Abstract
Snowpatch plant communities, which occur in parts of alpine landscapes where snow accumulates and persists well into the summer, are highly sensitive to climate change. The formation of persistent soil seed banks is recognised as a critical component of a plant community’s resilience to a changing environment. However, our understanding of the ecology of snowpatch soil seed banks and their potential role in the persistence of these threatened communities remains limited. To address this knowledge gap, we (1) characterised the density, diversity and composition of snowpatch soil seed banks along a snowmelt gradient (with early, mid, and late melt zones defined); and (2) contrasted their similarity with long-term vegetation surveys (2020, 2013, 2007) to assess the relationship between soil seed banks and standing vegetation over time. We found persistent soil seed banks in all snowmelt zones and that the snowmelt gradient significantly influenced their density, diversity and composition. Species density and diversity in soil seed banks were higher in the early and mid zones compared to the late zone. However, seedlings from the late zone emerged faster and more synchronously than those emerging from the early and mid zones. The species similarity between seed banks and standing vegetation was relatively high in the two most recent surveys (2020, 2013) compared to the initial survey (2007). However, the composition of life forms and regeneration strategies (i.e. sexual or vegetative reproduction) of seedlings that emerged from the soil seed banks was more similar to the composition of the initial standing vegetation survey (2007) than to the more recent surveys (2020, 2013). Our results suggest that although soil seed banks may be changing as the standing vegetation changes, they still have a compositional similarity to historical plant assemblages, contributing to the resilience of these endangered communities to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snow is one of the most important environmental drivers in alpine ecosystems, especially in temperate and high-latitude regions (Körner 2021). Patterns of snow distribution and duration create stable mosaics of vegetation types and influence their composition, phenology and productivity (Billings and Bliss 1959; Walker et al. 1993). Across alpine landscapes, there is often a greater accumulation of snow in shaded or sheltered areas of topographical features (e.g. slopes, cirques, cornices, or hillsides), forming banks or patches that can persist longer than the rest of the snowpack after winter (Billings and Bliss 1959). The vegetation associated with these patches is a distinct assemblage of plants, hereafter referred to as snowpatch plant communities, but also known in the literature as snowbanks or snowbeds communities (Billings and Bliss 1959; Björk and Molau 2007; Green and Pickering 2009b).
The diversity and composition of snowpatch plant communities vary along the snowmelt gradient (following snowmelt isolines), but they are generally dominated by specialised forbs, sedges and grasses, with the presence of non-vascular plants and occasionally some dwarf shrubs (Billings and Bliss 1959; Wahren et al. 2001; Green and Pickering 2009b; Venn et al. 2011; Pickering et al. 2014; Good et al. 2019; Verrall et al. 2023). These plant communities have adapted to the strong environmental filter imposed by extended snow cover (Körner 2021). Snowpatch plants are adapted to short snow-free growing seasons and use the timing of snowmelt as a cue to complete their life cycle (Venn and Morgan 2007; Winkler et al. 2018). As a consequence, these plants are highly sensitive to reductions in snow cover due to decreased precipitation and warmer temperatures, which are occurring in alpine environments worldwide (Hock et al. 2019). They are, therefore, considered threatened by climate change (Björk and Molau 2007; Williams et al. 2015; Matteodo et al. 2016). Studies on the effects of climate change on snowpatch plant communities are scarce (Verrall and Pickering 2020), but those that do exist have reported changes in diversity and composition (Sandvik and Odland 2014; Carbognani et al. 2014; Pickering et al. 2014; Verrall et al. 2023; Morgan and Walker 2023), productivity (Galen and Stanton 1995; Petraglia et al. 2013) and phenology (Galen and Stanton 1995; Petraglia et al. 2014).
In Australian alpine environments, snowpack depth and persistence has decreased significantly over the past 50 years (Sánchez-Bayo and Green 2013; Fiddes et al. 2015), thereby extending the growing season length in snowpatch areas (Verrall et al. 2023). In response, there has been a decrease in the abundance of snowpatch specialists and an increase in the presence and cover of common graminoids and shrubs in snowpatch plant communities (Pickering et al. 2014; Verrall et al. 2023; Morgan and Walker 2023). Morgan and Walker (2023) suggested that the detected changes in diversity and composition are driven by generalist species entering snowpatches via seed dispersal, with snowpatch specialists persisting through clonal reproduction despite the ongoing environmental change. However, persistence of the clonal snowpatch specialists is not guaranteed as snow cover will continue to decrease, and more competitive species may enter these areas (Morgan and Walker 2023).
In this context, regeneration from seed (i.e. sexual reproduction) will be critical for snowpatch species to adapt to new environmental conditions (either through gradual genetic adaptation of populations or phenotypic plasticity) or to shift in distribution with the snowmelt gradient (where possible) in response to climate change (Guisan and Theurillat 2000). Despite this, only a few studies have focused on plant recruitment from seed in snowpatch communities; contrasting species germination requirements along snowmelt gradients (Rosbakh et al. 2022) or between habitats with different snow cover persistence (Shimono and Kudo 2003, 2005). The changes in germination and seedling emergence due to climatic changes have also been explored (Bernareggi et al. 2015, 2016; Wang et al. 2018). The seed longevity of alpine snowpatch specialists may need to increase under future climatic conditions if they are to persist (Bernareggi et al. 2015), and as such, the soil seed banks of alpine snowpatch communities will likely play a vital role in this process as they are critical for plant communities resilience to environmental change (Gioria et al. 2022). However, studies investigating snowpatch soil seed bank ecology and their potential contribution to plant regeneration in these habitats are very rare (only few studies for arctic-alpine areas: Fox 1983; Welling et al. 2004).
Here, we address this knowledge gap by answering the following research questions: (1) How do the density, diversity, and composition of snow patch soil seed banks change along the snowmelt gradient? (2) How similar is the species composition of soil seed banks to that of standing vegetation, and how does this similarity change over time when compared to long-term surveys of standing vegetation (2007, 2013, and 2020)?
Materials and methods
Study area
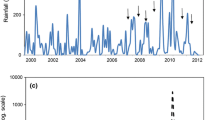
The study was conducted in the Main Range of Kosciuszko National Park (New South Wales, Australia; Fig. 1a). The region is part of the Australian Alps and is home to the highest mountain in Australia (Mt. Kosciuszko, 2,228 m a.s.l.). It is within this region that the longest-lasting snowpatches in Australia are found (Costin et al. 2000). The mean annual temperature in the area is 4.8 °C (1990–2020), with the hottest month being January and July the coldest. The mean annual precipitation is 1,274 mm (1990–2021), with most precipitation falling during the winter as snow (Thredbo meteorological station, Bureau of Meteorology, www.bom.gov.au). The winter snowpack forms between the end of May and July and becomes discontinuous in October (Costin et al. 2000).
Snowpatches and snowmelt zones
In Australia, snowpatch plant communities are classified as Endangered under the IUCN Red List criteria due to their limited distribution, occupying less than 1 per cent of Australian alpine areas (Williams et al. 2015), which cover less than 1 per cent of the whole continent (Costin et al. 2000). In the study area, four snowpatches located above 2000 m a.s.l. along a 10 km transect were selected for the study: Club Lake Cirque (Club), Cootapatamba Cornice (Coota), northeast ridge of Mount Kosciuszko (KNE) and Mawson Cirque (Mawson; Fig. 1b). The average size of the snow patches included in the study is 1,350 m2. On average, 50 per cent of this area corresponds to the early melt zone, 30 per cent to the mid melt zone and 20 per cent to the late melt zone (see below; Database S1). The orientation of snowpatches was south-southeast, with slopes between 19 and 25 degrees (Verrall et al. 2023).
Across snowpatches, snowmelt patterns create distinct zones with different growing season lengths that influence vegetation cover and species composition (Billings and Bliss 1959; Körner 2021). To account for this, three snowmelt zones were defined according to growing season length based on records from temperature loggers buried 10 cm below the soil surface within the selected snowpatches for 17 years (from 2003 to 2020): (1) early melt zone = > 140 days growing season length; (2) mid melt zone = 140 − 105 days; and (3) late melt zone = < 105 days (Fig. 1c; see Verrall et al. 2023). As well as the relative timing of the snow melt, each zone differs in species composition, bare ground and rock cover; the early zones are dominated by generalist graminoids with a cover of nearly 100%, the mid zones are composed of forbs and short graminoids (including some snowpatch specialists) with a cover of ~ 70%, and the late zones dominated by snowpatch specialists (forbs and graminoids) with a cover of ~ 25% and a high proportion of rock and bare ground (Green and Pickering 2009a; Verrall et al. 2023). Further details on the distribution of temperature loggers throughout the snowpatches, the methodology used to define the snowmelt zones, and the diversity and composition of communities along the snowmelt gradient can be found in Verrall et al. (2023).
Soil and standing vegetation sampling
Long-term standing vegetation surveys took place in 2020, 2013, and 2007. In all surveys, a stratified random sampling approach was used, where three 1 m2 quadrats were located at the same elevation across the slope targeting the early, mid and late snowmelt zones (Fig. 1c). The mean distance between the quadrats of the same zone was 4.5 ± 1.1 m. Within each established quadrat, the standing vegetation species richness and cover was visually assessed to 0.5 per cent using 100 equal cells of 10 × 10 cm. These data have previously been used in Pickering et al. (2014) and Verrall et al. (2023). A list of the recorded species in each survey is provided in Table S1. During the most recent survey (2020), we recovered buried temperature loggers that were later used alongside the previous 16 years of soil temperature data to define the early, mid and late snowmelt zones (see Sect. 2.2), so quadrats were not always established in the targeted zones (see Table S2 for the final sampling for each melt zone).
In 2020, after surveying the standing vegetation, we collected soil samples (~ 564 cm3) at the top and bottom of each established quadrat (one sample consisted of two bulked soil cores of 6 cm diameter * 10 cm depth, Fig. 1c). The soil cores were transported to the laboratory in labelled plastic bags at room temperature and in dark conditions.
Greenhouse study
In the laboratory, soil samples were air-dried at room temperature (20–25 °C) for six weeks. After drying, rocks and plant material were removed by sieving the soil through a 2 mm sieve. Seeds larger than 2 mm were returned to the soil samples. The soil samples were then cold stratified at 2 °C in the dark for 15 weeks to simulate an alpine winter under snow.
After the cold stratification, soil samples were spread over a 2 cm thick layer of sterilised sand in black plastic trays (20 × 30 cm). Each tray contained two soil samples, divided by plastic sheets, to avoid cross-contamination. Trays were randomly arranged on benches in an ambient greenhouse with a mean temperature of 18 °C during the night and 28 °C during the day). Six additional trays containing sterilised sand were used to control for contamination from exogenous seeds. Soil samples were watered manually to field capacity at the start of the experiment and then an automatic irrigation system was set to turn on for 1 min twice a day (7 am and 3 pm). The irrigation regime was changed on week 3 to 10 min twice a day (7 am and 3 pm) to maintain samples at field capacity as ambient conditions changed.
Every 1–3 weeks for 25 weeks, we recorded and identified all newly emerged seedlings. We marked them with toothpicks, and when their taxonomic identity was determined, we removed them from the trays. When reproductive structures were required for taxonomic identification, seedlings were transplanted to pots within the same greenhouse and kept alive until they were old enough to have their identity determined. Seedlings that died before being identified at the species level were recorded as members of a particular family or as unidentified monocots or dicots. Due to the large number of emerged seedlings of three common Poa species (Poa costiniana, P. fawcettiea, and P. hiemata) and the lack of space to grow them all to maturity, they were all included in a group labelled ‘Poa complex’. Species taxonomic classification followed Flora of Victoria (https://vicflora.rbg.vic.gov.au/). After 21 weeks in the greenhouse, we removed the moss growing on top of the trays and disturbed the soil with a fork to promote further germination. After 30 weeks, we stopped recording new germinants. We left the trays in the greenhouse for another 15 weeks, but no new seedlings were recorded.
Data analysis
The soil seed bank density per melt zone was determined by extrapolating the number of germinants of each soil sample (two soil cores of 28.27 cm2 of area and 264 cm3 of volume) to standard reporting measurements (squared metres and litres) and then calculating the mean and standard error using all replicates. We then contrasted the total number of emerged seedlings from the different snowmelt zones using a Generalized Linear Mixed Model (GLMM) with a negative binomial distribution and log-link function. In the model, snowmelt zones were treated as fixed effects and snowpatches as random effects. We then performed pairwise comparisons with confidence levels and p-value adjustments by Tukey corrections.
To characterise the composition of the soil seed banks, we classified the emerged seedlings according to their life form (forbs and graminoids; following Costin et al. 2000), life strategy (snowpatch specialist and generalist; following classification by Verrall et al. 2023), regeneration strategy (obligate seeders, mostly seeders, mostly vegetative and vegetative; following classification by Venn and Morgan 2010) and seed mass (small: <0.5 mg and large: >0.5 mg; following Venn and Morgan, 2017).
To describe the time when seedlings emerged during the greenhouse study, we calculated the median emergence time (T50; the time to reach 50 per cent of final emergence) and synchronisation index (values from 0 to 1, where a value 1 indicates that seedling emergence happened simultaneously and 0 indicates that all seedlings emerged at different times) where instead of germination records we used 3-weekly seedling emergence records. For these calculations, we only used the emergence data before we perturbated the soil trays (i.e. weeks 0 to 21). We contrasted the resulting values for the different snowmelt zones with two GLMMs, one with Poisson distribution (for T50 values) and another with beta distribution (useful for variables within a range of zero to one such as the synchronisation index values), with snowmelt zones treated as fixed effect and snowpatches as random effect. We then performed a posterior pairwise comparison with Tukey correction.
To measure the soil seed bank diversity, we constructed sample-sized rarefaction and extrapolation curves for the first three orders of Hills numbers (q = 0,1,2; Jost 2006). This allowed us to estimate the diversity of each melt zone on equal individual samples despite our sampling effort being different among the melt zones (see Table S2). The calculated q-orders are the equivalent to three traditional biodiversity indices: species richness (q = 0), Shannon diversity (q = 1, the exponential of Shannon entropy) and Simpson diversity (q = 2, the inverse of the one-complement of the Gini–Simpson index; Chao et al. 2023). Then, we contrasted the estimated number of effective species on a total sample of 1,500 individuals between snowmelt zones with a linear model with a similar structure to that specified above and posterior post-hoc tests with Tukey correction. The similarity among soil seed banks of the different zones was illustrated using a Venn diagram showing the number of shared species and a non-metric multidimensional scaling (NDMS) plot that used a Bray-Curtis matrix calculated from abundance data. We also performed an analysis of similarity (ANOSIMs) with presence-absence and abundance data.
Finally, to explore the similarity in species composition between the soil seed banks and the long-term vegetation surveys, we constructed NDMS plots that used a Jaccard dissimilarity matrix for presence-absence data and a Bray-Curtis matrix for abundance data. Furthermore, for each quadrat, we calculated the Jaccard qualitative similarity index (presence-absence data) and the quantitative similarity ratio (cover data) between the soil seed bank and the different long-term vegetation surveys (2007, 2013 and 2020). Further details on how these indices were calculated are in the supplementary materials (Supplementary Methods S1). Values of both indices range between 0 and 1, where zero means no similarities at all, and one means that the soil seed bank and the standing vegetation have the same species composition. To contrast the obtained values, we used GLMM with beta distribution and logit function. Because there were a few zeroes in the database (i.e. soil seed bank samples with no similarity with the standing vegetation), before fitting the model, we transformed the data with the formula (y·(n − 1) + 0.5)/n, where y is the obtained index value and n is the sample size, following Smithson and Verkuilen (Smithson and Verkuilen 2006).
Analyses were done in R (R Core Team 2022). We used the ‘lme4’ package (Bates et al. 2015) for the models with Poisson and negative binomial distribution and the ‘glmmTMB’ package (Brooks et al. 2017) for the beta distribution models. Diagnostics of good fit were performed using the ‘DHARMa’ package (Hartig, 2020). We followed the recommendations of Oberpriller et al. (2022) on keeping or not our random effects when fitting the models. Post hoc comparisons were performed using the ‘emmeans’ package (Lenth 2023). Median emergence time and synchronisation index were calculated using the package germinaR (Lozano-Isla et al. 2019). Rarefaction and extrapolation curves were calculated using the ‘iNEXT’ package (Hsieh et al. 2022). ANOSIMs and NDMSs were done in Past software (Hammer et al. 2001). All figures were constructed with ‘ggplot’ (Wickham 2016) and Microsoft Excel with further visual-only edition in Adobe Illustrator.
Results
Soil seed banks along the snowmelt gradient
Density and composition
A total of 2,118 seedlings of ten species emerged from the soil seed bank samples. Of these, 65% were identified to species level, 34% to genus but not species, 0.9% to family level, and 0.1% were only identified as morpho-species. In total, ten species/morpho-species were recorded (Table 1).
The overall seedling density was 1,038.40 ± 8.07 (mean ± s.e) per m2 (62.95 ± 11.06 per 1000 cm3). The seedling density varied along the snowmelt zones (Fig. 2a). The highest density was recorded in the early zone, followed by the mid zone, but without statistical difference. The late zone had significantly lower seedling density than the early and mid zones (Fig. 2a and Table S3).
The snowmelt gradient influenced the composition of the soil seed bank. Seedlings of graminoid species dominated the early zone, and their proportion decreased towards the late zone, where seedlings of forbs dominated the soil seed bank (Fig. 2b). Seedlings of generalist species were more abundant in the early zone, while seedlings of snowpatch specialists dominated the late zone (Fig. 2c). Smaller seeds dominated the early zone, while larger seeds were more abundant in the late zone (Fig. 2d). Seedlings of mostly seeders (i.e. species that are capable of vegetative reproduction but rarely do so) dominated the soil seed banks, with only one obligate seeder and no clonal species detected (Table 1). It is important to note that the patterns described above are strongly influenced by the dominant species found in each zone (e.g. the Poa complex in the early zone and Montia australasica in the late zone).
Soil seed bank densities (a) and composition (b-d) in the three melt zones. For (a), x shows the mean, and letters show differences from the post hoc analysis (see Table S3)
Seedlings emergence time
Emergence time and synchrony varied along the snowmelt gradient. The number of weeks needed to reach 50% of seedling emergence (T50) was shorter in the late zone and increased towards the early zone (early = 6.86 ± 0.94, mid = 4.56 ± 0.65, late = 2.86 ± 0.52; Fig. 3a). The synchronisation index indicated a higher degree of overlap in seedling emergence in the late zone, with the lowest synchrony values recorded for the early zone (Fig. 3b).
Cumulative seedling emergence during the 30-week greenhouse study (a), where the insert plot shows T50 values (mean ± s.e.) for each snowmelt zone. The vertical dashed line indicates when the soil was disturbed. Synchronisation index for the different snowmelt zones (b). Letters represent differences detected during the post hoc analysis (see Table S4)
Alpha and beta diversity
Soil seed bank diversity varied along the snowmelt zones. The early and mid snowmelt zones had the highest values for the three orders of q (Fig. 4a and b). In the late zone, the number of effective species for q = 0 was not significantly different from that in the mid and late zones but was significantly smaller when species abundances were considered (q = 1 and q = 2; Fig. 4a and b).
Snowmelt zones shared seven species (Fig. 4c). The ANOSIMs showed overall significant differences between the soil seed banks of the different zones (abundance data: R = 0.434, p = 0.0001 and presence-absence data: R = 0.311, p = 0.0002). However, pairwise comparisons (Table S5) and NDMS (Fig. 4d) revealed that the detected differences were driven by the seed bank of the late zone, which was significantly different from the early and mid zones, and no significant differences between the early and mid zones. In addition, the ordination showed high dissimilarity within the late soil seed bank samples (i.e. larger cluster area) compared to the early and mid zones (Fig. 4d).
Soil seed banks similarity with the standing vegetation
Similarity between soil seed banks and standing vegetation varied along the snowmelt gradient, with similarity decreasing from early to late zone (Fig. 5a and c, Table S6 and S7). Similarity also varied with vegetation survey time, with higher similarity values between soil seed banks and more recent vegetation surveys (2020, 2013) than with the initial vegetation survey (2007). Although this last pattern was similar in all melt zones, no significant changes were detected for the late zone (Fig. 5b and d). The two methods used gave similar results, but the values obtained with the quantitative method (i.e. cover data) were higher than those obtained with the qualitative method (i.e. presence-absence data).
The composition of life forms and regeneration strategies of the seedlings that emerged from the soil seed bank was more similar to the composition of the initial standing vegetation survey (2007) than to the more recent surveys (2020, 2013; Table S1 and Fig. 6). However, these results may reflect the dominance of specific species in the soil seed banks, such as M. australasica in the late melt zone.
Alpha (a-b) and beta (c-d) diversity of the snowmelt zones. (a) Rarefaction and extrapolation curves and (b) effective number of species estimated on a sample of 1500 seedlings for the three first orders of q and the three snowmelt zones. Fill areas (a) and error bars (b) represent the 95% CI obtained by bootstrap method where the sample size is fixed in each regenerated bootstrap sample. Letters indicate significance form overlaps of the calculated intervals. (c) Venn diagram and (d) ordination indicating the relationship in species composition between soil seed banks of the different snowmelt zones
Discussion
Our study shows that snowpatch plant communities have important persistent soil seed banks and that the snowmelt gradient influences their density, diversity, composition, and similarity with the standing vegetation. Furthermore, we show that for the early and mid zones, soil seed banks are more similar to recent (2020, 2013) than initial (2007) standing vegetation, while we detected no significant changes in similarity for the late zone over time.
Soil seed banks along the snowmelt gradient
In this study, the overall soil seed bank density was similar to that reported for snowbanks in a montane tundra in Alaska (1,115 seeds/m2; Fox 1983) and larger than that reported for arctic snowbeds in Finland (600 − 200 seeds/m2; Welling et al. 2004). Density was also comparable to that of other Australian alpine plant communities (Venn and Morgan 2010; Hoyle et al. 2013; Vázquez-Ramírez and Venn 2023).
The variation in soil seed bank density along the snowmelt gradient can be explained by the standing vegetation cover, with both seed bank density and vegetation cover decreasing from the early to the late zone (Verrall et al. 2023). A similar relationship between vegetation cover and seed bank density was reported for a snowmelt gradient in the arctic-alpine tundra (Fox 1983). However, this pattern contrasts with the reported lower seed production in early melt areas when compared to late melt areas in snowbeds in the Italian Alps (Tonin et al. 2019) and the reported negative correlation between soil seed bank size and vegetation cover in alpine plant communities along a snowmelt gradient in the Caucuses (Onipchenko et al. 1998). While plant numbers can explain this discrepancy (i.e. the total seed production of more plants producing less seeds could be larger than fewer plants producing more seeds), microsite conditions may also have an influence on our results. Due to low plant cover in the late zone, seeds have better light conditions and more competition free gaps, which is known to promote germination in snowpatch and alpine species (Hülber et al. 2011; Fernández-Pascual et al. 2021; Margreiter et al. 2021). As a consequence, the soil seed bank in the late melt zone may become depleted by constant germination events, independently of germinant survival rates. The increases in growing season length and soil temperatures in the late zones during the last 17 years (Verrall et al. 2023) could be driving this process. In contrast, in the early zone, where vegetation cover is close to 100%, most of the dispersed seeds would become part of the seed bank due to poor light conditions and the lack of gaps for germination.
Variations in soil seed bank composition along the snowmelt gradient can also be explained by the standing vegetation. Plants and seeds of generalist graminoids dominate the early zone, and plants and seeds of specialised forbs dominate the late zone. In the mid zone, a mixture of graminoids and forbs characterise both the soil seed banks and the standing vegetation (for standing vegetation data see Table S1 or Verrall et al. 2023). On the other hand, the species in the soil seed bank tend to be from those that commonly reproduce by seed, which differs from the high proportion of species in the standing vegetation which are thought to primarily regenerate clonally (Table S1). Such dominance of seed-bearing species in the soil seed bank, paired with the absence of seeds of the clonal species, has been reported before in other alpine environments (Venn and Morgan 2010; Vázquez-Ramírez and Venn 2023) and concurs with the reported lower investment of clonal plants in seed production when compared to non-clonal ones (Herben et al. 2015).
The difference in seedling emergence synchrony and time along the snowmelt gradient during the greenhouse study, suggests an adaptation of soil seed banks to the length of the growing season of each snowmelt zone. Seeds from the late zone tend to germinate quickly after the snowmelt in mid-summer, at a time when temperatures are warm and soil moisture is high from the melting snow, which increases the likelihood of successful seedling establishment before winter. In contrast, seeds from the early zone tend to germinate slowly and are not in sync, perhaps as a bet-hedging strategy in anticipation of a longer and more unpredictable snow-free season ahead. These findings concur with the reported dependence of snowpatch specialists on high soil temperatures for germination (i.e. summer conditions), whereas generalist alpine species have a broader germination niche (Shimono and Kudo 2001, 2003; Rosbakh et al. 2022). Higher synchrony and germination speed have also been reported for snowbed species when compared to species from a fellfield with shorter snow cover, at temperatures similar to our experiment (~ 25 °C; Shimono and Kudo 2005).
The soil seed bank species richness in this study is similar to the richness in an alpine-arctic snowbed in Finland (8 species; Welling et al. 2004) and a high alpine site in the Andes (9 species, Kalin Arroyo et al. 1999), but smaller than that reported for other Australian alpine plant communities (45 species in a short alpine herbfield, Hoyle et al. 2013; 18 and 24 species in a tall alpine herbfield and treeline respectively, Vázquez-Ramírez and Venn 2023; 14–24 species in alpine summits, Venn and Morgan 2010). The patterns of soil seed bank species diversity (q = 1,2,3) along the snowmelt gradient cannot be explained by the standing vegetation diversity. The soil seed bank has high diversity values in the early and mid zones, while the standing vegetation has high values in the mid and late zone (see Verrall et al. 2023). Low diversity values in the late zone when abundance of species is considered (q = 1 and 2), were driven by the snowpatch specialist and obligate seeder Montia australasica, that represents 90% of the soil seed bank.
Soil seed banks similarity with standing vegetation
Overall, the similarity values that we found between the standing vegetation and the soil seed banks, regardless of the zone and time period, are higher than those previously reported for other snow-dependent communities (Fox 1983; Welling et al. 2004) and other plant communities in the Australian Alps (Venn and Morgan 2010; Hoyle et al. 2013; Vázquez-Ramírez and Venn 2023). The observed significant difference in similarity values from 2007 to 2013, but not from 2013 to 2020, might be explained by the rapid changes in standing vegetation composition and the later stabilisation of standing vegetation in these snowpatches (Verrall et al. 2023).
From an ecological perspective, the higher dissimilarity between soil seed banks and older surveys suggests that soil seed banks are changing along with standing vegetation or that seed banks are driving changes in aboveground vegetation. Either way, the potential for soil seed banks in snowpatches to act as reservoirs of historical plant assemblages and to buffer environmental changes appears limited, similar to the findings in other temperate and cold plant communities worldwide (Plue et al. 2021). This could be due to the fact that alpine species are usually considered to be short-lived (Satyanti et al. 2018; Seglias 2022) and not all species tend to form persistent soil seed banks (Schwienbacher et al. 2010). Despite this finding, the life-forms and life-strategies of the seedlings forming the soil seed banks are very similar to those of the initial standing vegetation survey (2007), with a clear differentiation between melt zones. This contrasts with the reported direction of changes in the composition of standing vegetation in the mid and late melt zones, with a clear trajectory towards the early melt zone (i.e. competitive exclusion of snowpatch specialists by generalists). In other words, the reported increase of generalists graminoid species (e.g. Poa species) and decrease of snowpatch specialist forbs (e.g. Montia australasica) in the mid and late zones are not reflected in the composition of their seed bank yet, where forbs still dominate and graminoids are not an important part of it (only 23.4 per cent and 5 per cent of the total emerged seedlings in mid and late zones respectively, see Table S1 and Fig. 6). Hence, this compositional similarity in life forms and strategies between seed bank and standing plant communities may be an important functional buffer against climate change (Plue et al. 2021; Gioria et al. 2022).
Snowpatch soil seed banks in a future with less snow
As the growing season length (due to the reduction in snow cover) and ambient temperatures continue to increase in alpine regions worldwide (Hock et al. 2019), soil seed banks in snowpatches may increase in size and diversity (more markedly in the late zone). Changes in density and diversity could be driven by an increase in vegetation cover, increased productivity (Petraglia et al. 2013; Lluent et al. 2013), and the reported increase in alpine seed longevity in a warmer future alpine environment (Bernareggi et al. 2015). However, increased seed germinability and reduced dormancy as consequence of warmer temperatures (Bernareggi et al. 2016) need to be taken into account.
Seedling emergence and establishment from soil seed banks are likely to be affected in coming decades too. Decreases in the number of emerged seedlings and increases in dissimilarity between them and the standing vegetation could be expected as a consequence of shorter cold stratification periods and frost events (due to the lack of snow insulation), as has been shown for other alpine communities in the study area (Vázquez-Ramírez and Venn 2023). Moreover, earlier snowmelt and warmer temperatures could create the favourable microclimatic conditions necessary for germination of snowpatch specialists in the late melt zones much earlier in the snow-free season, forcing them to germinate when favourable spring conditions might not last, exposing them to an extended and unpredictable growing season, with potentially catastrophic consequences for plant recruitment (Shimono and Kudo 2003).
With respect to our methods, some seeds in the soil seed bank may not have germinated during the greenhouse phase of the study, as some may have required multiple cycles of cold stratification to break dormancy. Furthermore, at the time of soil collection (February 2020), the different snowmelt zones had been without snow cover for different lengths of time (e.g. early 3 months, mid 2 months and late 1 month), so the opportunity for seeds from the soil seed bank to germinate was uneven. Thus, we may have underestimated the soil seed bank density of the early and mid snowmelt zones, as some of the seeds ready to germinate are likely to have done so in the field prior to soil collection. Future research should aim to collect soils at a time when these differences can be considered (e.g. in late autumn before the snowpack is established or at different times in spring-summer, with a fixed period of time after snowmelt in each zone).
Our results suggest that although soil seed banks are potentially changing together with the standing vegetation, the compositional shifts in the standing vegetation are not yet reflected. Thus, snowpatch soil seed banks still have the capacity to act as a reservoir of diverse plant assemblages in a changing environment. Further studies that systematically collect soil seed banks in conjunction with long-term vegetation surveys are needed to improve our understanding of how quickly soil seed banks change and where the limits of this buffering capacity lie.
Data availability
Raw data supporting the results of this study can be found in the supplementary materials.
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67. https://doi.org/10.18637/jss.v067.i01
Bernareggi G, Carbognani M, Petraglia A, Mondoni A (2015) Climate warming could increase seed longevity of alpine snowbed plants. Alp Bot 125:69–78. https://doi.org/10.1007/s00035-015-0156-0
Bernareggi G, Carbognani M, Mondoni A, Petraglia A (2016) Seed dormancy and germination changes of snowbed species under climate warming: the role of pre- and post-dispersal temperatures. Ann Bot 118:529–539. https://doi.org/10.1093/aob/mcw125
Billings WD, Bliss LC (1959) An alpine snowbank environment and its effects on vegetation, plant development, and productivity. Ecology 40:388–397. https://doi.org/10.2307/1929755
Björk RG, Molau U (2007) Ecology of Alpine Snowbeds and the Impact of Global Change. Arctic, Antarct Alp Res 39:34–43
Brooks ME, Kristensen K, Benthem KJ, Van et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized Linear mixed modeling. R J 9:378. https://doi.org/10.32614/RJ-2017-066
Carbognani M, Tomaselli M, Petraglia A (2014) Current vegetation changes in an alpine late snowbed community in the south-eastern Alps (N-Italy). Alp Bot 124:105–113. https://doi.org/10.1007/s00035-014-0135-x
Chao A, Thorn S, Chiu C et al (2023) Rarefaction and extrapolation with beta diversity under a framework of Hill numbers: the iNEXT.beta3D standardization. https://doi.org/10.1002/ecm.1588. Ecol Monogr 93:
R Core Team (2022) R: A language and environment for statistical computing
Costin A, Gray M, Totterdell C, Wimbush D (2000) Kosciuszko Alpine Flora, 1st edn. CSIRO
Fernández-Pascual E, Carta A, Mondoni A et al (2021) The seed germination spectrum of alpine plants: a global meta‐analysis. New Phytol 229:3573–3586. https://doi.org/10.1111/nph.17086
Fiddes SL, Pezza AB, Barras V (2015) A new perspective on Australian snow. Atmos Sci Lett 16:246–252. https://doi.org/10.1002/asl2.549
Fox JF (1983) Germinable seed banks of Interior alaskan tundra. Arct Alp Res 15:405. https://doi.org/10.2307/1550835
Galen C, Stanton ML (1995) Responses of Snowbed Plant Species to changes in Growing-Season Length. Ecology 76:1546–1557. https://doi.org/10.2307/1938156
Gioria M, Osborne BA, Pyšek P (2022) Soil seed banks under a warming climate. Plant regeneration from seeds: a global warming perspective. Academic, London, pp 285–298
Good M, Morgan JW, Venn S, Green P (2019) Timing of snowmelt affects species composition via plant strategy filtering. Basic Appl Ecol 35:54–62. https://doi.org/10.1016/j.baae.2019.01.004
Green K, Pickering C (2009a) Vegetation, microclimate and soils associated with the latest-lying snowpatches in Australia. Plant Ecol Divers 2:289–300. https://doi.org/10.1080/17550870903019275
Green K, Pickering CM, Arctic (2009b) Antarct Alp Res 41:212–218. https://doi.org/10.1657/1938-4246-41.2.212
Guisan A, Theurillat J-P (2000) Assessing alpine plant vulnerability to climate change: a modeling perspective. Integr Assess 1:307–320. https://doi.org/10.1023/A:1018912114948
Hammer Ø, Harper DAT, Ryan PD (2001) PAST Paleontological Statistics Software Package for Education and Data Analysis
Herben T, Šerá B, Klimešová J (2015) Clonal growth and sexual reproduction: tradeoffs and environmental constraints. Oikos 124:469–476. https://doi.org/10.1111/oik.01692
Hock R, Rasul G, Adler C et al (2019) High Mountain areas. Intergovernmental Panel on Climate Change, Geneva, Switzerland
Hoyle GL, Venn SE, Steadman KJ et al (2013) Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Glob Chang Biol 19:1549–1561. https://doi.org/10.1111/gcb.12135
Hsieh T, Ma K, Chao A (2022) iNEXT: Interpolation and Extrapolation for Species Diversity. R package version 3.0
Hülber K, Bardy K, Dullinger S (2011) Effects of snowmelt timing and competition on the performance of alpine snowbed plants. Perspect Plant Ecol Evol Syst 13:15–26. https://doi.org/10.1016/j.ppees.2011.01.001
Jost L (2006) Entropy and diversity. Oikos 113:363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x
Kalin Arroyo MT, Cavieres LA, Castor C, Humaña AM (1999) Persistent soil seed bank and standing vegetation at a high alpine site in the central Chilean Andes. Oecologia 119:126–132. https://doi.org/10.1007/s004420050768
Körner C (2021) Alpine Plant Life. Springer International Publishing, Cham
Lenth R (2023) Emmeans: estimated marginal means. aka Least-Squares Means
Lluent A, Anadon-Rosell A, Ninot JM et al (2013) Phenology and seed setting success of snowbed plant species in contrasting snowmelt regimes in the Central Pyrenees. Flora - Morphol Distrib Funct Ecol Plants 208:220–231. https://doi.org/10.1016/j.flora.2013.03.004
Lozano-Isla F, Benites‐Alfaro OE, Pompelli MF (2019) GerminaR: an R package for germination analysis with the interactive web application GerminaQuant for R. Ecol Res 34:339–346. https://doi.org/10.1111/1440-1703.1275
Margreiter V, Walde J, Erschbamer B (2021) Competition-free gaps are essential for the germination and recruitment of alpine species along an elevation gradient in the European Alps. Alp Bot 131:135–150. https://doi.org/10.1007/s00035-021-00264-9
Matteodo M, Ammann K, Verrecchia EP, Vittoz P (2016) Snowbeds are more affected than other subalpine-alpine plant communities by climate change in the Swiss Alps. Ecol Evol 6:6969–6982. https://doi.org/10.1002/ece3.2354
Morgan J, Walker Z (2023) Early-melting snowpatch plant communities are transitioning into novel states. Sci Rep 13:16520. https://doi.org/10.1038/s41598-023-42808-5
Oberpriller J, de Souza Leite M, Pichler M (2022) Fixed or random? On the reliability of mixed-effects models for a small number of levels in grouping variables. Ecol Evol 12. https://doi.org/10.1002/ece3.9062
Onipchenko VG, Semenova GV, van der Maarel E (1998) Population strategies in severe environments: alpine plants in the northwestern Caucasus. J Veg Sci 9:27–40. https://doi.org/10.2307/3237220
Petraglia A, Carbognani M, Tomaselli M (2013) Effects of nutrient amendments on modular growth, flowering effort and reproduction of snowbed plants. Plant Ecol Divers 6:475–486. https://doi.org/10.1080/17550874.2013.795628
Petraglia A, Tomaselli M, Petit Bon M et al (2014) Responses of flowering phenology of snowbed plants to an experimentally imposed extreme advanced snowmelt. Plant Ecol 215:759–768. https://doi.org/10.1007/s11258-014-0368-1
Pickering C, Green K, Barros AA, Venn S (2014) A resurvey of late-lying snowpatches reveals changes in both species and functional composition across snowmelt zones. Alp Bot 124:93–103. https://doi.org/10.1007/s00035-014-0140-0
Plue J, Van Calster H, Auestad I et al (2021) Buffering effects of soil seed banks on plant community composition in response to land use and climate. Glob Ecol Biogeogr 30:128–139. https://doi.org/10.1111/geb.13201
Rosbakh S, Fernández-Pascual E, Mondoni A, Onipchenko V (2022) Alpine plant communities differ in their seed germination requirements along a snowmelt gradient in the Caucasus. Alp Bot 132:223–232. https://doi.org/10.1007/s00035-022-00286-x
Sánchez-Bayo F, Green K (2013) Australian snowpack disappearing under the influence of global warming and solar activity. Arct Antarct Alp Res 45:107–118. https://doi.org/10.1657/1938-4246-45.1.107
Sandvik SM, Odland A (2014) Changes in alpine snowbed-wetland vegetation over three decades in northern Norway. Nord J Bot 32:377–384. https://doi.org/10.1111/j.1756-1051.2013.00249.x
Satyanti A, Nicotra AB, Merkling T, Guja LK (2018) Seed mass and elevation explain variation in seed longevity of Australian alpine species. Seed Sci Res 28:319–331. https://doi.org/10.1017/S0960258518000090
Schwienbacher E, Marcante S, Erschbamer B (2010) Alpine species seed longevity in the soil in relation to seed size and shape - A 5-year burial experiment in the Central Alps. Flora Morphol Distrib Funct Ecol Plants 205:19–25. https://doi.org/10.1016/j.flora.2008.10.007
Seglias AE (2022) Can alpine plant species bank on conservation? Using artificial aging to understand seed longevity. Appl Plant Sci 10. https://doi.org/10.1002/aps3.11493
Shimono Y, Kudo G (2001) Pattern of seedling emergence of alpine plants: comparisons between fellfield and snowbed habitats (sci- entific note). Mem Natl Inst Polar Res 467–478
Shimono Y, Kudo G (2003) Intraspecific variations in Seedling Emergence and Survival of Potentilla matsumurae (Rosaceae) between Alpine Fellfield and Snowbed habitats. Ann Bot 91:21–29. https://doi.org/10.1093/aob/mcg002
Shimono Y, Kudo G (2005) Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecol Res 20:189–197. https://doi.org/10.1007/s11284-004-0031-8
Smithson M, Verkuilen J (2006) A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods 11:54–71. https://doi.org/10.1037/1082-989X.11.1.54
Tonin R, Gerdol R, Tomaselli M et al (2019) Intraspecific functional trait response to Advanced Snowmelt suggests increase of growth potential but decrease of seed production in Snowbed Plant Species. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.00289
Vázquez-Ramírez J, Venn SE (2023) Snow, fire and drought: how alpine and treeline soil seed banks are affected by simulated climate change. Ann Bot. https://doi.org/10.1093/aob/mcad184
Venn SE, Morgan JW (2007) Phytomass and phenology of three alpine snowpatch species across a natural snowmelt gradient. Aust J Bot 55:450–456. https://doi.org/10.1071/BT06003
Venn SE, Morgan JW (2010) Soil seedbank composition and dynamics across alpine summits in south-eastern Australia. Aust J Bot 58:349–362. https://doi.org/10.1071/BT10058
Venn SE, Morgan JW (2017) Alpine plant species have limited capacity for long-distance seed dispersal. PlantEcol 218:813–819. https://doi.org/10.1007/s11258-017-0731-0
Venn SE, Green K, Pickering CM, Morgan JW (2011) Using plant functional traits to explain community composition across a strong environmental filter in Australian alpine snowpatches. Plant Ecol 212:1491–1499. https://doi.org/10.1007/s11258-011-9923-1
Verrall B, Pickering CM (2020) Alpine vegetation in the context of climate change: a global review of past research and future directions. Sci Total Environ 748:141344. https://doi.org/10.1016/j.scitotenv.2020.141344
Verrall B, Green K, Pickering CM (2023) Temporal dynamics in alpine snowpatch plants along a snowmelt gradient explained by functional traits and strategies. Oecologia 201:155–171. https://doi.org/10.1007/s00442-022-05297-3
Wahren C-H, Williams RJ, Papst WA (2001) Alpine and subalpine snow patch vegetation on the Bogong High Plains, SE Australia. J Veg Sci 12:779–790. https://doi.org/10.2307/3236865
Walker DA, Halfpenny JC, Walker MD, Wessman CA (1993) Long-term studies of Snow-Vegetation interactions. Bioscience 43:287–301. https://doi.org/10.2307/1312061
Wang G, Baskin CC, Baskin JM et al (2018) Effects of climate warming and prolonged snow cover on phenology of the early life history stages of four alpine herbs on the southeastern tibetan Plateau. Am J Bot 105:967–976. https://doi.org/10.1002/ajb2.1104
Welling P, Tolvanen A, Laine K (2004) The Alpine soil seed Bank in Relation to Field seedlings and Standing Vegetation in Subarctic Finland. Arct Antarct Alp Res 36:229–238. https://doi.org/10.1657/1523-0430
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis
Williams RJ, Wahren C-H, Stott KAJ et al (2015) An International Union for the Conservation of Nature red list ecosystems risk assessment for alpine snowpatch herbfields South-Eastern Australia. Austral Ecol 40:433–443. https://doi.org/10.1111/aec.12266
Winkler DE, Butz RJ, Germino MJ et al (2018) Snowmelt timing regulates community composition, phenology, and physiological performance of Alpine plants. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01140
Acknowledgements
We acknowledge the Ngarigo and Wurundjeri people as First Nations and Traditional Custodians of the land on which the fieldwork and desktop research for this study were conducted. We pay our respect to their Elders past and present, and recognise the continuing connection to land, sea and culture, and that sovereignty was never ceded. We thank Jack Dodd and Scout Fisher for their assistance during the 2020 fieldwork and Emily Nicholson for her help during various stages of the study. We also thank two anonymous reviewers for their comments on the early draft of the manuscript. Research permits were obtained from the New South Wales National Parks and Wildlife Service (references numbers SL102366 and SL101511).
Funding
This work was made possible by funding from the Deakin University Faculty of Science, Engineering and Built Environment Research Grant 2020 and Australian Research Council Linkage Projects LP190100844 and LP170101143. Griffith University postgraduate funding for B.V. and a Deakin University Post Graduate Award for J.V-R supported the 2020 standing vegetation survey and glasshouse work.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
S.E.V. and B.V. conceived the study. S.E.V., B.V., J.V-R. performed the soil seed bank fieldwork, K.G, K.P. and B.V. contributed with the long-term standing vegetation data, J.V-R, E.N., S.E.V. performed the greenhouse study. J.V-R. curated the data and performed statistical analyses. J.V-R. drafted the manuscript. All authors read and commented on draft versions, contributed to, and approved the final version of the manuscript. S.E.V., T.W. obtained the funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vázquez-Ramírez, J., Verrall, B., Newling, E. et al. Soil seed banks reveal the legacy of shifting plant assemblages in late-lying alpine snowpatch communities. Alp Botany (2024). https://doi.org/10.1007/s00035-024-00321-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00035-024-00321-z