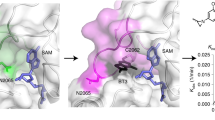
Abstract
NSD1, NSD2/MMSET/WHSC1, and NSD3/WHSC1L1 make up the nuclear receptor-binding Su(var)3-9, Enhancer-of-zeste and Trithorax domain family of histone methyltransferases, which are essential for regulating the chromatin. Abnormalities in histone methyltransferases function are increasingly being found to be associated with numerous pathological conditions, including carcinogenesis and tumor progression. NSD1, NSD2, and NSD3 are oncoproteins aberrantly expressed in numerous cancers in which selective inhibition may offer therapeutic opportunities, especially in cases of conditions with poor prognoses such as multiple myeloma. Histone methyltransferase inhibitors are scarce and selective inhibitors are being explored. NSD inhibitors are urgently needed. BIX-01294 is a G9a-like protein/G9a histone methyltransferase inhibitor commonly used to modulate H3K9 methylation in the context of cell reprogramming and cancer biology with in vitro IC50 values of 0.7 and 1.9 μM, respectively. Since the catalytic Su(var)3-9, Enhancer-of-zeste and Trithorax domain of the NSDs is highly related to that of G9a-like protein and G9a, we investigated the potentially differential NSDs inhibition by BIX-01294. In this study, we identified BIX-01294 as an NSD in vitro hit compound that differentially inhibits H3K36 methylation by NSD1, NSD2, and NSD3 with IC50 values of 40 ~ 112 μM. Furthermore, we investigated the molecular basis of inhibition by BIX-01294 on the NSDs and discuss the prospects of BIX-01294 derivatives for selective NSD inhibition.




Similar content being viewed by others
Abbreviations
- SET :
-
Su(var)3-9, Enhancer-of-zeste and Trithorax
- NSD :
-
Nuclear receptor-binding SET domain
- HMTase :
-
Histone lysine methyltransferase
- AdoMet :
-
S-adenosylmethionine
References
Allali-Hassani A, Kuznetsova E, Hajian T, Wu H, Dombrovski L, Li Y, Graslund S, Arrowsmith CH, Schapira M, Vedadi M (2014) A basic post-SET extension of NSDs is essential for nucleosome binding in vitro. J Biomol Screen 19(6):928–935
Angrand PO, Apiou F, Stewart AF, Dutrillaux B, Losson R, Chambon P (2001) NSD3, a new SET domain-containing gene, maps to 8p12 and is amplified in human breast cancer cell lines. Genomics 74(1):79–88
Chinnaiyan AM, Lnu S, Cao Q, Asangani I (2011) Compositions and methods for inhibiting MMSET. USA Patent US 20110207198 A1, filled 11 Feb 2011, issued 25 Aug 2011
Baujat G, Rio M, Rossignol S, Sanlaville D, Lyonnet S, Le Merrer M, Munnich A, Gicquel C, Cormier-Daire V, Colleaux L (2004) Paradoxical NSD1 mutations in Beckwith-Wiedemann syndrome and 11p15 anomalies in Sotos syndrome. Am J Hum Genet 74(4):715–720
Chang Y, Zhang X, Horton JR, Upadhyay AK, Spannhoff A, Liu J, Snyder JP, Bedford MT, Cheng X (2009) Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol 16(3):312–317
Chen P, Yao JF, Huang RF, Zheng FF, Jiang XH, Chen X, Chen J, Li M, Huang HF, Jiang YP, Huang YF, Yang XY (2015) Effect of BIX-01294 on H3K9me2 levels and the imprinted gene Snrpn in mouse embryonic fibroblast cells. Biosci Rep 35(5):e00257(1–9)
Cui J, Sun W, Hao X, Wei M, Su X, Zhang Y, Su L, Liu X (2015) EHMT2 inhibitor BIX-01294 induces apoptosis through PMAIP1-USP9X-MCL1 axis in human bladder cancer cells. Cancer Cell Int 15(1):4
di Luccio E (2015a) Inhibition of nuclear receptor binding SET domain 2/multiple myeloma SET domain by LEM-06 implication for epigenetic cancer therapies. J Cancer Prev 20(2):113–120
di Luccio E, Koehl P (2012) The H-factor as a novel quality metric for homology modeling. J Clin Bioinform 2(1):18
di Luccio E, Morishita M, Lee TH (2015b) Novel histone methyl transferase inhibitor and use thereof. PCT patent WO2015152437, filled 4 Jan 2014, issued 10 Aug 2015
Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, van Galen JF, Beverloo HB, Sonneveld E, Kaspers GJ, Trka J, Baruchel A, Zimmermann M, Creutzig U, Reinhardt D, Pieters R, Valk PJ, Zwaan CM (2011) NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood 118(13):3645–3656
Huang J, Zhang H, Yao J, Qin G, Wang F, Wang X, Luo A, Zheng Q, Cao C, Zhao J (2016) BIX-01294 increases pig cloning efficiency by improving epigenetic reprogramming of somatic cell nuclei. Reproduction 151(1):39–49
Jaju RJ, Fidler C, Haas OA, Strickson AJ, Watkins F, Clark K, Cross NC, Cheng JF, Aplan PD, Kearney L, Boultwood J, Wainscoat JS (2001) A novel gene, NSD1, is fused to NUP98 in the t(5;11)(q35;p15.5) in de novo childhood acute myeloid leukemia. Blood 98(4):1264–1267
Kang D, Cho HS, Toyokawa G, Kogure M, Yamane Y, Iwai Y, Hayami S, Tsunoda T, Field HI, Matsuda K, Neal DE, Ponder BA, Maehara Y, Nakamura Y, Hamamoto R (2013) The histone methyltransferase Wolf-Hirschhorn syndrome candidate 1-like 1 (WHSC1L1) is involved in human carcinogenesis. Genes Chromosomes Cancer 52(2):126–139
Krivov GG, Shapovalov MV, Dunbrack Jr. RL (2009) Improved prediction of protein side-chain conformations with SCWRL4. Proteins 77(4):778–795
Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T (2007) Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell 25(3):473–481
Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, Ohashi H, Naritomi K, Tsukahara M, Makita Y, Sugimoto T, Sonoda T, Hasegawa T, Chinen Y, Tomita Ha HA, Kinoshita A, Mizuguchi T, Yoshiura KK, Ohta T, Kishino T, Fukushima Y, Niikawa N, Matsumoto N (2002) Haploinsufficiency of NSD1 causes Sotos syndrome. Nat Genet 30(4):365–366
Lee JY, Lee SH, Heo SH, Kim KS, Kim C, Kim DK, Ko JJ, Park KS (2015) Novel function of lysine methyltransferase G9a in the regulation of Sox2 protein stability. PLoS One 10(10):e0141118
Loh SW, Ng WL, Yeo KS, Lim YY, Ea CK (2014) Inhibition of euchromatic histone methyltransferase 1 and 2 sensitizes chronic myeloid leukemia cells to interferon treatment. PLoS One 9(7):e103915
Martinez-Garcia E, Popovic R, Min DJ, Sweet SM, Thomas PM, Zamdborg L, Heffner A, Will C, Lamy L, Staudt LM, Levens DL, Kelleher NL, Licht JD (2011) The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood 117(1):211–220
Mirabella F, Wu P, Wardell CP, Kaiser MF, Walker BA, Johnson DC, Morgan GJ (2013) MMSET is the key molecular target in t(4;14) myeloma. Blood Cancer J 3:e114
Morishita M, di Luccio E (2011a) Cancers and the NSD family of histone lysine methyltransferases. Biochim Biophys Acta 1816(2):158–163
Morishita M, di Luccio E (2011b) Structural insights into the regulation and the recognition of histone marks by the SET domain of NSD1. Biochem Biophys Res Commun 412(2):214–219
Morishita M, Mevius D, di Luccio E (2014) In vitro histone lysine methylation by NSD1, NSD2/MMSET/WHSC1, and NSD3/WHSC1L. BMC Struct Biol 14(1):25
Pei H, Zhang L, Luo K, Qin Y, Chesi M, Fei F, Bergsagel PL, Wang L, You Z, Lou Z (2011) MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 470(7332):124–128
Shin JH, Kim PS, Kim ES, Park SJ, Jo YK, Hwang JJ, Park TJ, Chang JW, Seo JH, Cho DH (2015) BIX-01294-induced autophagy regulates elongation of primary cilia. Biochem Biophys Res Commun 460(2):428–433
Tao H, Li H, Su Y, Feng D, Wang X, Zhang C, Ma H, Hu Q (2014) Histone methyltransferase G9a and H3K9 dimethylation inhibit the self-renewal of glioma cancer stem cells. Mol Cell Biochem 394(1–2):23–30
Tisi D, Chiarparin E, Tamanini E, Pathuri P, Coyle JE, Hold A, Holding FP, Amin N, Martin AC, Rich SJ, Berdini V, Yon J, Acklam P, Burke R, Drouin L, Harmer JE, Jeganathan F, van Montfort RL, Newbatt Y, Tortorici M, Westlake M, Wood A, Hoelder S, Heightman TD (2016) Structure of the epigenetic oncogene MMSET and inhibition by N-alkyl sinefungin derivatives. ACS Chem Biol 11(11):3093–3105
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Upadhyay AK, Rotili D, Han JW, Hu R, Chang Y, Labella D, Zhang X, Yoon Y-s, Mai A, Cheng X (2012) An analog of BIX-01294 selectively inhibits a family of histone H3 lysine 9 Jumonji demethylases. J Mol Biol 416(3):319–327
Wiesmann UN, DiDonato S, Herschkowitz NN (1975) Effect of chloroquine on cultured fibroblasts: release of lysosomal hydrolases and inhibition of their uptake. Biochem Biophys Res Commun 66(4):1338–1343
Acknowledgements
Ms. Yeonjeong Roh is acknowledged for her technical support in processing the MALDI-TOF mass spectrometry data and for her assistance for the Fig. 1c.
Funding
This study is supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology to EDL (2015K2A1B8064967) and MM (2016R1D1A1B01014286). The School of Life Sciences, BK21 Plus KNU Creative BioResearch Group at Kyungpook National University is acknowledged for its support.
Author contributions
EDL and MM conceived and designed the experiments; MM, DM, YS, and SZ performed the experiments; EDL, MM, DM and SZ analyzed the data; and DM, YS, MM, and EDL drafted the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Masayo Morishita and Damiaan E. H. F. Mevius contributed equally to this work
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Morishita, M., Mevius, D.E.H.F., Shen, Y. et al. BIX-01294 inhibits oncoproteins NSD1, NSD2 and NSD3. Med Chem Res 26, 2038–2047 (2017). https://doi.org/10.1007/s00044-017-1909-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1909-7