubule structure and its stabilization. Org Biomol Chem 2:2153–2160
Arpicco S, Dosio F, Stella B, Cattel L (2011) Anticancer prodrugs: an overview of major strategies and recent developments. Curr Top Med Chem 11:2346–2381
Bohlin L, Rosen B (1996) Podophyllotoxin derivatives: drug discovery and development. Drug Discov Today 1:343–351
Dhamodharan R, Jordan M, Thrower D, Wilson L, Wadsworth P (1995) Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol Biol Cell 6:1215–1229
Eckhardt S (2002) Recent progress in the development of anticancer agents. Current medicinal chemistry. Anti-cancer Agents 2:419–439
Gerdes JM, Katsanis N (2005) Small molecule intervention in microtubule-associated human disease. Hum Mol Genet 14:R291–R300
Gourley M, Williamson JS (2000) Angiogenesis new targets for the development of anticancer chemotherapies. Curr Pharm Des 6:417–439
Hamel E (1996) Antimitotic natural products and their interactions with tubulin. Med Res Rev 16:207–231
Hartmann DJT, Lipp HP (2006) Camptothecin and podophyllotoxin derivatives. Drug Saf 29:209–230
Holthuis JJM (1988) Etoposide and teniposide. Int J Clin Pharm 10:101–116
Honore S, Pasquier E, Braguer D (2005) Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci 62:3039–3056
Hyder I, Yedlapudi D, Kalivendi SV, Khazir J, Ismail T, Nalla N, Miryala S, Sampath Kumar HM (2015) Synthesis and Biological evaluation of novel 4β-[(5-substituted)-1,2,3,4-tetrazolyl] podophyllotoxins as anticancer compounds. Bioorg Med Chem Lett 25:2860–2863
Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L (1996) Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56:816–825
Kamal A, Reddy MK, Shaik TB, Rajender, Srikanth YV, Reddy VS, Kumar GB, Kalivendi SV (2012) Synthesis of terphenyl benzimidazoles as tubulin polymerization inhibitors. Chem Inform 50:9–17
Kamal A, Shaik AB, Jain N, Kishor C, Nagabhushana A, Supriya B, Kumar GB, Chourasiya SS, Suresh Y, Mishra RK (2015a) Design and synthesis of pyrazole–oxindole conjugates targeting tubulin polymerization as new anticancer agents. Eur J Med Chem 92:501–513
Kamal A, Shaik AB, Polepalli S, Kumar, Reddy VS, Mahesh R, Garimella S, Jain N (2015b) Synthesis of arylpyrazole linked benzimidazole conjugates as potential microtubule disruptors. Bioorg Med Chem 23:1082–1095
Khaled M, Jiang ZZ, Zhang LY (2013) Deoxypodophyllotoxin: a promising therapeutic agent from herbal medicine. J Ethnopharmacol 149:24–34
Kozielski F, Arnal I, Wade RH (1998) A model of the microtubule–kinesin complex based on electron cryomicroscopy and X-ray crystallography. Curr Biol 8:191–198
Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W (2012) Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149:1269–1283
Lin HY, Bai LF, Wang F, Wu X, Han L, Baloch SK, Yang YH, Wang XM (2015) Semi-synthesis and anti-lung cancer activity evaluation of aryl dihydrothiazol acyl podophyllotoxin ester derivatives. RSC Adv 5:27775–27784
Liu L, Wang D, Wang J, Ji H, Zhang Y (2015a) NOAD, a novel nitric oxide donor, induces G2/M phase arrest and apoptosis in human hepatocellular carcinoma Bel-7402 cells. Toxicol in Vitro 29:1289–1297
Liu YQ, Tian J, Qian K, Zhao XB, Morris-Natschke SL, Yang L, Nan X, Tian X, Lee KH (2015b) Recent progress on C-4-modified podophyllotoxin analogs as potent antitumor agents. Med Res Rev 35:1–62
Ma Y, Fang S, Li H, Han C, Lu Y, Zhao Y, Liu Y, Zhao C (2013) Biological evaluation and molecular modeling study of podophyllotoxin derivatives as potent inhibitors of tubulin polymerization. Chem Biol Drug Des 82:12–21
Méchali M, Lutzmann M (2008) The cell cycle: now live and in color. Cell 132:341–343
Mokale SN, Sanap PT, Shinde DB (2010) Synthesis and hypolipidemic activity of novel 2-(4-(2-substituted aminothiazole-4-yl) phenoxy) acetic acid derivatives. Chem Inform 45:3096–3100
Nam NH (2003) Combretastatin A-4 analogs as antimitotic antitumor agents. Curr Med Chem 10:1697–1722
Pettit GR, Toki B, Herald DL, Verdier-Pinard P, Boyd MR, Hamel E, Pettit RK (1998) Antineoplastic agents. 379. Synthesis of phenstatin phosphate1a. J Med Chem 41:1688–1695
Stoner EJ, And PJS, Cooper AJ (1999) Synthesis of ABT-378, an HIV protease inhibitor candidate: avoiding the use of carbodiimides in a difficult peptide coupling. Org Process Res Dev 3:145–148
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A (2015) Global cancer statistics, 2012. Cancer J Clin 65:87–108
Wang F, Wang X, Zhang MX, Yang YH, Zhu HL (2015) Synthesis, biological evaluation and molecular modeling of 1 H-benzo [d] imidazole derivatives as novel anti-tubulin polymerization agents. RSC Adv 5:74425–74437
Zuo D, Guo D, Jiang X, Guan Q, Qi H, Xu J, Li Z, Yang F, Zhang W, Wu Y (2014) 3-(3-hydroxy-4-methoxyphenyl)-4-(3,4,5-trimethoxyphenyl)- 1,2,5-selenadiazole (G-1103), a novel combretastatin A-4 analog, induces G2/M arrest and apoptosis by disrupting tubulin polymerization in human cervical HeLa cells and fibrosarcoma HT-1080 cells. Chem Biol Interact 227C:7–17
Acknowledgements
The authors are grateful to the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R27), the National Natural Science Foundation of China (31470384, 31171161, and 31670298), and the Fundamental Research Funds for the Central Universities (020814380002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Cui Hu and Xiang Zhu contributed equally to this work.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Hu, C., Zhu, X., Wang, GH. et al. Design, synthesis and anti-cancer evaluation of novel podophyllotoxin derivatives as potent tubulin-targeting agents. Med Chem Res 27, 351–365 (2018). https://doi.org/10.1007/s00044-017-1992-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1992-9