Abstract
Rarity as a multidimensional concept has not been discussed for the ostracods along with the effects of physicochemical factors and sedimentation rates of waters on their distribution. We randomly sampled 91 aquatic sites including eight habitat types in the Çanakkale Province to study ostracod species rarity, commonness, and ecological preferences, and to test the relationships between the rarity index (average of geographic range, habitat specificity, and population size indices) and environmental factors. A total of 44 ostracod taxa (25 living) were reported from 75 sites. Among the species, Candonocypris caledonica was recorded for the first time in Türkiye. Sixty percent of 25 living species exhibited cosmopolitan characteristics, making significant contributions to the diversity of ostracods. The well-known cosmopolitan species, Heterocypris salina (rr = 0.268), Cypridopsis vidua (rr = 0.289), Limnocythere inopinata (rr = 0.330), Ilyocypris bradyi (rr = 0.370), and Psychrodromus olivaceus (rr = 0.390), were identified as common species based on the rarity index (rr) values. Of the environmental variables, only sediment grain sizes (×2000, ×500, and ×63 μm) were found to be effective on the distribution of ostracod species. The Shannon index exhibited a positively significant association with ×2000, while the population size index displayed a linear relationship with ×63 μm grain size, indicating a small population size and rarity. Associations among index values, sediment grain size, and water quality variables showed that comprehensive studies on ostracods, analyzing both water and sediment, can provide a clearer and more precise information for evaluating the rarity and ecological preferences of species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conservation of biodiversity suggests the protection of a great number of species, encompassing both common and rare species. Rare species are species of increasing importance in conservation biology (Maciel 2021) because the decline of a population of a rare species to below viable levels causes the loss of taxonomic, genetic, and functional diversity within a habitat (Longton and Hedderson 2000). Rarity is not easy to biologically define since it is indicated as the numeric ratio of a species to other sympatric species, and so many animal and plant species are classified as rare species (Drury 1974) because rare species are represented by a few individuals, while common species are represented by many individuals in ecological communities (Flather and Sieg 2007). For this reason, the method for determining rare species should be clear and understandable for scientists and decision-makers (Maciel 2021).
Beyond the abundance of species, rarity is a multidimensional concept and is described in seven forms of species rarity, relating to geographic range (restricted or widely distributed), local population density (abundance based and small or large populations), and habitat specialization (specialist or generalist), as well as combinations of these (Rabinowitz 1981). The purpose of the geographic range is to assign a species based on the area where it was found relative to a sampled area (Maciel and Arlé 2020). To classify species as habitat specialists and generalists, habitat specificity is utilized to detect the habitat preference of a species for a kind of habitat or microhabitat (Vitt 1981). The last one, population size, is based on the traditional rarity parameter, the number of individuals (Rabinowitz 1981), and Maciel (2021) also stated the usage of body size and density of a species to describe rare species. On the other hand, species distributed in a wide geographical range, occupying many habitats, and forming large populations or population sizes are called common, abundant, or cosmopolitan species (Işık 2011).
Causes of rarity are as follows: (i) the inherent biological and ecological characteristics of a species, such as its traits (e.g., slow life histories), and the traits of its ecosystem (e.g., low carrying capacity), and (ii) anthropogenic and/or extrinsic causes such as habitat loss or fragmentation, human exploitation for control and collecting, pollution, and introduction of exotic species (Pärtel et al. 2005). Species under the pressure of the extrinsic causes are more threatened than intrinsically rare species since intrinsically rare species are adapted to low species abundance to complete their life history when extrinsic rare species do not (Flather and Sieg 2007).
Biodiversity not only includes the number of species but also the phylogenetic and functional diversities. In this sense, the influence of a species on the spatial distribution of biodiversity can be shown in two ways, as follows: (i) the functional or phylogenetic distinctiveness of the species and (ii) the distribution of the species in geographic space (White et al. 2023). Therefore, identifying the contributions of common and rare species to the spatial distribution of biodiversity allows us to estimate the groups of indicator species for local and national monitoring schemes. For example, common species with 100% occupancy add little or no variance to a spatial species richness pattern, whereas species with 50% occupancy will add a large amount of variance (White et al. 2023). In addition, Lawler et al. (2003) stated that “Indicators of biodiversity have been proposed as a potential tool for selecting areas for conservation when information about species distributions is scarce.” Marcot and Flather (2007) utilized common species as indicators to find the location of rare species. All studies show that it is not only important whether a species is common or rare, but also how it is used. Each species is therefore an important taxonomic unit, as one species can be an indicator of another. Additionally, rare and common species both hold significance in ecosystem functioning, as emphasized in various studies (e.g., Lyons et al. 2005; Flather and Sieg 2007; Macdougall et al. 2013; Leitão et al. 2016). The loss of a species, regardless of its rarity, can seriously impact ecosystem functioning due to its unique functional roles (Violle et al. 2017). This decline in biodiversity not only affects ecosystem function but also human well-being (Ceballos et al. 2015). Determining the rarity or commonness of a species is very important for conservation efforts and understanding its importance in the ecosystem.
For numerous organisms, whether a species is rare or common often relies on limited parameters such as abundance or occurrence frequency (e.g., Özuluğ et al. 2018; Xiao et al. 2023), indicating the necessity of comprehensive assessment methods. This issue is also apparent in the case of ostracods. Ostracods with a length range of 0.3–8 mm and low-Mg calcite shells are encountered in a variety of aquatic bodies, including rivers, streams, ponds, rice fields, groundwater, oceans, lakes, springs, and semi-terrestrial habitats (Meisch 2000; Martens and Horne 2016). The ubiquity of ostracods relies on their carapaces, two shells connected to each other by a hinge at the dorsal part, with high preservation potential as fossils date to the early Ordovician period, approximately 485 million years ago (Williams et al. 2008). The geochemical information of shells is controlled by aquatic variables since they show relationships with major and minor elements in waters, such as magnesium (Mg2+), strontium (Sr2+), and calcium (Ca2+) (De Deckker et al. 1988), as well as the oxygen content of water (Delorme 1978) and chemical properties of water including temperature, pH, salinity, hydraulic conditions, and bottom grain sizes or sedimentation rates (Ruiz et al. 2013). During their distribution, ostracod species display species-specific responses to the above-mentioned aquatic variables (Külköylüoğlu 2013). The distribution of living ostracod species is also influenced by their ability to disperse, as these organisms can produce resting eggs (Meisch 2000). Therefore, fossil and modern (living) ostracods can be powerful and reliable indicators to reconstruct and estimate climate and environmental changes in past and present conditions, respectively (Holmes and Chivas 2002; Külköylüoğlu 2004). They are separated into two main indicator groups: positive [cosmopolitan (or cosmoecious) species with wider geographical distribution and tolerance levels to different environmental variables] and negative (noncosmopolitan species with limited tolerance levels and relatively narrow geographical distribution) (Külköylüoğlu et al. 2020). At this point, the question “Do cosmopolitan species tends to be common, whereas other species are considered rare?” arises.
Regarding this question, a rarity index developed by Maciel (2021) was used to evaluate the rarity of ostracod species in the Çanakkale Province (Türkiye). During the present study, this new index, a multiparameter index that does not depend on a single parameter, relating to the geographic range, habitat specificity, and population size indices, was used for the first time for the ostracods in the literature. In addition to studying both rare and common species, assessing the rarity of ostracod species with significant indicator potential will enable us to understand whether their rarity is inherent to their ecological characteristics or influenced by environmental variables. This will be achieved by conducting multidimensional statistical analyses. Therefore, we aimed (i) to assess the rarity and commonness of ostracod species in the Çanakkale Province (Türkiye) during the autumn season of 2022, (ii) to explore the relationships among rarity index and its three dimensions (the geographic range, the habitat specificity, and the population size) values with environmental variables, and (iii) to determine distribution and ecological preferences of individual ostracod species.
Material and methods
Study area
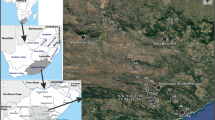
Çanakkale Province (Fig. 1) is located in the northwest of Türkiye, between 25°40′–27°30′ longitude and 39°27′–40°45′ latitude, separating the continents of Europe and Asia with the 61-km-long Dardanelles. It has 9933 km2 of surface area, and approximately 55% of this area is covered by forests, while the remaining areas consist of meadows, pastures, and arable land. The total coastline length of the province is 671 km. The provincial center, the Çanakkale, with about 2 m of elevation, covers 928 km2, constituting 10% of the total surface area. The largest island of Türkiye in the Aegean Sea, Gökçeada, as well as Bozcaada and Tavşan islands, are found within the province’s borders. The westernmost point of the Anatolian Peninsula, Baba Burnu, and the westernmost point of Türkiye, Avlaka Burnu on Gökçeada, are also within the provincial borders. The discharge of province’s rivers and streams increases during the fall season rains and the melting of snow in the April and May months, dropping to a few hundred liters per second at other times. There are a few lakes, and the existing ones are salt lakes on the Gelibolu Peninsula and Gökçeada, which dry up in the summer. The climate of the Çanakkale Province exhibits transitional climate characteristics due to its location. Although it generally reflects Mediterranean climate features, the presence of the province further north results in lower average temperatures during the winter. The minimum and maximum temperatures are −4.2 and +35.8 °C in February and August, respectively, when the annual average temperature and humidity are equal to 14.7 °C and 72.6%, respectively. A distinctive climatic feature of the climatic condition of the Çanakkale Province is the prevalence of windy weather (e.g., northeasterly wind, south–southwest wind) for a significant part of the year, compared with other close provinces. The annual average rainfall varies between 662.8 m3 (Gökçeada) and 854.9 m3 (Ayvacık). Rainfall significantly decreases during the summer months, with the highest precipitation occurring in December, January, and February. The number of days with snow cover is mostly limited, reaching a maximum of 8 days (ÇİKTM 2024).
Measurement and sampling
A total of 91 aquatic sites, including dam (d), stream (st), pond (p), trough (t), canal (ca), lake (l), spring (s), and creek (c), were visited for ostracod sampling from 28 October to 2 November (autumn) of 2022 (see Figs. 1 and S1). Firstly, a global positioning system, GARMIN Etrex 30, was used to record the elevation and coordinates (latitudes and longitudes). Air temperature (Ta, °C), moisture (%), and wind speed (Mph) were determined with the aid of a Kestel-3000 anemometer. Then, the water quality parameters of each sampled sites, representing pH, dissolved oxygen concentration (DO, mg L−1), water temperature (Tw, °C), electrical conductivity (EC, μS cm−1), salinity (psu), total dissolved solids (TDS, mg L−1), and oxidation–reduction potential (ORP, mV), were measured with a HANNA HI-9829 multiparameter device. Polyethylene bottles with a volume of 100 ml were utilized to collect water samples from each site for chemical analyses (calcium (Ca2+, mg L−1), magnesium (Mg2+, mg L−1), potassium (K1+, mg L−1), strontium (Sr2+, mg L−1) and ferrous iron (Fe2+, mg L−1)), maintaining in the containers at +4 °C until the analysis was done. After that, surface sediment (about 2–3 cm) was gathered with a rectangular hand net with a 150 μm mesh size for ostracod specimens from the littoral areas of each sampling site up to a 1 m depth. About 200 ml of collected sediments were put into the 250 ml plastic bottles and fixed with 70% ethanol to prevent the deterioration of the ostracod specimens for further studies. For sediment grain size analysis, we collected the sediment remaining after getting the ostracod samples in 100 ml plastic bottles.
Analyses in laboratory
ArcGIS and ArcMAP software with a 30 m Tandem-X Digital Elevation Model (DEM) was used to create the map of the sampled aquatic sites in Çanakkale Province (Fig. 1). The analyses of Ca2+, Mg2+, K1+, Sr2+, and Fe2+ were carried out with the aid of the Dionex ICS-1100 ion chromatography system at the Eskişehir Technical University (Türkiye), following the Standard Methods for the Examination of Water and Wastewater provided in APHA (2012). Hereafter, we used abbreviation of physico-chemical variables in the text. To determine the sediment grain size, we followed the Wentworth classification (Wentworth 1922), 2000–1000 µm very coarse sand–gravel, 1000–500 µm coarse sand, 500–250 µm medium sand, 250–125 µm fine sand, 125–63 µm very fine sand, 63–39 µm silt, and < 39 µm clay. The collected sediment samples were sieved through three separate sieves with sizes of 2000, 500, and 63 µm to get the average grain size and the results were given in terms of weight. These grain sizes are presented as ×2000, ×500, and ×63 µm in the text. Grain size measurements were performed at the Middle East Technical University (Türkiye) central laboratory using a particle size analyzer (Malvern Mastersizer 2000 device and Malvern Nano ZS90 device) both in dry and wet conditions.
Sediment samples collected for the ostracods were filtered through four standard-sized sieves (0.5, 1.0, 1.5, and 2.0 mm mesh size), washed under tap water, and then put in 250 ml plastic bottles with 70% ethanol. Subsequently, ostracod specimens were separated from the sediments with dissecting needles and a Pasteur pipette under an Olympus ACH 1X stereo microscope, and then summed in glass vials containing 70% ethanol. To conduct taxonomic identification, adult ostracod specimens were dissected in lactophenol solution to prepare permanent slides with soft body parts under a light microscope (Olympus BX-51). After dissection, the separated valves of each species were kept in micropaleontological slides to get scanning electron microscopy (SEM) illustrations, using a Carl Zeiss EVO-50 type SEM–EDX at the Department of Geological Engineering, Hacettepe University, Türkiye. We followed the taxonomic keys provided in Meisch (2000) and Karanovic (2012) to identify ostracod specimens to species or genus levels, using soft body parts and carapace features, along with checking the taxonomic nomenclature of species according to Meisch et al. (2019). All prepared permanent slides and individuals in glass vials are kept in the Limnology Laboratory of Bolu Abant İzzet Baysal University (Türkiye).
Statistical analyses
In all statistical analyses, adult ostracod individuals with complete soft body parts were considered. The Shannon index value of Çanakkale Province was calculated, along with the proportional contribution of the individual species to the alpha diversity using Species Diversity and Richness 4 software (Seaby and Henderson 2006).
To assess the rare ostracod species in the present study, the rarity index (Maciel 2021) based on the scheme of Rabinowitz (1981), relating to three dimensions of rarity proposed by her as follows: the geographic range, the habitat specificity, and the population size, were applied. Firstly, the geographic range index (gri) (Eq. 1), habitat specificity index (hsi) (Eq. 2), and population size index (psi) (Eq. 3) were calculated for each species.
where: gri is the geographic range index, latrange is the range of the two extreme latitude points and longrange is the range of the two extreme longitude points of the ith species. c is a constant equaling to 1, used to eliminate the sum of latrange and longrange being smaller than 1 since some species are found only one time and so they have only one latitude and longitude.
where: hsi is the habitat specificity index and hmax is the maximum habitat number where the ith species is encountered.
where: psi is the population size index and pmax is the maximum population of the ith species that occurred anywhere.
Then, an average index value, rarity index (rr) (Eq. 4), was computed from these mentioned indices for each species. The rr value ranges from 0 to 1, where an index value closer to 0 means a more common species and closer to 1 indicates a rarer species. This evaluation is also accepted for other above-mentioned indices (Maciel 2021). The R package called rrindex was utilized for the calculations of these indices (Maciel 2021).
Before the statistical analyses performed below, multicollinearity amid the environmental variables was checked, and variables with an inflation factor larger than 10, indicating a possibility of multicollinearity, were not used in the analyses, with the aid of Ecological Community Analysis II Software (Seaby and Henderson 2007). Accordingly, 11 (pH, dissolved oxygen concentration, electrical conductivity, water temperature, elevation, calcium, magnesium, strontium, and ×2000, ×500, and ×63 amount of sediment particles remained on the mesh with 2000, 500 and 63 μm sizes, respectively) environmental variables were used.
A fourth-corner analysis (Legendre et al. 1997) was used to test the associations or correlations between values of indices (gri, hsi, psi, and rr) and environmental variables. This method originally combined three tables into a matrix, representing species distribution across samples, environmental characteristics of samples, and species traits. We utilized the above-mentioned index values calculated for each species instead of species traits. The significance of the relationships was quantified by 999 permutations based on model 1 of Legendre et al. (1997), where the abundances of each species were permuted within each row to test the null hypothesis stating that environmental variables do not show any associations and/or control on the index values (gri, hsi, psi, and rr) of individual species. This analysis was run with aid of the R package ade4 (Dray and Dufour 2007). Beyond the fourth-corner analysis, we conducted a nonparametric Spearman rank correlation analysis using the IBM SPSS statistics package version 21 to examine the pattern of bivariate correlations between index values (gri, hsi, psi, and rr) and environmental variables (physico-chemical variables and sediment grain size), as well as among physico-chemical variables, Shannon index values, and sediment grain size. To assess the type of relationships (e.g., linear, non-linear, S-shaped) among index values and environmental variables, generalized additive models (GAMs) were applied based on the generalized cross validation (GCV) criterion and significance of explanatory variables in the Çanakkale Province, using the R package mgcv (Wood 2017).
The longest gradient length of the detrended correspondence analysis was larger than 4.0, suggesting the suitability of the species data for the application of unimodal technique, a canonical correspondence analysis (CCA) (Lepš and Šmilauer 2003). Environmental variables used in the CCA analysis were log transformed with PAST 3.26 software (Hammer et al. 2001) to eliminate skewness and achieve a normal distribution, except pH (ter Braak and Šmilauer 2002). Then, we performed CCA along with the forward selection of the Monte Carlo permutation test (499) using CANOCO 4.5 software to understand the relationships between environmental variables and ostracod species that occur more than two times. Optimum and tolerance levels of individual ostracod species, found at least two times, for different environmental variables were estimated with aid of C2 software (Juggins 2003), utilizing the weighted-averaging regression model.
Results
Descriptive statistical analysis results (minimum, maximum, mean, and standard deviation) of water quality variables obtained from the measurements in the field and analyzed in the laboratory are given in Table 1. A total of 44 ostracod taxa, comprising 25 living and 19 subfossil, were reported in 75 out of the 91 aquatic sites sampled in the present study. These taxa are classified into three superfamilies: Cypridoidea (39 taxa in 17 genus), Cytheroidea (4 taxa in genus Cyprideis, Limnocythere, and Semicytherura), and Darwinuloidea (1 taxa in genus Darwinula). SEM illustration of living species is provided in Fig. S2. Among living species, Cypridopsis vidua (O.F. Müller, 1776) was the most recorded species with 11 occurrences, followed by Heterocypris salina (Brady, 1868) (9 times), Limnocythere inopinata (Baird, 1843) (9 times), and Ilyocypris bradyi Sars, 1890 (5 times). Of which, H. salina was the only species found in seven of the eight habitat types during the present study (Tables 2 and S1). Fifteen of 25 living species were characterized with cosmopolitan characteristics (showed a wide geographical distribution) (Table 2). In the present study, mostly cosmopolitan species, such as L. inopinata, C. vidua, I. bradyi, and H. salina, made more contributions proportionally to the Shannon index (H′) value of the Çanakkale Province, equal to 2.559 (see Table 2).
According to geographic range index (gri), only one species, H. salina (gri = 0.466) had an index value lower than 0.5 (Fig. 2a, Table 2). About half of the species displayed a habitat specificity index (hsi) value equal to 1, indicating rarity. Conversely, the most common species, such as C. vidua (hsi = 0.250), H. salina (hsi = 0.333), L. inopinata (hsi = 0.333), and I. bradyi (hsi = 0.333), exhibited index values closer to 0, representing commonness (Fig. 2b, Table 2). In contrast to gri and hsi results, about 80% of species had population size index (psi) values lower than 0.4 (closer to 0), suggesting common species, except for Neglecandona lindneri (Petkovski, 1969), Ilyocypris monstrifica (Norman, 1862), and Potamocypris similis G.W. Müller, 1912, whose index values equal to 1 (Fig. 2c, Table 2). The rarity index (rr), obtained by averaging these three indices, varied from 0.268 to 1 for the 25 ostracod species in the present study. Neglecandona lindneri and P. similis were classified as rare species due to the rr index value corresponding to 1. Cosmopolitan species (Cypria opthalmica (Jurine, 1820), Cyprideis torosa (Jones, 1850), Ilyocypris inermis Kaufmann, 1900, Potamocypris villosa (Jurine, 1820), and Prionocypris zenkeri (Chyzer and Toth, 1858)) had rr index values larger than 0.650 indicating their rarity in the Çanakkale Province. Five well-known cosmopolitan species, H. salina (rr = 0.268), C. vidua (rr = 0.289), L. inopinata (rr = 0.330), I. bradyi (rr = 0.370), and P. olivaceus (Brady and Norman, 1889) (rr = 0.390), were classified as the common species based on rr index values. Additionally, Candona candida (O.F. Müller, 1776), H. incongruens (Ramdohr, 1808), I. gibba (Ramdohr, 1808), and Candonocypris caledonica (Méhes, 1939) showed rr index values below 0.5, suggesting their higher commonness in the present study (see Fig. 2d, Table 2).
Scatter plots provided by the indices’ values ranged from 0 to 1 (y axis) for 25 ostracod species (x axis) in the Çanakkale Province. The indices on the y axis are the geographic range index (gri; a), habitat specificity index (hsi; b), population size index (psi; c), and the rarity index (rr; d). Abbreviations of species codes are given in Table 1
Habitat specificity index (hsi) and population size index (psi) showed positive correlations with Mg2+ and ×63 μm sediment grain size, respectively, based on fourth-corner analysis. Meanwhile, geographical range index (gri), hsi, and rarity index (rr) exhibited moderately negative significant correlations with EC according to Spearman rank correlation analysis. In the same analysis, gri and psi indices displayed negatively significant correlations with ×2000 μm sediment grain size and Ca2+, respectively. Among the physico-chemical variables of water, Ca2+ and Sr2+ showed a statistically significant positive correlation with the ×2000 μm sediment grain size, while EC displayed positive correlation with the ×500 μm sediment grain size. The Shannon index value demonstrated a notable correlation of 41.8% with coarse particulate sediment, ×2000 μm sediment grain size (see Fig. 3).
Fourth-corner and Spearman rank correlation analyses showing the associations of geographic range index (gri), habitat specificity index (hsi), population size index (psi), and the rarity index (rr) with environmental variables, and among the Shannon index value, physico-chemical variables of water, and sediment grain sizes. In the fourth-corner analysis, red and grey colors indicate positive and no correlations, respectively. Abbreviations for environmental variables can be found in Table 1
Only statistically significant relationship patterns are visualized in Fig. 4 based on the generalized additive model (GAM) analysis. GAM analysis revealed statistically meaningful nonlinear relationship of gri and rr indices with pH. The associations of hsi and rr indices were mostly quadratic concave with Sr2+. Among the indices, psi demonstrated a positively linear relationship with ×63 μm sediment grain size (Fig. 4).
The first two axes of CCA explained 85.5% of correlations between species and environmental variables (see Table S2). Among them, sediment grain sizes [×2000 (λ = 0.57, F = 2.68, p = 0.004), ×500 (λ = 0.43, F = 2.06, p = 0.006), and ×63 (λ = 0.37, F = 1.80, p = 0.030)] exhibited significant influence on the distribution of ostracod species in the Çanakkale Province, when the other nine variables [Elev (λ = 0.28, F = 1.35, p = 0.200), Tw (λ = 0.27, F = 1.39, p = 0.202), DO (λ = 0.29, F = 1.43, p = 0.166), Ca2+ (λ = 0.17, F = 0.87, p = 0.512), Sr2+ (λ = 0.20, F = 1.02, p = 0.440), EC (λ = 0.18, F = 0.91, p = 0.542), Mg2+ (λ = 0.25, F = 1.25, p = 0.282) and pH (λ = 0.06, F = 0.30, p = 0.984)] did not show significant effects (Fig. 5). Among the species, N. neglecta (Sars, 1887) with a moderate rr index value (0.554) (Table 2) was positioned at the center of the CCA diagram, suggesting that its distribution is not significantly correlated with the environmental variables measured. Cypria opthalmica (rr = 0.807) and I. monstrifica (rr = 0.749), classified as rare species due to rr index values (Table 2), were located on the positive side of axis 1 without close associations with environmental variables measured. Of the common species, P. olivaceus displayed a negative correlation with ×63 μm sediment grain size, while I. bradyi exhibited a close association with ×2000 μm sediment grain size. Species with a rr index value close to 0.5 (Table 2), I. decipiens Masi, 1905 (0.512) and P. arcuata (Sars, 1903) (rr = 0.554) demonstrated close relationships with the negative side of axis 2 correlating with DO (see Fig. 5). In conformity with CCA results, P. arcuata (Opt = 12.92 mg L−1) and I. decipines (Opt = 10.16 mg L−1) were among the species with highest optimum levels for DO. Psychrodromus olivaceus had the lowest optimum level (0.09 g) for ×63 μm sediment grain size, while I. bradyi was one of the species with the highest optimum level (23.65 g) for ×2000 μm sediment grain size. Cypria opthalmica displayed the lowest optimum (369.50 μS cm−1) and tolerance (20.51 μS cm−1) levels for EC (for more information, see Table 3).
Distribution of ostracod species (▲, filled-up triangles) in the Çanakkale Province according to the effect of sediment grain sizes, ×63, ×500, and ×2000 μm, pH, elevation (Elev), dissolved oxygen (DO), electrical conductivity (EC), water temperature (Tw), strontium (Sr2+), calcium (Ca2+), and magnesium (Mg2+) on the CCA diagram. The arrows indicate environmental variables and dashed arrows represent the variables with statistically nonsignificant effects. Sampled sites (○, circles) are shown on the diagram and coded as 40st where “40” is the site and lowercase letters are ca, canal; c, creek; d, dam; l, lake; p, pond; s, spring; st, stream; and t, trough. Codes of species are provided in Table 1
Discussion
Until now, there have been five published studies (Gülen 1985; Kılıç et al. 2000; Altınsaçlı and Griffiths 2002; Rasouli et al. 2014; Perçin-Paçal et al. 2017) and one unpublished dataset (Altınsaçlı unpublished data) related to ostracods in the study area. However, most of these studies are not comprehensive and have sampled only a few sites within the province. An exception is the work of Perçin-Paçal et al. (2017), who conducted seasonal samplings at 28 sites on Gökçeada Island in 2007. The present study represents the first comprehensive and extensive ostracod investigation in the Çanakkale Province during the autumn season of 2022. Previous studies have documented a total of 28 non-marine ostracod species. Nine of which were also found in the present study (Table 2). Sixteen of 25 living species in the present study are new records for the Çanakkale Province. Of these species, Candonocypris caledonica is a new species for the non-marine ostracod fauna of Türkiye. With the findings of the present study, the total number of non-marine ostracod species in the province was increased to 44 species.
The 60% of the species that exhibit cosmopolitan characteristics in the present study (Table 2) underscores their significant contribution to overall species diversity (e.g., Yavuzatmaca 2019; Külköylüoğlu et al. 2020). For example, 11 out of 15 species contributing proportionally to the Shannon index value of the Çanakkale Province in the autumn season are cosmopolitan (Table 2). This contribution can be attributed to the frequent occurrence of cosmopolitan species with high abundances compared with non-cosmopolitans. The importance of these species beyond their contribution to diversity is that they can also be utilized as valuable indicators. It was emphasized that P. olivaceus, one of the cosmopolitan species found in this study, may be used an indicator of aquatic systems in both lotic and lentic habitats, while H. salina may be an indicator species of fluctuations in ecological variables and changes such as the case in EC (Yavuzatmaca 2020). While these findings highlight the importance of cosmopolitan species, their status as biodiversity indicators and their roles in the ecosystem functioning should be examined in future studies.
According to the geographic range (gri) and habitat specificity (hsi) indices, one and four cosmopolitan species, respectively, have index values below 0.5, suggesting they may be common species according to these indices. However, based on the population size index (psi), almost all species except three species appear to be common species in the present study (see Table 2 and Fig. 2). This demonstrates how the assessment of rarity or commonness varies when using different variables. When the rarity index (rr) (Maciel 2021), an average of these three indices was considered, only eight cosmopolitan and one non-cosmopolitan species have lower index values approaching commonness compared with other species. This observation highlights the reliability and feasibility of employing multiple parameters, rather than relying on a single one to evaluate the rarity and commonness of species. In a compilation of 188 studies from the literature by Özuluğ et al. (2018), commonly found cosmopolitan species in Türkiye, N. neglecta (recorded in 74 papers), C. torosa (in 67 papers), C. vidua (in 69 papers), and H. incongruens (in 63 papers), were characterized as common species based on their occurrence frequencies. The first two species in this study had rr index values above 0.5, while the last two species showed rr index values below 0.5 (Table 2). This indicates that while cosmopolitan species have the potential to become common species, not all cosmopolitan species achieve this status. This potential of cosmopolitans to become common can be explained by their uniform distribution capacity over space due to their long-distance colonization ability (Durrett and Levin 1998). This is evident in the case of ostracods. For example, Yavuzatmaca et al. (2015) and (2017b) found random and clump distributional patterns of ostracods among sampling sites in Adıyaman and Burdur provinces (Türkiye), respectively. These distributional patterns were attributed to the prevalence of species characteristics, with 51.85% of species exhibiting non-cosmopolitan characteristics in Adıyaman, while 63.64% of species with cosmopolitan characteristics in Burdur. Considering the wide geographical distribution and broad ecological preferences of cosmopolitan species (Külköylüoğlu 2013), it should not be assumed that cosmopolitan species are exempt from protection or are not endangered. This is because if cosmopolitan species are not taken under protection without understanding whether they are common or rare in a specific region, the loss of a species, even if it is cosmopolitan, will be inevitable and may negatively affect the functioning of the ecosystem, considering that a species may have a unique functional trait (Violle et al. 2017).
The fourth-corner and Spearman’s correlation analyses revealed that physico-chemical variables in water exert effects on the values of the indices (gri, hsi, psi, and rr) in the autumn of 2022 (Fig. 3). Among them, hsi shows a positive correlation with Mg2+, indicating that an increase in Mg2+ values correspond to an increase in the index value and suggesting an augmentation in rare species. Conversely, hsi indicates a quadratic convex relationship with Sr2+, implying that an increase in Sr2+ values initially enhance the index, but beyond a certain point, it starts to decrease. This suggests that habitats meeting the ecological preferences of species inhabit numerous species, opposed to species found in nearly in all habitats, such as H. salina in the present study. The low levels of these variables in the water facilitate the adaptation of species with wider tolerance levels, while adversely affecting other species. These variables, (e.g., low Mg2+found as trace element of the ostracod carapace) are closely tied to Mg2+ and Sr2+ ratios in water (Chivas et al. 1985; De Deckker et al. 1988, 1999; Holmes and De Deckker 2012). Similarly, a negatively significant correlation was observed between psi and Ca2+ (Fig. 3). An increase in the Ca2+ content of water leads to low values of psi, as presented in Table 2. This means that Ca2+ can directly or indirectly be effective on the number of ostracod individuals. The dissolution of limestone by carbonic acid elevates levels of ionized Ca2+ and bicarbonate (HCO31−) in water, promoting photosynthesis with a subsequent rise in carbonate (CO32−) and hydroxyl (OH1−). The presence of Ca2+ limits pH increase by precipitating CO32− as calcium carbonate (CaCO3) (Wetzel 2001; Boyd et al. 2016), indicating close relationships among Ca2+, pH, and DO. Accordingly, high values of Ca2+ in the water bodies correlates to low pH and DO values, illustrating why many species in this study exhibit low psi index values (Table 3). This is because all species, except C. caledonica (6.40 mg L−1), had optimum values above 7 for DO, while I. gibba exhibited the lowest optimum level (8.45) for pH compared with others with higher values. Such kind of relationships among physico-chemical variables in waters help to explain the nonlinear relationship of pH with gri and rr indices (Fig. 3) and the quadratic concave association of Sr2+ with rr in the present study (Fig. 4). All these findings recommend that both individual ostracod species and index values are affected by the water quality variables, since ostracods seem to be related to specific ions in the waters that they inhabit to calcify their low magnesium calcite carapaces (Turpen and Angell 1971). Additionally, the sum of all dissolved ion concentrations, EC, also indicated negative correlations with hsi and rr indices (Fig. 3), demonstrating its effect on the distribution of ostracod species, as previously proposed by Çapraz et al. (2022).
Sediment grain sizes displayed influential effects on the distribution of ostracod species in the present study (Fig. 5). Ostracods utilize sediments to escape from predators through burrowing (Mbahinzireki et al. 1991) and to find a suitable location to explore nutrients (Roca and Danielopol 1991). The grain size of sediment exhibits various effects on the ostracod assemblages (Ruiz et al. 2013). For instance, Suren (1992) highlighted the high abundance of ostracods in gravel habitats, while Geranmayeh and Varnosfaderany (2022) found a negatively significant correlation of ostracods’ density with percentage of gravel and sand but positively with the amount of silt and clay. Among sediment grain sizes, fine sediment content with reduced porosity and hydraulic permeability restricts nutrients and oxygen exchange between surface and subsurface sediments. The deposition fine sediments provide a homogenized habitat for taxa tolerating low DO and the ability to burrow in sediments (Mathers et al. 2019). On the other hand, finer sediments limit interstitial habitats, precluding the larger-bodied organisms (Boulton 2007). The lowest survivorship of ostracods was observed in finer sediments compared with coarse because of vigorous movement of ostracods, clogging of respiratory appendages, and the lower value of DO (Donohue and Irvine 2003). Casado-Martinez et al. (2016) emphasized that the presence of clay as proxy for finer sediments decreases the growth of ostracods due to high organic matter in fine-grained sediments and the greater adsorption capability of fine sediments for pollutants than coarser ones. The linear relationship of psi and ×63 μm sediment grain size in the present study (Fig. 4) supports the negative effect of finer sediment on ostracod population size. An increase in the ratio of finer sediment ascends the value of psi, signifying rare species with small population size, as only ostracod species tolerate conditions of habitats with finer sediment (e.g., low DO). The positive and significant correlation of Shannon index value with ×2000 μm sediment grain size (Fig. 3) illustrates that sediment with large grain size has a positive effect on ostracod diversity. Mao et al. (2021) underlined the importance of sediment grain size, along with the water depth and hydrological conditions, on the distribution of ostracods in Dalongchi Lake, northwestern China. In the same study, authors pinpointed the preference of Ilyocypris sp. for relatively coarse substrate. In the present study, two species of the genus Ilyocypris (I. bradyi and I. gibba) showed close associations with coarse particulate sediments (Fig. 5). Both species are also among the species with highest tolerance levels to ×2000 μm sediment grain size (Table 3). The close association of P. kraepelini G.W. Müller, 1903 with larger grain size was reported by Szlauer-Łukaszewska and Pešić (2020). In the present study, the species displayed a similar preference for larger grain size sediments, oriented on the side of ×2000 μm sediment grain size (Fig. 5), while having lowest optimum level (0.23) after P. olivaceus (0.09) for ×63 μm sediment grain size (Table 3).
Considering that the transfer of gases and ions continues until an equilibrium is established among water, air, and sediment (Boyd 2020), the importance of sediment for benthic ostracods becomes evident. As seen in Fig. 3, the correlations of water chemicals with sediment grain size indicate that water and sediment should be considered together when discussing benthic organisms. This relationship impacts both the distribution of species and their levels of rarity and commonness. For example, sediment phosphate content was reported to be the most effective factor in the distribution of ostracods in Sinop Province (Türkiye), along with magnesium and water temperature (Yavuzatmaca et al. 2017a). Therefore, when water and sediment analyses are conducted simultaneously and then distribution and ecology of ostracods are studied, valuable information will be obtained regarding the indicator potential of these organisms.
Conclusions
A new addition to the non-marine ostracod fauna of Türkiye is C. caledonica, reported for the first time. The direct impact of cosmopolitan species on local species diversity has been observed. Indices used to assess species rarity and commonness, such as gri, hsi, psi, and rr, have demonstrated distinct relationships with the physico-chemical properties of water. Also, it is strongly recommended to use multiple parameters rather than one to evaluate the rarity of species for a more accurate assessment. While cosmopolitan species have the potential to become common compared with others, it is essential to assess this potential, considering that not every cosmopolitan ostracod species becomes common. Sediment grain size displayed notable correlational patterns with ostracod distribution and clear relationships with water quality parameters. Therefore, we recommend conducting comprehensive studies on ostracods by simultaneously analyzing both water and sediment to provide clearer and more precise information in future studies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Altınsaçlı S, Griffiths HI (2002) A review of the occurrence and distribution of the recent non-marine Ostracoda (Crustacea) of Turkey. Zool Middle East 27:61–76. https://doi.org/10.1080/09397140.2002.10637941
Boulton AJ (2007) Hyporheic rehabilitation in rivers: restoring vertical connectivity. Freshw Biol 52:632–650. https://doi.org/10.1111/J.1365-2427.2006.01710.X
Boyd CE (2020) Water quality, third. Springer International Publishing, Auburn, AL, USA
Boyd CE, Tucker CS, Somridhivej B (2016) Alkalinity and hardness: critical but elusive concepts in aquaculture. J World Aquac Soc 47:6–41. https://doi.org/10.1111/JWAS.12241
ter Braak J, Šmilauer P (2002) CANOCO reference manual and canodraw for windows user’s guide: software for canonical community ordination (version 4.5). Plant Research International, Wageningen, The Netherlands
Çapraz Ç, Külköylüoğlu O, Akdemir D, Yavuzatmaca M (2022) Determining effective environmental factors and ecology of non-marine Ostracoda (Crustacea) in Giresun. Turkey Int J Limnol 58:3. https://doi.org/10.1051/limn/2022002
Casado-Martinez MC, Burga-Pérez KF, Bebon R et al.(2016) The sediment-contact test using the ostracod Heterocypris incongruens: effect of fine sediments and determination of toxicity thresholds. Chemosphere 151:220–224. https://doi.org/10.1016/j.chemosphere.2016.01.126
Ceballos G, Ehrlich PR, Barnosky AD et al.(2015) Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci Adv. https://doi.org/10.1126/SCIADV.1400253/SUPPL_FILE/1400253_SM.PDF
Chivas AR, De Deckker P (1985) Shelley JMG (1985) Strontium content of ostracods indicates lacustrine palaeosalinity. Nat 3166025(316):251–253. https://doi.org/10.1038/316251a0
ÇİKTM (2024) Çanakkale İl Kültür ve Turizm Müdürlüğü. https://canakkale.ktb.gov.tr/TR-70467/cografya.html#:~:text=%C3%87anakkale%3B%20Balkan%20Yar%C4%B1madas%C4%B1’n%C4%B1n%20Do%C4%9Fu,Bal%C4%B1kesir%20il%20s%C4%B1n%C4%B1rlar%C4%B1%20ile%20%C3%A7evrilidir. Accessed 8 Jan 2024
De Deckker P, Chivas AR, Shelley JMG, Torgersen T (1988) Ostracod shell chemistry: a new palaeoenvironmental indicator applied to a regressive/transgressive record from the gulf of Carpentaria, Australia. Palaeogeogr Palaeoclimatol Palaeoecol 66:231–241. https://doi.org/10.1016/0031-0182(88)90201-5
De Deckker P, Chivas AR, Shelley JMG (1999) Uptake of Mg and Sr in the euryhaline ostracod Cyprideis determined from in vitro experiments. Palaeogeogr Palaeoclimatol Palaeoecol 148:105–116. https://doi.org/10.1016/S0031-0182(98)00178-3
Delorme LD (1978) Distribution of freshwater ostracodes in lake erie. J Great Lakes Res 4:216–220. https://doi.org/10.1016/S0380-1330(78)72187-8
Donohue I, Irvine K (2003) Effects of sediment particle size composition on survivorship of benthic invertebrates from Lake Tanganyika, Africa. Arch Für Hydrobiol 157:131–144. https://doi.org/10.1127/0003-9136/2003/0157-0131
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20. https://doi.org/10.18637/JSS.V022.I04
Drury WH (1974) Rare species. Biol Conserv 6:162–169. https://doi.org/10.1016/0006-3207(74)90061-5
Durrett R, Levin S (1998) Spatial aspects of interspecific competition. Theor Popul Biol 53:30–43. https://doi.org/10.1006/TPBI.1997.1338
Flather CH, Sieg CH (2007) Species rarity: definition, causes, and classification. In: Raphael MG, Molina R (eds) Conservation of rare or little-known species: biological, social, and economic considerations. Island Press, Washington, DC, pp 40–66
Geranmayeh A, Varnosfaderany MN (2022) Monitoring the frequency changes of ostracod shells in the sediment core (Case study: gavkhooni international Wetland). Iran J Appl Ecol 11:45–59. https://doi.org/10.47176/IJAE.11.1.8651
Gülen D (1985) The species and distribution of the group of Podocopa (Ostracoda-Crustacea) in freshwaters of western Anatolia. İstanbul Üniversitesi Fen Fakültesi Mecmuası Seri B 50:65–80
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:178
Holmes JA, Chivas AR (2002) Ostracod shell chemistry—overview. Geophysical monograph series. American Geophysical Union (AGU), pp 185–204
Holmes JA, De Deckker P (2012) The chemical composition of ostracod shells: applications in quaternary palaeoclimatology. Dev Quat Sci 17:131–143. https://doi.org/10.1016/B978-0-444-53636-5.00008-1
Işık K (2011) Rare and endemic species: why are they prone to extinction? Turk J Botany 35:411–417. https://doi.org/10.3906/bot-1012-90
Juggins S (2003) Software for ecological and palaeoecological data analysis and visualization—C2 User Guide. Newcastle University, Newcastle upon Tyne, UK
Karanovic I (2012) Recent freshwater ostracods of the world. Springer Berlin Heidelberg, Berlin, Heidelberg
Kılıç M, Altınsaçlı S, Balkıs H, Balkıs N (2000) The ostracods species collected from coasts of the Gökçeada (Imbroz) island (Aegean Sea). Turkish J Mar Sci 6:87–102
Külköylüoğlu O (2004) On the usage of ostracods (Crustacea) as bioindicator species in different aquatic habitats in the Bolu region, Turkey. Ecol Indic 4:139–147. https://doi.org/10.1016/j.ecolind.2004.01.004
Külköylüoğlu O (2013) Diversity, distribution and ecology of non-marine Ostracoda (Crustacea) in Turkey: application of pseudorichness and cosmoecious species concepts. Recent Res Dev Ecol 4:1–14
Külköylüoğlu O, Akdemir D, Yavuzatmaca M (2020) Non-marine Ostracoda (Crustacea) as indicator species group of habitat types. Aquat Ecol 54:519–533. https://doi.org/10.1007/s10452-020-09757-x
Lawler JJ, White D, Sifneos JC, Master LL (2003) Rare species and the use of indicator groups for conservation planningespecies raras y el uso de grupos indicadores para la planeación de la conservación. Conserv Biol 17:875–882. https://doi.org/10.1046/J.1523-1739.2003.01638.X
Legendre P, Galzin R, Harmelin-Vivien ML (1997) Legendre, Galzin, Harmelin-Vivien-1997—relating behavior to habitat solutions to thefourth-corner problem. Ecology 78:547–562. https://doi.org/10.1890/0012-9658(1997)078[0547:RBTHST]2.0.CO;2
Leitão RP, Zuanon J, Villéger S et al.(2016) Rare species contribute disproportionately to the functional structure of species assemblages. Proc Biol Sci. https://doi.org/10.1098/RSPB.2016.0084
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press
Longton RE, Hedderson TA (2000) What are rare species and why conserve them? Lindbergia 25:53–61. https://doi.org/10.2307/20150038
Lyons KG, Brigham CA, Traut BH, Schwartz MW (2005) Rare species and ecosystem functioning. Conserv Biol 19:1019–1024. https://doi.org/10.1111/j.1523-1739.2005.00106.x
Macdougall AS, McCann KS, Gellner G, Turkington R (2013) Diversity loss with persistent human disturbance increases vulnerability to ecosystem collapse. Nature 494:86–89. https://doi.org/10.1038/NATURE11869
Maciel EA (2021) An index for assessing the rare species of a community. Ecol Indic 124:107424. https://doi.org/10.1016/J.ECOLIND.2021.107424
Maciel EA, Arlé E (2020) Rare7: an R package to assess the forms of rarity in a community. Ecol Indic 115:106419. https://doi.org/10.1016/J.ECOLIND.2020.106419
Mao X, Liu X, Li J et al.(2021) Population age structure of ostracods in lake sediment and its implication for within-lake transport of microfossils. Ecol Indic 131:108182. https://doi.org/10.1016/j.ecolind.2021.108182
Marcot BG, Flather CH (2007) Species-level strategies for conserving rare or little-known species [Chapter 6]. In: Raphael MG, Molina R (eds) Conservation of rare or little-known species: Biological, social, and economic considerations. Island Press, Washington, DC, pp 125–164
Martens K, Horne DJ (2016) Collecting and processing living, non-marine ostracods. J Crustac Biol 36:849–854. https://doi.org/10.1163/1937240X-00002488
Mathers KL, Hill MJ, Wood CD, Wood PJ (2019) The role of fine sediment characteristics and body size on the vertical movement of a freshwater amphipod. Freshw Biol 64:152–163. https://doi.org/10.1111/FWB.13202
Mbahinzireki G, Uiblein F, Winkler H (1991) Microhabitat selection of ostracods in relation to predation and food. Hydrobiologia 222:115–119
Meisch C (2000) Freshwater ostracoda of Western and Central Europe (Süswasserfauna von mitteleuropa). Spektrum Akademischer Verlag, Heidelberg
Meisch C, Smith RJ, Martens K (2019) A subjective global checklist of the extant non-marine ostracoda (Crustacea). Eur J Taxon 2019:1–135. https://doi.org/10.5852/EJT.2019.492
Özuluğ O, Kubanç SN, Kubanç C, Demirci Gİ (2018) Checklist of quaternary and recent ostracoda (Crustacea) species from Turkey with information on habitat preferences. Turkish J Biosci Collect 2:51–100
Pärtel M, Kalamees R, Reier Ü et al.(2005) Grouping and prioritization of vascular plant species for conservation: combining natural rarity and management need. Biol Conserv 123:271–278. https://doi.org/10.1016/J.BIOCON.2004.11.014
Perçin-Paçal F, Altınsaçlı S, Altınsaçlı S (2017) Distribution, diversity and some ecological characteristics of ostracods (Crustacea: Ostracoda) in Gökçeada (Imbros) Island (Northern Aegean Sea, Turkey). Biol 72:774–789. https://doi.org/10.1515/biolog-2017-0088
Rabinowitz D (1981) Seven forms of rarity. In: Synge H (ed) The biological aspects of rare plants conservation. Wiley, pp 205–217
Rasouli H, Aygen C, Külköylüoǧlu O (2014) Contribution to the freshwater ostracoda (Crustacea) Fauna of Turkey: distribution and ecological notes. Turkish J Fish Aquat Sci 14:11–20. https://doi.org/10.4194/1303-2712-v14_1_02
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA), Washington, D.C., USA
Roca JR, Danielopol DL (1991) Exploration of interstitial habitats by the phytophilous Ostracod Cypridopsis vidua (O.F. Millier): experimental evidence. Annls LimnoL 27:243–252. https://doi.org/10.1051/limn/1991018
Ruiz F, Abad M, Bodergat AM et al.(2013) Freshwater ostracods as environmental tracers. Int J Environ Sci Technol 10:1115–1128. https://doi.org/10.1007/s13762-013-0249-5
Seaby R, Henderson P (2006) Species diversity and richness version 4. Pisces-Conservation Ltd., Lymington, England
Seaby RM, Henderson PA (2007) Community analyses package (CAP 4.1. 3.). Engl Pisces Conserv Ltd, Lymington, England
Suren AM (1992) Meiofaunal communities associated with bryophytes and gravels in shaded and unshaded alpine streams in New Zealand. New Zeal J Mar Freshw Res 26:115–125. https://doi.org/10.1080/00288330.1992.9516507
Szlauer-ŁUkaszewska A, Pešić V (2020) Habitat factors differentiating the occurrence of Ostracoda (Crustacea) in the floodplain of a small lowland River Kra piel (N-W Poland). Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2020012
Turpen JB, Angell WR (1971) Aspects of molting and calcification in the ostracod heterocypris. Biol Bull 140:331–338. https://doi.org/10.2307/1540077
Violle C, Thuiller W, Mouquet N et al.(2017) Functional rarity: the ecology of outliers. Trends Ecol Evol 32:356–367. https://doi.org/10.1016/J.TREE.2017.02.002
Vitt LJ (1981) Lizard reproduction: habitat specificity and constraints on relative clutch mass on JSTOR. Am Nat 117:506–514
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Wetzel R (2001) Limnology: lake and river ecosystems. Academic Press, Elsevier Science, USA
White HJ, McKeon CM, Pakeman RJ, Buckley YM (2023) The contribution of geographically common and rare species to the spatial distribution of biodiversity. Glob Ecol Biogeogr 32:1730–1747. https://doi.org/10.1111/geb.13734
Williams M, Siveter DJ, Salas MJ et al.(2008) The earliest ostracods: the geological evidence. Senckenb Lethaea 88:11–21. https://doi.org/10.1007/BF03043974
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. Chapman and Hall/CRC Press, New York
Xiao J, Zhong Z, Wang C et al.(2023) Rare species are significant in harsh environments and unstable communities: based on the changes of species richness and community stability in different sub-assemblages. Sustain 15:13994. https://doi.org/10.3390/SU151813994
Yavuzatmaca M (2019) Comparative analyses of non-marine ostracods (Crustacea) among water basins in Turkey. Acta Zool Acad Sci Hungaricae 65:269–297. https://doi.org/10.17109/AZH.65.3.269.2019
Yavuzatmaca M (2020) Species assemblages of Ostracoda (Crustacea) from west-site of Turkey: their indicator potential for lotic and lentic habitats. Biologia (bratisl) 75:2301–2314. https://doi.org/10.2478/s11756-020-00494-y
Yavuzatmaca M, Külköylüoğlu O, Yılmaz O (2015) Distributional patterns of non-marine Ostracoda (Crustacea) in Adiyaman Province (Turkey). Ann Limnol - Int J Limnol 51:101–113. https://doi.org/10.1051/limn/2015005
Yavuzatmaca M, Külköylüoğlu O, Yılmaz O, Akdemir D (2017a) On the relationship of ostracod species (Crustacea) to shallow water ion and sediment phosphate concentration across different elevational range (Sinop, Turkey). Turkish J Fish Aquat Sci 17:1333–1346. https://doi.org/10.4194/1303-2712-v17_6_40
Yavuzatmaca M, Külköylüoğlu O, Yılmaz O (2017b) Estimating distributional patterns of non-marine Ostracoda (Crustacea) and habitat suitability in the Burdur province (Turkey). Limnologica 62:19–33. https://doi.org/10.1016/J.LIMNO.2016.09.006
Acknowledgements
We thank to Mr. Ömer Lekesiz from Osmaniye Korkut Ata University, Türkiye for his help to create the map. Also, we would like to express our gratitude to Mrs. Filiz Batmaz and Mr. Ahmet Özdilek for their kind help in the laboratory. This research was supported by The Scientific and Technological Research Council of Türkiye (Project no: 121Y430).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The study was designed by M.Y. and O.K. The field data and samples were collected by M.Y., A.At., and A.Ay. Field samples were analysed by M.Y., O.K., A.At., A.Ay., and İ.Ö.Y. Sediment grain size analysis was performed by İ.Ö.Y. Statistical analyses and first draft of manuscript were performed and written, respectively, by M.Y., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yavuzatmaca, M., Külköylüoğlu, O., Ataman, A. et al. Assessment of rarity and ecological preferences of the non-marine ostracods (Crustacea) in Çanakkale Province (Türkiye). Aquat Sci 86, 74 (2024). https://doi.org/10.1007/s00027-024-01088-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01088-5