Abstract
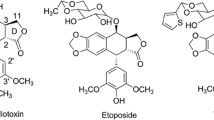
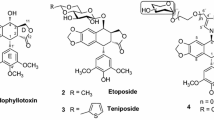
Twenty novel podophyllotoxin derivatives(1―20) were designed and synthesized. The anti-proliferation activities of these compounds were evaluated against three human cancer cell lines(HepG2, Calu-1 and MCF-7) using podophyllotoxin and Combretastatin A4(CA-4) as positive controls. Among all the compounds, compound 2 displayed more significant anti-proliferation activities against MCF-7 and Calu-1 cell lines and showed lower toxicity towards non-cancer cells. Furthermore, the cell cycle and apoptosis analysis results revealed that compound 2 can cause cell arrest at G2/M phase, leading to cancer cell apoptosis. Meanwhile, it can also reduce the adhesive ability of Calu-1 cells to fibronectin and laminin. The docking simulation results demonstrated that compound 10 can nicely bind to the colchicine site of tubulin. The podophyllotoxin derivatives are worthy to be further investigated to obtain more potent anti-cancer drugs.
Similar content being viewed by others
References
Karikas G. A., J. Buon., 2010, 15(4), 627
Mishra B. B., Tiwari V. K., Eur. J. Med. Chem., 2011, 46, 4769
Zhang P. N., Li Z. F., Zhao C. Q., Nat. Prod. Res. Dev., 2004, 16(1), 80
Gordaliza M., Clin. Transl. Oncol., 2007, 9(12), 767
Shang H., Chen H., Zhao D. M., Tang X. W., Liu Y. F., Pan L., Cheng M. S., Arch. Pharm. Chem. Life Sci., 2012, 345(1), 43
Bohlin L., Rosen B., Drug Discov. Today, 1996, 1(8), 343
Kumar A., Kumar V., Alegria A. E., Malhotra S. V., Curr. Med. Chem., 2011, 18(25), 3853
Sang C. Y., Liu J. F., Qin W. W., Zhao J., Hui L., Jin Y. X., Chen S. W., Eur. J. Med. Chem., 2013, 70, 59
You Y., Curr. Pharm. Des., 2005, 11(13), 1695
Liu Y. Q., Yang L., Tian X., Curr. Bioact. Compd., 2007, 3(1), 37
Gordaliza M., Castro M. A., del Corral J. M., Feliciano A. S., Curr. Pharm. Des., 2000, 6(18), 1811
Berkowitz D. B., Maeng J. H., Dantzig A. H., Shepard R. L. Norman B. H., J. Am. Chem. Soc., 1996, 118, 9426
Kamal A., Tamboli J. R., Nayak V. L., Adil S. F., Vishnuvardhan M. V., Ramakrishna S., Bioorg. Med. Chem., 2014, 22(9), 2714
Kamal A., Tamboli J. R., Vishnuvardhan M. V., Adil S. F., Nayak V. L., Bioorg. Med. Chem. Lett., 2013, 23, 273
Kamal A., Suresh P., Ramaiah M. J., Srinivasa Reddy T., Kapavarapu R. K., Rao B. N., Imthiajali S., Lakshminarayan R. T., Bioorg. Med. Chem., 2013, 21(17), 5198
Wang H. W., Nogales E., Nature, 2005, 435, 911
Gourley M., Williamson J. S., Curr. Pharm. Des., 2000, 6, 417
Lin C. M., Ho H. H., Pettit G. R., Hamel E., Biochemistry, 1989, 28(17), 6984
Pettit G. R., Singh S. B., Hamel E., Lin C. M., Alberts D. S., Experientia, 1989, 45(2), 209
Pettit G. R., Toki B., Herald D. L., Verdier-Pinard P., Boyd M. R., Hamel E., Pettit R. K., J. Med. Chem., 1998, 41(10), 1688
Nam N. H., Curr. Med. Chem., 2003, 10(17), 1697
Abad A., López Pérez J. L., del Olmo E., García-Fernández L. F., Francesch A., Trigili C., Barasoain I., Andreu J. M., Díaz J. F., San Feliciano A., J. Med. Chem., 2012, 55(15), 6724
Castro M. A., del Corral J. M. M., Gordaliza M., García P. A., Gómez-Zurita M. A., García-Grávalos M. D., de la Lglesia-Vicente J., Gajate C., An F. Y., Mollinedo F., San Feliciano A., J. Med. Chem., 2004, 47(5), 1214
Ettinger D. S., Finkelstein D. M., Ritch P. S., Lincoln S. T., Blum R. H., Lung Cancer, 2002, 37(3), 311
Che C., Yang G. Q., Thiot C., Lacoste M. C., Currie J. C., Demeule M., Regina A., Beliveau R., Castaigne J. P., J. Med. Chem., 2010, 53(7), 2814
Qian Y., Zhang H. J., Lv P. C., Zhu H. L., Bioorg. Med. Chem., 2010, 18(23), 8218
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.21376112), the Natural Science Foundation of the Colleges and Universities in Jiangsu Province, China(No.13KJB350002) and the Natural Science Foundation of the Colleges and Universities Major Project in Jiangsu Province, China(No.15KJA350001).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bai, L., Wang, R., Zou, Y. et al. Design, synthesis and antitubulin activity of novel podophyllotoxin derivatives as potent anticancer agent. Chem. Res. Chin. Univ. 31, 964–969 (2015). https://doi.org/10.1007/s40242-015-5325-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-015-5325-6