Abstract
The Central Australian desert ant Melophorus bagoti maintains ground-nesting colonies in the semi-desert habitat. These ants manage waste by dumping items outside the nest. To examine this process, we placed organic and non-organic materials that are associated with either low or high pathogenic risk around or into the nest and observed the nest’s response. We found that generally, ants dumped high-pathogenic-risk materials (dead larvae, dead ants of the colony, foraged food, moth, and non-nest cicada exoskeleton) further from the nest than low-pathogenic-risk ones (sand, buffel grass, cookies), with the exception of (organic) larval shells from their own nest, which were also dumped close to the nest. This pattern of dumping suggests that these ants choose their dumping distance based on how spoilable the experimental materials are.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Significance statement
Eusocial insects maintain hygiene in their nests with cleaning behaviours. One such behaviour is the disposal of refuse material (food waste), dead nestmate bodies, and other waste outside the nest. We observed the heat-loving Australian desert ant, the red honey ant, dump experimentally provided materials placed in or near their nest. The ants carried all experimental materials in their mandibles for some distance from the entrance and dumped them with a stereotypical motion. Materials associated with more pathogenic risk, such as dead larvae and moths, were dumped at longer distances than were materials of low pathogenic risk, such as grass and non-organic sand. Spoilability of the dumped material determined dumping distance, suggesting that dumpers were sensitive to the possible harm of dumped materials’ proximity to the nest.
Introduction
Waste is a source for disease (Kannowski 1972; Visscher 1983), and as a result of these risks, many animals have evolved behavioural strategies for removing waste items from their burrows or nests (López-Riquelme and Fanjul-Moles 2013). Eusocial societies, living in large multi-member colonies, generate a large amount of waste material that includes the excreta of the members of the colony, food waste, larval cocoon casings, and their own dead members (ants: (Hölldobler and Wilson 1977; Hölldobler and Wilson 1990; Möglich and Hölldobler 1975; Rettenmeyer 1963; Weiss 2006; stingless bees: Kolmes and Sommeijer 1992; other subsocial insects: Tallamy and Wood 1986). Such waste materials in the proximity of colony members create a potential source of infection and the spread of fungal, bacterial, or parasitic diseases (Cremer 2019).
To mitigate the elevated risk of diseases stemming from colony waste, social insects have evolved collective nest hygiene behaviours as part of their social immunity (Cremer 2019). In Apis dorsata, healthy adult workers’ defaecation rarely occurs within nests; the rectum is significantly distended in winter and the bees defaecate in early spring days on short cleansing flights (Lindauer 1975). In vertebrate animals, naked mole-rats, the best known eusocial mammal, live in colonies containing several subordinate females that help to take care of the pups of the queen (Watarai et al. 2018). Naked mole rat colonies exhibit highly structured organisations of their nest chambers with designated toilet chambers for defaecation (Yu et al. 2017). The pregnant dominant female manipulates subordinates to partake in alloparental care of the pups using chemical cues transmitted in her faeces. Naked mole-rats eat faeces regularly to supplement their diet, re-absorbing leftover nutrients. Several ants dispose of their faeces systematically. Leaf-cutting ants dig special underground waste disposal chambers (Atta cephalotes) or maintain outside waste dumps (Atta colombica and A. mexicana) (Hart and Ratnieks 2001). Fire ant (Solenopsis invicta) larvae produce droplets which consist of excreta. These droplets are not solicited by workers, but are picked up by passing ants and carried out from the nest (Porter 1984). Some ants defaecate in particular areas such as the borders of the nest or in designated refuse dumps. Social insects deal with waste by several methods, including necrophobia (avoidance of dead or injured conspecifics), intraspecific necrophagy (cannibalism of dead conspecifics), burial behaviour, and necrophoresis (the removal of dead conspecifics) (López-Riquelme and Fanjul-Moles 2013).
Many nesting insects remove more than dead conspecifics. Besides dead nestmates (Diez et al. 2014; Pull et al. 2018), workers also remove food waste and foreign materials (Czaczkes et al. 2015; Hart and Ratnieks 2002). The invasive garden ants, Lasius neglectus, detect and prevent infected brood from causing a systemic colony infection by employing an efficient multicomponent behaviour called destructive disinfection (Pull et al. 2018). These ants specifically identify infected pupae during the pathogen’s non-contagious incubation period, utilising chemical ‘sickness cues’ emitted by pupae. They then remove the pupal cocoon, perforate its cuticle and administer antimicrobial poison, which enters the body and prevents pathogen replication from the inside out. In Cataglyphis bicolor, a desert ant, workers carry dead ants or larvae away from the nest and drop them up to 35 m from the nest (Harkness 1977). Red honey ants Melophorus bagoti, the study species in this account, show two distinct types of waste management strategies: transporting waste materials away from the nest during the summer months when the nest is active above ground and also depositing waste in latrine channels/tunnels in the underground, 10–12 cm below ground level (personal observations), likely throughout the year because the excavation on which the observations were based was done in the southern summer.
M. bagoti colonies are widespread in the Central Australian semi-desert. They forage solitarily during the hot summer days, scavenging dead insects and collecting seeds, sugary plant exudates, and other miscellaneous items (Muser et al. 2005; Schultheiss and Nooten 2013; Sommer and Wehner 2012). While we know that they navigate using path integration and terrestrial visual cues (Freas et al. 2019), as yet little is known about their waste management strategies. Here we studied how M. bagoti workers react to a variety of ‘waste’ materials placed near or in their nest.
In this study, we tested the nest maintenance behaviour of M. bagoti, focusing on their propensity to remove waste or foreign items when such items are placed at the nest entrance and dump them away from the nest. Dumping refers to transporting an object outside of the nest and dropping it on the ground. We tested whether ants’ dumping differs depending on the type of material encountered by experimentally placing materials in or at the nest entrance. We tested whether the ants reacted differently to pathogenic risk in the waste materials they removed from the nest, predicting that higher-risk waste would be carried farther from the nest entrance before being dumped. Lacking a lab in the field for testing for pathogens, we categorised the pathogenicity or pathogen risk level of the substances based on the proportion of animal materials contained in the item. Dead ants and larvae from the nest, larva cocoons, cicada shells, and scavenged arthropod food that foragers had brought back consisted entirely of animal materials and were classified as high pathogenic risk. Buffel grass and sand pellets contained no animal materials and were classified as low pathogenic risk. Cookie crumbs contained only a small amount of animal food (milk solids) and were found unrefrigerated on supermarket shelves; they were classified as being of low pathogenic risk to the colony as well.
Methods
The experiments with M. bagoti took place from January to March 2018 on a field site with tussocks of buffel grass (Pennisetum cenchroides) interspersed with bushes, Acacia spp., and scattered large Eucalyptus trees on the outskirts of Alice Springs, Northern Territory, Australia (Deeti and Cheng 2021a; Deeti and Cheng 2021b; Deeti et al. 2020). We designed the experiment to examine dumping behaviour at two monodomus nests (locations at 23°45.448′ S, 133° 52.908′ E and 23° 45.497′ S, 133° 52.971′ E).
Experimental materials were divided into two categories. Category 1 consisted of animal-based organic materials classified as of high pathogenic risk: locust moths (Chortoicetes terminifera), dead ants and larvae from the nest, foraged food, cicada shells (Cyclochila australasiae), and empty cocoons (waste left from pupated larvae) of M. bagoti. The foraged food was taken from outbound workers carrying food, so that the food was presumed to be on the way to being dumped. We checked to ensure that what was being carried was food and not inorganic materials such as sand pellets. Category 2 consisted of plant-based organic and inorganic materials: buffel grass, cookie crumbs (Arnott’s Granita), and sand pellets. The cookie crumbs were made up of wheat flour, vegetable oil, sugar, wheatmeal (which contains wheat), wheat flakes, golden syrup, salt, malt extract (from barley), baking powder, food acid (citric acid), emulsifier (soy lecithin), natural flavours, food colour (paprika extract), antioxidant (E307b from soy), and milk solids (fats 20.9 g/100 g; protein 7.4 g/100 g). Despite these cookies containing a small quantity of animal product, given the overall makeup of these cookies, we categorized them as plant-based organic materials and thus low pathogenic risk to the ants. When foragers came out in search for food, they usually found the experimentally provided buffel grass, cicada shells, dead moths, and cookie crumbs. These buffel grass, cicada shells, moths (Fig. 1D), and cookie crumbs (Fig. 1C) were placed around the nest within ~ 5 cm from the nest entrance in eight cardinal directions of N, NE, E, etc. (Fig. 1C, D, Table 1). We placed the cookie crumbs, moths, foraged food pieces together or alone within the 5 cm area at the same time on each day of experimentation. Whenever the ants took away the experimental items, we replaced those items with another item. Workers, which we call dumpers, regularly carry out empty cocoons of M. bagoti larvae and sand pellets from inside the nest and dumping them. For this reason, the experimental empty cocoons and sand pellets were dropped into the nest entrance. These items were dropped into the nest one at a time. Once the removal of one experimental item was observed, the next item was placed into the nest. The experimental sand pellets looked different from the sand pellets inside the colony because the former stemmed from outside the colony and are not coated with ants’ saliva and enzymes. All the experimental materials and times (N = 16) of testing were chosen at random, constrained by the activity schedules of the ants. The experiment went on over multiple non-consecutive days until we had at least eight replications for each material from each nest. All the ants carrying experimental materials were followed individually. We measured the distance at which ants dumped experimental materials.
Illustration of different experimental materials placed or dumped around the nest entrance. A Cookie pieces distributed around the nest in eight cardinal directions. B Black circle indicating an ant carrying out a cookie piece that then switched to carrying a dead moth C A dead moth was taken away by workers (circle denotes ants picking up and carrying the dead moth with their mandibles). D Black circle denotes a worker taking a dead ant away from the nest
We calculated the mean dumping distances of each experimental material of the two categories from the two nests. The differences in the distances across test conditions were analysed. In statistical analysis, the two nests formed a factor. Each dumping event was considered independent for statistical analyses, even though some animals might have participated in multiple bouts of dumping. It was difficult to catch dumping ants for identification (by painting them) before they returned the often short distance to their nest. The experiment was conducted over multiple non-consecutive days to gain a good degree of true independence in the behaviour. Dumping distances were transformed to a log scale. The Brown-Forsythe test for heterogeneity of variance of the log-transformed data for different materials found significant heterogeneity of variance (df1 = 7, df2 = 55.3, p ≤ 0.0005). We thus conducted a Welch’s ANOVA (two-way) on log-transformed data, which is suitable for data with significant heterogeneity of variance, followed by Games-Howell post-hoc tests (also suitable for conditions with heterogeneity of variance) for a pairwise comparison of the experimental materials’ dumping distances. We predicted longer dumping distances for the materials categorised as pathogenic. We report distances from the nest entrance as means ± standard deviation in meters to the nearest cm. We also performed a separate Welch’s ANOVA with categories as a factor and materials as nested within categories.
Results
We observed that whenever these ants removed items from, or around the nest, they followed a stereotypical procedure: they gripped an item with their mandibles, re-positioned it until it could be lifted from the ground, then raised it. Once raised, ants walked with the object for the intended dumping distance and then tossed it with a stereotypical lunge (https://osf.io/uzw9x/files/). Ants always responded to our experimentally introduced material by dumping it away from the nest. The distance at which they dumped an item, however, varied by material type.
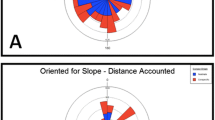
The Welch’s ANOVA comparing our a priori categories showed no significant difference between the distance of dumped group-1 items as compared to group-2 (F (1, 119) = 0.018, p = 0.916). Within categories, however, significant differences were found (F (7, 113) = 8.31, p = 0.0005). We then conducted an ANOVA comparing all experimental materials, without the category factor. The ANOVA found a significant effect of the experimental materials (F (7, 113) = 161.28, p < 0.0005), but no significant effect of nest (F = 6.07, p = 0.058) and no significant interaction between nest and experimental materials (F = 6.57, p = 0.131). Pairwise Games–Howell HSD comparisons across materials revealed 3 groups, statistically speaking: a short-distance group (Buffel grass: 0.06 m ± 0.20 m, and Sand: 0.06 m ± 0.23 m), two items that differed from one another in the intermediate distance group (Empty cocoon: 0.20 m ± 0.07 m, and Cookie: 1.1 m ± 0.46 m), and a long-distance group (Cicada shell: 5.02 m ± 1.25 m, Moth: 6.06 m ± 1.6 m, Foraged food: 6.56 m ± 1.24 m, and Dead ants 6.58 ± 1.03 m, Fig. 2, Table S1). The post-hoc comparisons between experimental items can be summarised as Buffel grass, Sand < Empty cocoon < Cookie < Cicada shell, Moth, Foraged food, Dead ants, with “ < ” indicating a significant difference (Table S1). All the materials classified as low pathogenic risk fell in the short-distance or intermediate groups. One material classified as high pathogenic risk in our predictions, larva cocoon, belonged to the intermediate distance group, while the rest of the high-pathogenic-risk waste materials fell into the long-distance group.
Dumping distances from the nest entrance from two different nests. Boxes show medians and upper and lower quartiles; whiskers extend to the minimum to maximum values. The distance from the nest entrance at which each experimental material was dumped is shown on a logarithmic scale but with linear axis units. Pairs of materials differ significantly by the Games–Howell post hoc test (p < 0.05) if they do not share a letter in common
Discussion
Except for the case of larva cocoons and cookie crumbs, our results on dumping distances supported our initial predictions based on our assessment of potential pathogenic risk. The broad pattern of results suggests that dumping in ants likely serves to reduce clutter in the nest and around the nest entrance as well as to minimise the chances of contact with potential pathogens. For decluttering, rejected items can be dumped anywhere around the nest beyond 5 cm of the entrance. Indeed, in various ant species, including Melophorus ants (see Fig. 1), sand piles can be found in a circular ring around the nest (5–10 cm). For reducing contact with pathogenic materials, however, it pays to dump these items far enough away from the nest entrance.
The observed waste removal patterns likely reflect an adaptive prophylactic behaviour that evolved to reduce the risk of pathogens entering and infecting the nest colony. This would explain why materials of potentially high pathogenic risk are deposited further from the nest, even in the absence of an experimentally introduced pathogenic agent. Our results suggest that this behavioural response occurs regardless of the detection of pathogens on the waste, though an interesting follow-up study could be constructed to experimentally introduce contaminated and control waste materials. Such an experiment could untangle if these behaviours are based on potential risks alone or also encompass pathogenic detection when removing waste. Dumping at a faraway distance not only removes a source of possible pathogens and reduces the chances of any nest member contacting contaminated items, but also reduces the chance of nestmates bringing back the spoiled item. Although pathogenicity was not directly measured, the pattern of data is mostly consistent with the idea that spoilability contributes to determining the distance from the nest at which a substance is dumped. The clear separation of dumping distances between the long-distance group and the other two groups (short-distance group and intermediate items) of dumped materials reflects the two functions of dumping, reducing clutter and avoiding potential pathogens.
The two exceptions in what we have called the intermediate group, noted earlier, deserve consideration. With regard to cookie crumbs, we take a ‘glass-half-full’ perspective. These items were dumped at an average distance that was about fivefold shorter than the average of the long-distance group. An intermediate distance makes some functional sense because the cookies do contain a small amount of animal matter, milk solids.
With regard to M. bagoti larval cocoons, on the other hand, we take a ‘glass-half-empty’ perspective and need to discuss why their dumping distance does not conform to initial predictions. We initially rated this as a high risk of pathogenic growth. In retrospect, we may have overestimated the pathogenicity of larval cocoon, which comprises the fabric of fine silk thread, a compound which may be poor nurturing grounds for pathogens. Additionally, these larval cocoons are continuously fed and cleaned by the colonies’ adults, including hygienic cleaning of the cocoon surface (Hölldobler and Wilson 1990). Some ants are known to disinfect larval brood with formic-acid-rich poison—poisonous to the pathogens, not to the brood—or antimicrobial materials found in the environment, both of which would make these substrates resistant to pathogenic growth (Tragust et al. 2013a, b). Thus, these cocoons are not only likely to be of low pathogenic risk compared with other organic materials but might also be resistant to pathogenic growth, making them a low risk to the colony (Armitage et al. 2012; Tragust et al. 2013a, b). This possible pathogenic resistance would align with the observed short-distance waste disposal of this item category. Compared with larva cocoons of M. bagoti, however, cicada shells were dumped at longer distances. This may be because these non-nest materials have gathered contaminants that may decompose. They are also not protected by workers’ disinfectant agents. A limitation of our study is that we were unable to measure pathogenicity of the experimental materials in the field. Future studies should collect samples of dumped materials, preserve them, and test their pathogenicity in a lab.
Social insects are known to be capable of detecting pathogens and altering their behaviours to limit transmission within the colony (Stroeymeyt et al. 2018; Conroy and Holman 2022). Both ants and honeybees have been shown to exhibit hygienic behaviours to remove infected larval brood and nest mates from the hive/nest (Tragust et al. 2013b; Conroy and Holman 2022), indicating that workers are able to recognize some types of pathogenic infections, either through direct detection or possibly via signals actively or passively emitted by infected individuals. Similar pathogenic signals might also be detectable on waste materials. The detectability of such signals would mean that workers may make behavioural decisions regarding dumping distance based on both the associated risk a material has of carrying potential pathogens as well as detection of the actual presence/absence of pathogenic agents on the material. If foragers can detect these pathogenic agents, in a potential future test using artificially contaminated materials, we would expect that contaminated items would be carried further away than non-contaminated control items irrespective of waste type.
It is currently not clear how social insects recognize pathogens. The process may vary depending on the context of exposure, for example, whether the pathogens are encountered in food. Studies on ants have shown that they detect and communicate information via chemical signals from their metapleural gland and venom glands (Hölldobler and Engel 1978; Hölldobler and Wilson 1990; Hughes and Goulson 2001). Honey bees and ants are known to use cuticular hydrocarbons (CHCs) for signalling and chemical communication (Howard and Blomquist 2005; Wang et al. 2016). Ants may be able to signal infections from wounds via cuticular hydrocarbons (Frank et al. 2022), although this work has not been peer reviewed yet. Can they also transmit information about pathogenicity of materials?
Mechanistically, insects rely on and communicate with chemical cues in the exploitation of food sources and in the defence of colonies against predators, competitors, and diseases (Blum 1969; Breed et al. 2004). Chemical cues from the products of decomposition, like saturated fatty acids, trigger necrophoric behaviour (transport of spoiled materials) by ant workers. In addition, in the Argentine ant Linepithema humile, the rapid waning in dead ants of cuticular chemical compounds associated with living ants also triggers necrophoric behaviour before decomposition sets in (Choe et al. 2009). Workers recognize and remove dead adult nestmates on the basis of a lack of life-related cuticular compounds in addition to an accumulation of death-related compounds. Other social insects also distinguish between nestmates and non-nestmates through cuticular hydrocarbons (Singer 1998), and other death-related cues form aversive odours which remain on an individual’s surface for a period of time following death (Diez et al. 2013). CHCs are also known to provide information about species identity and influence behavioural response (Hölldobler and Wilson 1990). In Reticulitermes flavipes, freshly dead individuals belonging to a competitor species, Reticulitermes virginicus, trigger intensive burial behaviour in workers. Soldiers are recruited to guard the burial site (Sun et al. 2013). On the other hand, dead conspecifics, regardless of the nest of origin, are taken inside into a chamber so that nutrients can be extracted. In contrast, all materials placed near or into a M. bagoti nest, including cookies, elicit removal by ant workers. It is likely that something more than chemical cues from the removed items triggers such behaviour because foraging ants bring back as food some of these substances. Cookie crumbs, in particular, have been used as a food source for foraging M bagoti ants in numerous studies. The place at which these substances are found may be a factor, but this question needs further investigation.
Another question remains: how does an ant, once it has decided whether to dump something, determine when and where to drop the refuse item? These ants have a robust path integration system (Cheng et al. 2009; Collett and Collett 2000; Narendra 2007; Wystrach et al. 2012) that could make such distance estimations (Wittlinger et al. 2007). They also use view-based navigation based on the surrounding panorama (Cheng et al. 2009; Collett and Collett 2000; Freas et al. 2017; Freas et al. 2019; Wystrach et al. 2011; Wystrach et al. 2012), which could also be used to pinpoint a dumping location. Using artificially constructed views (Graham and Cheng 2009; Schultheiss et al. 2016) is one way to dissociate views and distance. In this regard, it is important to note in future studies the directions in which individual ants take materials for dumping. Do dumpers prefer to dump materials in roughly the same direction from the nest (in the same sector) like foragers (Muser et al. 2005; Wehner et al. 2004), a strategy that would be consistent with learning one or a few views along the route? Future studies should collect directional information and also track repeated dumping episodes of identified individuals.
In summary, we examined the distance at which red honey ants dump experimentally provided materials. Except for the case of shells of the ants’ own larvae, items of animal origin were dumped at average distances over 5 m, while items containing no or little content of animal origin were dropped at average distances less than 1.2 m. We think that dumping serves to reduce clutter within the nest and to reduce the chances of any nest member contacting potentially pathogenic materials.
Data availability
Supplementary videos are available at Open Science framework: https://osf.io/uzw9x/files/. The videos recorded 600 frames per seconds and when we run, they play 20-fold slower.
References
Armitage SA, Fernández-Marín H, Wcislo WT, Boomsma JJ (2012) An evaluation of the possible adaptive function of fungal brood covering by attine ants. Evolution 66(6):1966–1975
Blum MS (1969) Alarm pheromones. Annu Rev Entomol 14(1):57–80
Breed MD, Guzmán-Novoa E, Hunt GJ (2004) Defensive behavior of honey bees: organization, genetics, and comparisons with other bees. Annu Rev Entomol 49:271
Cheng K, Narendra A, Sommer S, Wehner R (2009) Traveling in clutter: navigation in the Central Australian desert ant Melophorus bagoti. Behav Proc 80(3):261–268
Choe D-H, Millar JG, Rust MK (2009) Chemical signals associated with life inhibit necrophoresis in Argentine ants. Proc Natl Acad Sci USA 106(20):8251–8255
Collett M, Collett TS (2000) How do insects use path integration for their navigation? Biol Cybern 83(3):245–259
Conroy TE, Holman L (2022) Social immunity in the honey bee: do immune-challenged workers enter enforced or self-imposed exile? Behav Ecol Sociobiol 76(2):1–9
Cremer S (2019) Social immunity in insects. Curr Biol 29(11):R458–R463
Czaczkes TJ, Heinze J, Ruther J (2015) Nest etiquette—where ants go when nature calls. PLoS ONE 10(2):e0118376
Deeti S, Cheng K (2021a) Observations on relocation of a nest by a colony of red honey ants (Melophorus bagoti). Northern Territory Naturalist 30:100–107
Deeti S, Cheng K (2021b) Learning walks in an Australian desert ant, Melophorus bagoti. J Exp Biol 224(16):242177
Deeti S, Fujii K, Cheng K (2020) The effect of spatially restricted experience on extrapolating learned views in desert ants, Melophorus bagoti. Anim Cogn 23(6):1063–1070
Diez L, Moquet L, Detrain C (2013) Post-mortem changes in chemical profile and their influence on corpse removal in ants (report). J Chem Ecol 39(11–12):1424
Diez L, Lejeune P, Detrain C (2014) Keep the nest clean: survival advantages of corpse removal in ants. Biol Lett 10(7):20140306
Frank ET, Kesner L, Liberti J, Helleu Q, LeBoeuf A.C., Dascalu, A., Azuma, F., Economo, E.P., Waridel, P., Engel, P., Schmitt, T. and Keller, L. (2022) Infection signaling and antimicrobial wound care in an ant society. bioRxiv, 2022.2004.2026.489514.
Freas CA, Cheng K (2017) Learning and time-dependent cue choice in the desert ant, Melophorus bagoti. Ethology 123(8):503
Freas CA, Fleischmann PN, Cheng K (2019) Experimental ethology of learning in desert ants: Becoming expert navigators. Behav Proc 158:181–191
Graham P, Cheng K (2009) Ants use the panoramic skyline as a visual cue during navigation. Curr Biol 19(20):R935–R937
Harkness RD (1977) The carrying of ants Cataglyphis bicolor by others of the same nest. J Zool (London) 183:419–430
Hart AG, Ratnieks FLW (2001) Task partitioning, division of labour and nest compartmentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav Ecol Sociobiol 49(5):387–392
Hart AG, Ratnieks FL (2002) Waste management in the leaf-cutting ant Atta colombica. Behav Ecol 13(2):224–231
Hölldobler B, Engel H (1978) Tergal and sternal glands in ants. Psyche 85(4):285–330
Hölldobler B, Wilson EO (1977) Colony-specific territorial pheromone in the African weaver ant Oecophylla longinoda (Latreille). Proc Natl Acad Sci USA 74(5):2072–2075
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Hughes W, Goulson D (2001) Polyethism and the importance of context in the alarm reaction of the grass-cutting ant, Atta capiguara. Behav Ecol Sociobiol 49:503–508
Kannowski PB (1972) A symbiosis: Gardening Ants, the Attines. Neal A. Weber. American Philosophical Society, Philadelphia, 1972. xx, 146 pp., illus. $8. Memoirs of the American Philosophical Society, vol. 92. Science 178(4063), 856–856
Kolmes SA, Sommeijer MJ (1992) Ergonomics in stingless bees: changes in intranidal behavior after partial removal of storage pots and honey in Melipona favosa (Hym. Apidae, Meliponinae). Insectes Soc 39(2):215–232
Lindauer M (1975) The social behavior of the bees: a comparative study (book review). 25:458-458
López-Riquelme GO, Fanjul-Moles ML (2013) The funeral ways of social insects. Social strategies for corpse disposal. Trends Entomol 9:71–129
Möglich M, Hölldobler B (1975) Communication and orientation during foraging and emigration in the ant Formica fusca. J Comp Physiol A 101(4):275–288
Muser B, Sommer S, Wolf H, Wehner R (2005) Foraging ecology of the thermophilic Australian desert ant Melophorus bagoti. Australian J Zool 53(5):301–311
Narendra A (2007) Homing strategies of the Australian desert ant Melophorus bagoti I. Proportional path-integration takes the ant half-way home. J Exp Biol 210(10):1798
Porter S (1984) Fire ant polymorphism: the ergonomics of brood production. ProQuest Dissertations Publishing 16(4):323-336
Pull CD, Ugelvig LV, Wiesenhofer F, Grasse AV, Tragust S, Schmitt T, Brown MJF, Cremer S (2018) Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. Elife 7:e32073
Rettenmeyer CW (1963) Behavioral studies of army ants. Estudios de comportamiento de hormigas guerreras. Universit Kansas Scienc Bullet 44:281–465
Schultheiss P, Nooten SS (2013) Foraging patterns and strategies in an Australian desert ant. Austral Ecol 38(8):942–951
Schultheiss P, Stannard T, Pereira S, Reynolds AM, Wehner R, Cheng K (2016) Similarities and differences in path integration and search in two species of desert ants inhabiting a visually rich and a visually barren habitat. Behav Ecol Sociobiol 70(8):1319–1329
Singer TL (1998) Roles of hydrocarbons in the recognition systems of insects. Am Zool 38(2):394–405
Sommer S, Wehner R (2012) Leg allometry in ants: Extreme long-leggedness in thermophilic species. Arthropod Struct Dev 41(1):71–77
Stroeymeyt N, Grasse AV, Crespi A, Mersch DP, Cremer S, Keller L (2018) Social network plasticity decreases disease transmission in a eusocial insect. Science 362(6417):941–945
Sun Q, Haynes KF, Zhou X (2013) Differential undertaking response of a lower termite to congeneric and conspecific corpses. Sci Rep 3(1)
Tallamy DW, Wood TK (1986) Convergence patterns in subsocial insects. Annu Rev Entomol 31(1):369–390
Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S (2013a) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol 23(1):76–82
Tragust S, Ugelvig LV, Chapuisat M, Heinze J, Cremer S (2013b) Pupal cocoons affect sanitary brood care and limit fungal infections in ant colonies. BMC Evol Biol 13(1):1–10
Visscher PK (1983) The honey bee way of death: Necrophoric behaviour in Apis mellifera colonies. Anim Behav 31(4):1070–1076
Wang Q, Goodger JQD, Woodrow IE, Elgar MA (2016) Location-specific cuticular hydrocarbon signals in a social insect. Proc R Soc B 283(1827):20160310
Watarai A, Arai N, Miyawaki S, Okano H, Miura K, Mogi K, Kikusui T (2018) Responses to pup vocalizations in subordinate naked mole-rats are induced by estradiol ingested through coprophagy of queen’s feces. Proc Natl Acad Sci USA 115(37):9264–9269
Wehner R, Meier C, Zollikofer C (2004) The ontogeny of foraging behaviour in desert ants, Cataglyphis bicolor. Ecol Entomol 29(2):240–250
Weiss MR (2006) Defecation behavior and ecology of insects. Annu Rev Entomol 51:635–661
Wittlinger M, Wehner R, Wolf H (2007) The desert ant odometer: a stride integrator that accounts for stride length and walking speed. J Exp Biol 210(Pt 2):198–207
Wystrach A, Beugnon G, Cheng K (2011) Landmarks or panoramas: what do navigating ants attend to for guidance? Front Zool 8(1):1–11
Wystrach A, Beugnon G, Cheng K (2012) Ants might use different view-matching strategies on and off the route. J Exp Biol 215(1):44–55
Yu C, Wang S, Yang G, Zhao S, Lin L, Yang W, Cui S (2017) Breeding and rearing naked mole-rats (Heterocephalus glaber) under laboratory conditions. J Am Assoc Lab Anim Sci 56(1):98–101
Acknowledgements
We acknowledge the traditional custodians of the land upon which this research was conducted, the Arrernte people. Their culture and customs have nurtured and sustained this land since the Dreamtime and continue to do so today. We pay our respects to their Elders past and present. We thank the Centre for Appropriate Technology at Alice Springs, Australia for letting us work on their property and providing some storage space, and the CSIRO Arid Zone Research at Alice Springs for administrative support. We also thank two anonymous reviewers for helpful comments that have especially improved the statistical treatment of data and the interpretation of the pattern of results.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported by a grant from the Australian Research Council (DP 1598700), by AUSMURIB000001 associated with ONR MURI grant N00014-19-1-2571 (Trevor and Sudhakar both supported in part by this grant) and by Macquarie University.
Author information
Authors and Affiliations
Contributions
Experimental design: SD, KC. Data collection: SD. Writing: SD, TM, CF and KC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Ethical approval
Australia has no ethical regulations regarding work with insects. The study was non-invasive and no long-term aversive effects were found on the nests or on the individuals studied.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deeti, S., Freas, C., Murray, T. et al. Dumping behaviour of Australian desert ants (Melophorus bagoti) (Hymenoptera:Formicidae). Insect. Soc. 70, 225–232 (2023). https://doi.org/10.1007/s00040-023-00911-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-023-00911-w