Abstract
Lake ecology can be affected by exchange flows driven by horizontal temperature gradients in lake–wetland interfaces. In this work, we investigate the hypothesis that thermally driven flows modulate the horizontal migration patterns of freshwater zooplankters. A 48-h field campaign in a shallow lake (Lake Vela, Quiaios, Portugal) was carried out to test this hypothesis. Thermal differences between the littoral and limnetic areas were measured along two transects featuring a Schoenoplectus lacustris and a Myriophyllum aquaticum stand in the littoral. In parallel, the physiochemistry and chlorophyll a, as a proxy for food availability differences between the littoral and the limnetic zones, were monitored. Zooplankton samples were collected for assessing overall and group-specific number-density differences. The diel period (day or night) and the site (littoral or limnetic zone) did not interact significantly to modulate the variation patterns for the studied physiochemical variables, indicating that these parameters should not explain horizontal zooplankton distribution patterns. The expected patterns for zooplankton diel horizontal migration as driven by the presence of visual predators were occasionally confirmed by our limnetic versus littoral abundance records through time, depending on the transect. Group-specific abundance patterns indicate particular features: copepods always preferred the littoral over the limnetic zone regardless of the diel period; chydorids always preferred the littoral zone regardless of the macrophyte stand involved; bosminids tended to preferentially concentrate in the limnetic zone. No consistent relationship was identified between the expected flow direction due to temperature differences and zooplankton abundance changes, although it occasionally occurred through the dataset.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural convection in lentic water bodies can influence water quality (Naghib et al. 2018) and may have substantial ecological impacts (Mao et al. 2019). In a lake, mixing and transport of particles can be promoted by density currents driven by variations in water temperature (Mortimer 1974; Okely and Imberger 2007; Tsydenov et al. 2016; Mao et al. 2019). Horizontal differences (limnetic vs littoral areas) in temperature create density gradients, which promote the establishment of horizontal surface exchange flows (Farrow 2004; Okely and Imberger 2007). The faster heating of the water in shallower areas than in the deeper areas generates convective currents from the littoral to the open lake at the surface. Temperature differences as small as 0.5 °C lead to velocity magnitudes of the order of a few centimetres per second (Pálmarsson and Schladow 2008). Also, aquatic plants are able to induce convective motion by promoting differential shading and by reducing wind in shallower regions (Lovstedt and Bengtsson 2008; Lightbody et al. 2008; Zhang and Nepf 2009). Lovstedt and Bengtsson (2008) observed average temperature differences of 0.8 °C and mean velocities of 0.8 ± 0.5 cm/s in surface currents towards the vegetated littoral in a shallow lake in southern Sweden. These thermal flows can transport nutrients, chemicals and pollutants through the surface of water across lentic water bodies (Mao et al. 2019), and can influence zooplankton distribution patterns (Podsetchine and Schernewski 1999).
The distribution of zooplankters has been argued to be driven by abiotic and/or biotic factors (Viljanen and Karjalainen 1993; Pinel-Alloul 1995; Thackeray et al. 2004; Gabaldón et al. 2019; Rollwagen-Bollens et al. 2020), relating to two major zooplankton movement patterns: diel horizontal migration (DHM) and diel vertical migration (DVM) (Pinel-Alloul 1995; Hembre and Megard 2003; Pinel-Alloul et al. 2004; Emily et al. 2017; Ermolaeva et al. 2019). DVM describes the movement into deeper and darker sites in the water column during the light time (day) to avoid visual predators, but at night the opposite movement typically occurs towards the water surface for improved acquisition of food resources (O’Brien 1979; Viljanen and Karjalainen 1993; Cuker and Watson 2002). In shallow lakes, DVM is less meaningful, since light may easily reach the bottom of the water column (Lauridsen et al. 1998; Burks et al. 2002). Rather, DHM is a major mechanism, described as the movement of zooplankton from limnetic zones into the littoral zones where macrophyte stands occur or vice versa (Wojtal et al. 2003; Jensen et al. 2010). DHM has been argued to depend on factors such as oxygen availability, pH, water turbidity, food resource availability and predatory pressure (Lauridsen et al. 1996; Burks et al. 2002, 2006; Ermolaeva et al. 2019). Fish predators can have a strong role in inducing zooplankton to move horizontally (Wojtal et al. 2003), and this pressure is higher in shallow than in deep lakes, since the relative density of fish is higher (Jeppesen 1998; Burks et al. 2002). Zooplankters indeed perceive chemical cues released by predators, as demonstrated by the induction of morphological and behavioural defences, as well as physiological adjustment (Engelmayer 1995; Lauridsen and Lodge 1996; Sakwińska 1998; Laforsch et al. 2006; Pijanowska et al. 2006; Castro et al. 2007a; Bell et al. 2019).
Although there is evidence of the establishment of typical DHM (see above), consistent preference for the limnetic zone by zooplankters regardless of the light cycle has also been found in shallow lakes and during spring, when predatory cues are greatest (Antunes et al. 2007; Castro et al. 2007b). The inconsistent evidence in DHM patterns may relate to the limited examination of conditioning factors, while DVM has been studied in more detail. For example, the role of thermal-driven surface exchange flows in modulating DHM of zooplankters in shallow lakes has not been comprehensively studied, despite occasional reference related to DVM (Pinel-Alloul 1995). This is an important gap considering the swimming activity constraints of zooplankters; they can float, drift or indeed swim, but their active swimming capacity has been assumed to be limited, leaving them at the mercy of currents to a significant extent (Thorp and Covich 2001). In fact, zooplankton exhibit movement patterns that can be generally understood as their swimming mode, which can be active (e.g. for cruise feeders and feeding-current zooplankters) or passive (e.g. for ambush feeders; Tiselius and Jonsson 1997; Visser and Thygesen 2003; Kiørboe 2011; Kjellerup and Kiørboe 2012; van Someren Gréve et al. 2017). There are species-specific, gender-specific and size-specific variations regarding swimming capacity and patterns (Folt and Burns 1999; Bianco et al. 2014; Almeda et al. 2017; Heuschele et al. 2017; McCloud et al. 2018; Ekvall et al. 2020), but the true capacity of actively swimming against flows to follow a given stimulus (e.g. food availability or predation avoidance) can be questioned.
In this context, the use of thermal surface exchange flows as a locomotory mechanism to zooplankters seems reasonable. This is an underlying hypothesis of the present study, which we explore by conducting an experimental campaign in Lake Vela, a shallow lake that exhibits temperature differences between littoral and pelagic areas, mostly when the former is covered with Myriophyllum.
Materials and methods
Study area
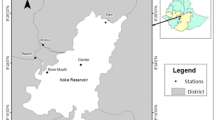
The study area is located in the southern end of Lake Vela (40.259984, −8.792322; Fig. 1), where dense, non-coincident littoral stands of the emergent macrophyte Schoenoplectus lacustris (hereinafter coded as S) or the submerged macrophyte Myriophyllum aquaticum (hereinafter coded as M) are established. A 48-h-long sampling campaign covering these two stands was run during July 2019. The time frame defined for the study was defined considering the typical plankton dynamics in Lake Vela and the climatic evolution of the spring season observed in 2019. In this way, a period of high abundance of zooplankton of a wide range of sizes, in its typical growing season, was targeted and successfully verified.
Location of the study area at Lake Vela (Quiaios, Portugal). The positioning of the temperature sensors in the limnetic (out) or littoral areas (in), relating to the Myriophyllum aquaticum stand (M) or to the Schoenoplectus lacustris stand (S) is shown, and a schematic detail of the deployment at each site is provided
Lake Vela is a freshwater shallow lake with 70 ha surface area included in the Natura 2000 network (PTCON0055) (CM—Ministries Council 2000), which is integrated in the Quiaios system of interconnected reservoirs (Figueira da Foz, Portugal; Fig. 1).
The west bank of the lake is covered by a Pinus pinaster and Acacia spp. stand, and the east bank comprises agricultural fields (Abrantes et al. 2006b, 2010). Littoral vegetation across the lake includes Phragmites australis, Cladium mariscus, Nymphaea alba, Myriophyllum sp. and species of the Poaceae family (Antunes et al. 2003; Abrantes et al. 2006a; Castro et al. 2007b). The lake harbours several fish species including several dominant non-indigenous fish species: the pumpkinseed sunfish Lepomis gibbosus, the mosquitofish Gambusia holbrooki, the carp Cyprinus carpio and the largemouth bass Micropterus salmoides (Abrantes et al. 2006a; Castro et al. 2007a, b). The pumpkinseed sunfish, and even more the mosquitofish, are voracious visual omnivorous/planktivorous fish that typically exert strong predatory pressure on zooplankton (García-Berthou 1999; García-Berthou and Moreno-Amich 2000). The pumpkinseed sunfish was reported to comprise more than half of the fish assemblage in Lake Vela about 15 years ago (Castro et al. 2007b). No systematic records are available since then, but occasional surveys show that this species is declining, a trend possibly related to the increase in catfish populations following introduction, although large populations of mosquitofish are frequently observed (J Pereira and F Gonçalves, personal observation). The introduction of these fish species has displaced native species such as Barbus spp., Chondrostoma spp. and Squalius spp. (Castro et al. 2007a).
Water temperature measurements
The water temperature was monitored every minute during the 48 h of the field campaign through dedicated data loggers (HOBO® Pendant, MX2201) placed 5 cm below the water surface. Lake Vela is confirmedly polymictic (Castro et al. 2007b); hence major vertical temperature gradients are not expected. Four temperature loggers were deployed to record water temperature, inside the stand of each plant species (‘S-in’ and ‘M-in’, respectively) and in the corresponding limnetic zone (‘S-out’ and ‘M-out’) following littoral-limnetic axes; the sensors were placed in the lower surface of floating boards, which were moving structures guided by poles fixed in the lake sediment (Fig. 1). For the data analysis, the water temperature was sampled in time intervals of 7 min, which is the response time of the sensors in water.
Physiochemistry: sampling and analysis
Water physiochemistry was monitored along the 48 h of each field campaign at each sampling site (i.e. near each temperature logger), every 4 h (i.e. two measurements through the campaign at 0:00, 4:00, 8:00, 12:00, 16:00, 20:00). Dissolved oxygen, pH, total dissolved solids (TDS) and electrical conductivity were recorded in situ using a multiparameter field probe (AquaRead AP2000). Water samples were collected at a depth of 20 cm below the free surface. Water was collected into plastic bottles and vacuum-filtered (Nalgene 6132-0020, 36 cc) in the field through glass microfibre filters (1.2 µm pore size; 47 mm ø). Filtered and unfiltered sub-samples, as well as the filters, were stored and transported into the laboratory at 4 °C in the dark for further analysis. The residue was used to quantify total suspended solids (TSS; APHA 2017) and chlorophyll a (Chl a) content (Lorenzen 1967). The filtrate was used to quantify coloured dissolved organic carbon (cDOC; Williamson et al. 1999). Unfiltered water samples were used to estimate turbidity through the absorption coefficient at 450 nm (Brower et al. 1998). Additional aliquots of unfiltered samples were mineralized with potassium persulfate (Ebina et al. 1983) for quantification of total phosphorous content through the tin(II) chloride method (APHA 2017) and total nitrogen content through the cadmium reduction method (Lind 1979).
Zooplankton: sampling and analysis
Zooplankton was sampled following the same design and schedules as described above for water samples. For this purpose, 20 L of lake water was pumped (underwater electric pump, Nuova Rade 500 GPH, 1900 LPH, 12 V) and filtered in situ through a 200-µm mesh sieve. The sieve was then washed, and the retained organisms were preserved with alcohol (70% v/v). The sample volume was based on previous experience of the research team in the same lake, considering the feasibility of seasonal and long-term zooplankton sampling with diverse research focuses using volumes ranging from 5 to 25 L (Antunes et al. 2003; Abrantes et al 2006a; Castro et al. 2007b). Preserved samples were sorted under a stereoscope (Olympus SZX9), and the organisms belonging to the four major zooplankter groups in the lake assemblage (Copepoda, Bosminidae, Daphniidae and Chydoridae) were counted. Whole samples were analysed, and reference zooplankton keys were used as a support to assign organisms to these groups (Sandercock and Scudder 1996; Bledzki and Rybak 2016).
Data analysis
Data were grouped by diel period (night samples assumed as replicates for the night group and day samples assumed as replicates for the day group) and by macrophyte stand focused, both for value averaging or ranging and for the statistical analysis. A two-way analysis of variance (ANOVA) approach was applied to assess the effect of these two factors (diel period, with two levels; site, with four levels) and their interaction. Each dataset was transformed as necessary to meet ANOVA assumptions of normality of the distribution and homoscedasticity. Provided a significant effect of a given factor, the main effects for this factor within each level of the other were further inspected using the post hoc Tukey test. All analyses considered an alpha level of 0.05. A water temperature value was assigned to each sample, which was obtained as the average temperature of the readings obtained in the 15-min period preceding the sampling.
Results
Physiochemical context
The field campaign for the present study was carried out at the end of the spring season, when the daily mean atmospheric temperatures were around 20 °C, as measured at 5-min intervals by a nearby weather station. Over the period of 10 days before the study, the station recorded a mean air temperature of 18.3 ± 3.5 °C (mean ± standard deviation; n = 3328; data not provided). The water temperatures in the same period ranged within 21.0–28.2 °C (22.6 ± 1.7 °C). Although these ranges are typical in Lake Vela, they are slightly higher than the records commonly observed earlier in the season (Castro et al. 2007b). Electrical conductivity and pH varied within 600–643 µS/cm and 6.61–8.22, respectively (Table 1), the latter fulfilling the national criteria for good ecological potential (INAG 2009). However, high values for total dissolved solids were recorded (391–417 mg/L), as well as high TSS and cDOC levels, consistent with the observed low transparency of the water and nutrient levels (Table 1). Total N and total P were found at concentrations above national criteria for good ecological potential in northern lakes (INAG 2009) and typical of eutrophic to hypereutrophic water bodies, with Chl a levels as a phytoplankton biomass surrogate confirming this picture (Thomas et al. 1996; USEPA 2000).
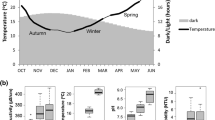
In general, there is no remarkable spatial (among sites) or diel (day vs night) variation regarding the physiochemical variables recorded in the present study (see Table 1 for measured ranges). The single exception to this finding was barely found for pH, which differed significantly among sites (Table S1) only during the night, but these differences were between unrelated sites (Fig. 2).
Mean pH, dissolved oxygen and chlorophyll a (Chl a) levels recorded at the study sites during the day (n = 8; white bars) or during the night (n = 4; grey bars). The error bars represent the standard deviation and the letters denote significant differences between sites during the night (Tukey test; p < 0.05)
Dissolved oxygen was found to change significantly with the diel period by the omnibus two-way ANOVA (Table S1), with the mean levels always lower during the night (Fig. 2). The lowest dissolved oxygen levels (2.10–3.38 mg/L) at all sites occurred at 8:00 following the second night of the field campaign. Generally, the levels rose and peaked between 12:00 and 20:00 (maximum = 9.74 mg/L).
Since the variables that characterize sites and diel periods did not exhibit significant variations (Table S1, supplementary material), there is no evidence that spatial and temporal variation in the studied physiochemical parameters (except in some cases, discussed in Sect. 3.2, Chl a as a surrogate for food availability) can constrain horizontal zooplankton distribution patterns, serving as DHM drivers. There was room then to consider that predatory pressure (Castro et al. 2007a, b) could be a main biotic driver of any potential DHM patterns in Lake Vela, and that thermally driven currents could also be an important factor to explain the DHM patterns.
Horizontal distribution of zooplankton in Lake Vela
In the present study, no significant differences depending on the diel period or the sites were found in the zooplankton abundance (Table S2, supplementary material). The graphical analysis of sample abundance through time shows that theoretical patterns of whole zooplankton DHM driven by visual predators are followed very occasionally (Fig. 3). For example, within the Myriophyllum axis, zooplankton indeed seem to prefer (higher density) the littoral over the limnetic zone during the day in the first and last diurnal period, but there is one inversion of this preference in the second day time period (24 h). The expected (based on potential predation cues) reversion of the trend during the night was not observed; within the Schoenoplectus axis, preference patterns of whole zooplankton are even less clear, since it was common to observe similar abundance records in the littoral and the limnetic zones through time. It is noteworthy that sample size can be claimed as a bias source in the capturing of migration patterns in general. In the present study, we established sample volumes based on previous experience in Lake Vela (see Sect. 2.4). Moreover, our abundance records (see Fig. 3) are within the same order of magnitude as those obtained in previous studies in Lake Vela for equivalent seasonal periods and the same taxa (Antunes et al. 2003; Abrantes et al. 2006a); in some cases, we recorded lower abundance, but recent seasonal sampling campaigns confirm that the zooplankton assemblage in Lake Vela is declining coincidently with an increasing frequency of cyanobacteria blooms.
Abundance of zooplankters, and then group-specific zooplankters in the littoral (in) or the limnetic (out) area of Lake Vela along the Myriophyllum (M) and the Schoenoplectus (S) axis through the sampling period. Grey lines were added between the data points for clarity purposes only, hence not reflecting record continuity. Grey shadowing was used to mark the nocturnal period
In summary, our results suggest that no DHM can be clearly recognized as a mechanism driven by interspecific relationships adopted by the zooplankton in Lake Vela.
Group-specific abundance patterns are not consistent with the trends found for the overall zooplankton assemblage, and the site had a significant effect in the abundance of Copepoda, Daphniidae and Chydoridae (Table S2). Copepods always preferred the littoral over the limnetic zone regardless of the diel period (Fig. 3); their abundance was significantly different between the two zones within the Myriophyllum axis in both the diurnal (Tukey test; p < 0.001) and the nocturnal (Tukey test; p = 0.005) period, and within the Schoenoplectus axis during the day (Tukey test; p < 0.001).
Daphnids showed an inconsistent abundance pattern, preventing any conclusions on their preference towards the littoral or the limnetic zone. In the Myriophyllum axis, abundance was higher or similar in the littoral throughout the whole sampling period, except at the beginning of the second night, when the organisms apparently preferred the limnetic zone (Fig. 3); this particular time point apparently configured the expected DHM pattern, but the statistics could not distinguish M-in and M-out in any of the diel periods (Tukey test: p = 0.372 for diurnal data; p = 0.853 for nocturnal data). In the Schoenoplectus axis, a significant preference for the limnetic zone was observed during the night (Fig. 3 and Tukey test with p = 0.036), consistent with the expected DHM, but this preference did not invert during the day (or abundance was similar in both zones).
Chydorids were consistent throughout the whole sampling period by preferring the littoral zone regardless of the macrophyte stand involved, with very little numbers found in the limnetic zone. This translated into significantly higher abundance records in the littoral for the Myriophyllum axis (Tukey test: p < 0.001 for diurnal data; p = 0.019 for nocturnal data), but the abundance did not differ statistically for the Schoenoplectus axis. Apart from particular time points at the beginning of the sampling period concerning the Schoenoplectus axis, bosminids tended to preferentially concentrate in the limnetic zone (Fig. 3), but the statistics did not confirm that site had a significant effect constraining abundance (Table S2).
Surface thermal flows in Lake Vela and their relationship with zooplankton distribution
Figure 4 presents the water temperature measured during the field campaign in the sampling sites (see Fig. 1), while Fig. 5 shows the discrete difference in the 15-min average water temperature in littoral and limnetic areas, for both axes, for each sampling time, allowing a more direct comparison with zooplankton discrete data.
The temperature records in the Schoenoplectus axis (S-in and S-out) indicate that the surface water is always warmer in the limnetic area than in the littoral where there is a vegetation cover, even during the night. The only exception was registered at the time of the first sampling (12:00 on 01/07/2019) when the records showed a short period with water temperature higher in the littoral than in the limnetic area. This might be a consequence of perturbations induced by the start of the campaign. The temperature differences observed vary between 0.2 and 0.5 °C during the day and are around 0.1 °C during the night. Although the temperature difference reduces during the night, the expected behaviour of the vegetation in relevantly slowing the cooling during the night was not observed, which may be related to the specific heat features of Schoenoplectus. Regarding the Myriophyllum axis (M-in and M-out), the temperature difference pattern is not consistent with the expected: during the day the water was warmer in the littoral than in the limnetic area, and during the night, temperatures in the littoral and in the limnetic area did not differ. The temperature differences observed during the day are significantly larger than those registered in the Schoenoplectus axis, with nearly 2 °C difference registered on the first day, corresponding to a mean density variation of 996.8 – 997.2 = −0.4 kg/m3 or −0.04%. During the night, the differences between surface water temperature in the littoral and limnetic areas were also around −0.1 °C.
Considering the temperature differences as the main driving force to induce surface exchange flows, no obvious density current would be expected in the Schoenoplectus axis during the entire observation period. This obviously excludes thermally driven currents as a contributor to explain whole and group-specific horizontal distribution of zooplankton in the Schoenoplectus axis. In the Myriophyllum axis, a surface current from the littoral to the limnetic area should be expected during the day, basically ceasing from late afternoon to morning. Correlating the observed distribution of the temperature differences with the horizontal variation in whole zooplankton abundance, no strong relation was found, as no consistent increase in the zooplankton abundance in the limnetic area and corresponding decrease in the littoral was observed in the Myriophyllum axis during the day. Furthermore, significant variations in zooplankton abundance were identified during the night period when weak or no surface currents were expected. Higher abundance of copepods, daphnids and chydorids was found in the Myriophyllum littoral area during the day, which is contrary to what would be expected if DHM patterns of these species were driven by thermal currents. Bosminids are always more abundant in limnetic areas, which meets the thermal flow expected in the Myriophyllum axis during the day, but is inconsistent with the expected absence of thermal flows therein during the night and in the Schoenoplectus axis. Thus, the acquired dataset does not allow definitive conclusions on the role of thermally driven exchange flows on the zooplankton horizontal migration patterns in shallow lakes.
Discussion
Concerning the physiochemical context of the study, the observed trend of higher pH values in vegetated littoral areas than in limnetic zones has been documented previously (Kairesalo 1980; Frodge et al. 1990; Arcifa et al. 2013). This generally relates to whether macrophytes are submerged, and thus rooted in the substrate (normally prevalent in shallower areas), or floating-leaf forms, for example, and thus relatively unaffected by the size of the water column. Regarding the dissolved oxygen, the lower values observed during the night correspond to the typical pattern in lakes, reflecting continuous oxygen consumption by respiration through the night, with limited input compensation versus maximum phytoplankton photosynthesis releasing oxygen to the water during the day (Andersen et al. 2017). While dissolved oxygen levels below 3.5 mg/L may challenge the survival of aquatic animals in the long term, zooplankters generally withstand these hypoxia levels for short periods (Burks et al. 2002). The diel variation observed in oxygen levels was consistent with the corresponding variation in Chl a (Fig. 2), but no consistent spatial pattern between littoral zones and limnetic zones was observed, which is in line with previous records in Lake Vela (Castro et al. 2007b) and in other lakes (Šorf and Devetter 2011; Špoljar et al. 2011).
High zooplanktivorous fish density is known to indirectly impact zooplankton communities due to the corresponding predation pressures, stimulating zooplankton movement to escape predation (Castro et al. 2007b; Jensen et al. 2010). In polymictic lakes, there is no effective depth gradient of abiotic conditions (e.g. temperature, light penetration), which is why it is frequently assumed that zooplankton move horizontally to find refuge, following a DHM featuring movement towards limnetic zones at night and towards the vegetated littoral during the day (Lauridsen et al. 1996; Burks et al. 2002). Reverse DHM patterns have also been reported and argued to be related to non-visual benthic invertebrate predators such as Chaoborus (Ringelberg 2009; Antón-Pardo et al. 2021) that typically swim at lower depth closer to lake banks, but the depth of the sampling sites in Lake Vela is homogeneous (50–70 cm, depending on the macrophyte stand covered) within the littoral–limnetic axes in our sampling area, rendering this alternative pattern unlikely. Moreover, in water bodies with high fish load, invertebrate predators are also preyed upon, which decreases the likelihood of a reverse DHM driven by non-visual invertebrate predators (Nurminen et al. 2007; Montiel-Martínez et al. 2015), and in Lake Vela in particular. No evidence of DHM was observed in Lake Vela at first; however, this analysis was based on the entire zooplankton assemblage. Recognizing that different zooplankters have different ‘swimming’ abilities and hence distinct capacity to actively move along horizontal axes (Tiselius and Jonsson 1997; Visser and Thygesen 2003; Kiørboe 2011; Kjellerup and Kiørboe 2012; van Someren Gréve et al. 2017), it is reasonable to examine potential spatial patterns among the different zooplankton groups.
The abundance of copepods was higher in the littoral than in the limnetic zone regardless of the diel period. Our expectation was that this group would consistently reflect predator-based DHM patterns regardless of any other potential drivers due to their long antennae allowing propulsive force of suitable magnitude and direction to enable an active protective behaviour. Indeed, copepods are able to exploit the mechanical energy to capture as many food items (algae) as possible, while expending as little energy as possible (Jiang et al. 2002b, 2002a; Jiang and Strickler 2007); they can detect and locate food particles (chemoreception and mechanoreception), swimming towards it (Bundy and Vanderploeg 2002). This suggests that food availability, which was slightly higher in the littoral as indicated by higher Chl a concentration in both macrophyte axes and regardless of the diel period, may drive copepod preference for the littoral over the limnetic zone.
Daphnids, as the largest cladocerans, are particularly susceptible to visual predation; hence, this group is generally expected to exhibit diel migration responding to refuge needs. It has been shown that patches of vascular macrophytes growing in littoral zones constitute a refuge to large cladocerans, and that the pursuit for protection is directly proportional to the density and structural complexity of these habitats, i.e. to the predatory capacity by fish within the patches (Manatunge et al. 2000; Padial et al. 2009). Submerged macrophytes are a more effective refuge for zooplankton than emergent plants (Hanson and Butler 1994; Jacobsen et al. 1997; Stansfield et al. 1997; Jeppesen et al. 1998; Ardohain et al. 2021), and our results are consistent in this context. First, the submerged density and complexity of the Myriophyllum patch was much higher and likely provided a more efficient refuge than the Schoenoplectus patch, largely limiting the capacity of even small fish exploring the littoral to actively prey upon zooplankters (e.g. Gambusia sp.). This may explain the preference of daphnids for the littoral regardless of the diel period within the Myriophyllum axis. Second, the shadowing within the Schoenoplectus patch is lower and would likely provide similar visual fields for predators between the limnetic and the littoral zone relative to the Myriophyllum stand. This may explain the preference, or similar distribution, of daphnids through these two zones within the Schoenoplectus axis during diurnal or nocturnal periods, respectively.
Chydorids and bosminids have similar mean body size ranges, being amongst the smallest cladoceran representatives, and definitively smaller than Daphniidae (Rizo et al. 2019). In this context, clear patterns of DHM driven by predatory cues would not be expected for these two groups, as they would be inherently protected from visual predators by their small size (Rizo et al. 2019), although this perspective has been challenged (Jeppesen et al. 1998). In the present study, chydorids preferred the littoral zone, with higher abundance generally recorded within Myriophyllum paths, and bosminids preferred the limnetic zone in general; differences should likely be due to their ecology. This difference can be primarily explained by the ecological niches involved. As compiled e.g. by Nevalainen (2010) or Klemetsen et al. (2020), chydorids evolved coupled with the diversification of aquatic plants, and the group is composed mostly of littoral species exploiting different substrates through their various locomotor and feeding adaptations. Our results agree with the preference for the littoral by chydorids (note that limnetic abundance of these organisms was very low throughout the sampling campaign) and also with the role of vegetation in shaping distribution (Tremel et al. 2000; Adamczuk 2014), with higher abundance generally recorded within the Myriophyllum stand than the Schoenoplectus stand. Bosmina have a different feeding flexibility and locomotory behaviour than other cladocerans because they feed more like a raptorial predator than a passive collector, and they can select food items upon availability, which is an energy-efficient mechanism allowing them to share habitat with competitors without the need for costly spatial migration (DeMott and Kerfoot 1982).
Challenges to and inconsistencies with zooplanktonic DHM theory are well known and relate to the contrast between predation and prey refuge (Burks et al. 2002; Nurminen and Horppila 2002; Meerhoff et al. 2006; Castro et al. 2007b; Jensen et al. 2010; Arcifa et al. 2013; Antón-Pardo et al. 2021), as well as to the role of water transparency in moderating the relationship. Turbidity, which is high in Lake Vela, has a consistent negative effect on prey capture by visually oriented predators, and there is also evidence that high turbidity leads to reduced prey capture in non-visual predators (Ortega et al. 2020). The behaviour of dominant planktivorous fish in Lake Vela may also contribute to the inconsistencies, because young pumpkinseed sunfish tend to prey in the littoral (García-Berthou and Moreno-Amich 2000), as do mosquitofish, mostly upon littoral cladocerans (García-Berthou 1999). Unfortunately, there are no systematic records on the fish assemblage of Lake Vela at the time of the sampling, and mosquitofish were consistently observed near both vegetated areas during the sampling period, while pumpkinseed sunfish were more rarely observed. The role of wind and thermal currents in modulating the spatial heterogeneity of zooplankton distribution in lakes has been postulated (Okely and Imberger 2007), but these ideas have also been questioned as factors affecting zooplankton spatial distribution (Lévesque et al. 2010).
In a shallow lake with littoral regions populated by emergent vegetation, differential solar heating can produce near-surface temperature differences between vegetated and non-vegetated regions. During the day, especially on sunny days, the shadowing effect on the littoral areas should reduce surface water heating relative to the limnetic areas, leading to the generation of horizontal exchange flows towards the vegetated areas (Zhang and Nepf 2009). During the night, the cooling effect is expected to be more efficient in the limnetic areas, leading to a surface flow from littoral to lake open areas. Nevertheless, the water temperature measurements of the present work did not follow that expected pattern. In the Schoenoplectus axis, the surface water in the limnetic area was always warmer than in the littoral. In the Myriophyllum axis, the water was warmer in the littoral than in the limnetic area during the day, whereas the temperatures were similar in the littoral and limnetic areas during the night. This behaviour is likely related to the type of vegetation and its specific heat features. Myriophyllum aquaticum is characterized by very dense plant distributions with short canopies. A potential large heat absorption by the plants might contribute to a faster water heating process compared with the limnetic area.
The correspondence between the expected surface exchange flow based on the water temperature differences and the abundance of the whole and group-specific zooplankton was not definitive with respect to the role of thermally driven exchange flows on the zooplankton horizontal migration patterns. Lake circulation is prone to the influence of several factors leading to complex flow (Zhang and Nepf 2009; Mao et al. 2019; Naghib et al. 2018). In shallow lakes in Mediterranean regions with high temperatures and exposure to annually prevalent winds (North Atlantic Anticyclone combined with North Atlantic Oscillation), the wind pattern and intensity may have a significant influence on the surface exchange flow and, therefore, on the spatial distribution of zooplankton. The potential role of the wind in this process will require further analysis and additional study.
Availability of data and materials
Data can be requested contacting the corresponding author.
References
Abrantes N, Antunes SC, Pereira MJ, Gonçalves F (2006a) Seasonal succession of cladocerans and phytoplankton and their interactions in a shallow eutrophic lake (Lake Vela, Portugal). Acta Oecologica 29:54–64
Abrantes N, Pereira R, Gonçalves F (2006b) First step for an ecological risk assessment to evaluate the impact of diffuse pollution in lake Vela (Portugal). Environ Monit Assess 177:411–431
Abrantes N, Pereira R, Gonçalves F (2010) Occurrence of pesticides in water, sediments, and fish tissues in a lake surrounded by agricultural lands: concerning risks to humans and ecological receptors. Water Air Soil Pollut 212:77–88
Adamczuk M (2014) Niche separation by littoral-benthic Chydoridae (Cladocera, Crustacea) in a deep lake-potential drivers of their distribution and role in littoral-pelagic coupling. J Limnol 73:490–501
Almeda R, van Someren Grève H, Kiørboe T (2017) Behavior is a major determinant of predation risk in zooplankton. Ecosphere 8:e01668
Andersen MR, Kragh T, Sand-Jensen K (2017) Extreme diel dissolved oxygen and carbon cycles in shallow vegetated lakes. Proc R Soc B Biol Sci 284:20171427
Antón-Pardo M, Muška M, Jůza T, Vejříková I, Vejřík L, Blabolil P, Čech M, Draštík V, Frouzová J, Holubová M (2021) Diel changes in vertical and horizontal distribution of cladocerans in two deep lakes during early and late summer. Sci Total Environ 751:141601
Antunes SC, Abrantes N, Gonçalves F (2003) Seasonal variation of the abiotic parameters and the cladoceran assemblage of Lake Vela: comparison with previous studies. Ann Limnol 39:255–264
Antunes SC, de Figueiredo DR, Marques SM, Castro BB, Pereira R, Gonçalves F (2007) Evaluation of water column and sediment toxicity from an abandoned uranium mine using a battery of bioassays. Sci Total Environ 374:252–259
APHA (2017) Standard methods for the examination of water and wastewater. American Public Health Association American Water Works Association, Water Environment Federation, Washington DC
Arcifa M, Bunioto T, Perticarrari A, Minto W (2013) Diel horizontal distribution of microcrustaceans and predators throughout a year in a shallow neotropical lake. Braz J Biol 73:103–114
Arcifa MS, Perticarrari A, Bunioto TC, Domingos AR, Minto WJ (2016) Microcrustaceans and predators: diel migration in a tropical lake and comparison with shallow warm lakes. Limnetica 35:281–296
Ardohain DM, Gabellone NA, Claps MC (2021) Main drivers in the structure and dynamics of the zooplankton community in a pampean seepage shallow lake throughout an annual cycle during turbid and clear water regimes. Int Aquat Res 13:53–70
Bell ATC, Murray DL, Prater C, Frost PC (2019) Fear and food: Effects of predator-derived chemical cues and stoichiometric food quality on Daphnia. Limnol Oceanogr 64:1706–1715
Bianco G, Mariani P, Visser AW, Mazzocchi MG, Pigolotti S (2014) Analysis of self-overlap reveals trade-offs in plankton swimming trajectories. J R Soc Interface 11:20140164
Bledzki LA, Rybak JI (2016) Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to species identification, with notes on ecology, distribution, methods and introduction to data analysis. Springer
Brower JE, Zar JH, Von Ende CN (1998) Field and laboratory methods for general ecology. WCB McGraw-Hill
Bundy MH, Vanderploeg HA (2002) Detection and capture of inert particles by calanoid copepods: the role of the feeding current. J Plankton Res 24:215–223
Burks RL, Lodge DM, Jeppesen E, Lauridsen TL (2002) Diel horizontal migration of zooplankton: cost and benefits of inhabiting the littoral. Freshw Biol 47:343–365
Burks RL, Mulderij G, Gross E, Jones I, Jacobsen L, Jeppesen E, Van Donk E (2006) Center stage: the crucial role of macrophytes in regulating trophic interactions in shallow lake wetlands. In: Bobbink R, Beltman B, Verhoeven JTA, Whigham DF (eds) Wetlands: functioning, biodiversity, conservation and restoration. Ecological Studies (Analysis and Synthesis). Springer, pp 37–59
Castro BB, Consciência S, Gonçalves F (2007a) Life history responses of Daphnia longispina to mosquitofish (Gambusia holbrooki) and pumpkinseed (Lepomis gibbosus) kairomones. Hydrobiologia 594:165–174
Castro BB, Marques SM, Goncalves F (2007b) Habitat selection and diel distribution of the crustacean zooplankton from a shallow Mediterranean lake during the turbid and clear water phases. Freshw Biol 52:421–433
CM-Ministries Council (2000) Resolução do Conselho de Ministros n◦ 76/2000. Diário da República I Série-B, Portugal
Cuker BE, Watson MA (2002) Diel vertical migration of zooplankton in contrasting habitats of the Chesapeake Bay. Estuaries 25:296–307
DeMott WR, Kerfoot WC (1982) Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecol 63:1949–1966
Ebina J, Tsutsui T, Shirai T (1983) Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res 17:1721–1726
Ekvall MT, Sha Y, Palmér T, Bianco G, Bäckman J, Åström K, Hansson LA (2020) Behavioural responses to co-occurring threats of predation and ultraviolet radiation in Daphnia. Freshw Biol 65:1509–1517
Emily H, Hrabik TR, Li Y, Lawson ZJ, Carpenter SR, Vander Zanden MJ (2017) The effects of experimental whole-lake mixing on horizontal spatial patterns of fish and Zooplankton. Aquat Sci 79:543–556
Engelmayer A (1995) Effects of predator-released chemicals on some life history parameters of Daphnia pulex. Hydrobiologia 307:203–206
Ermolaeva NI, Zarubina EY, Bazhenova OP, Dvurechenskaya SY, Mikhailov VV (2019) Influence of abiotic and trophic factors on the daily horizontal migration of zooplankton in the littoral zone of the novosibirsk reservoir. Inland Water Biol 12:418–427
Farrow DE (2004) Periodically forced natural convection over slowly varying topography. J Fluid Mech 508:1–21
Fish Biology 55: 135–147.
Folt C, Burns C (1999) Biological drivers of zooplankton patchiness. Trends Ecol Evol 14:300–305
Frodge JD, Thomas GL, Pauley GB (1990) Effects of canopy formation by floating and submergent aquatic macrophytes on the water quality of two shallow Pacific Northwest lakes. Aquat Bot 38:231–248
Gabaldón C, Devetter M, Hejzlar J, Šimek K, Znachor P, Nedoma J, Seďa J (2019) Seasonal strengths of the abiotic and biotic drivers of a zooplankton community. Freshw Biol 64:1326–1341
García-Berthou E (1999) Food of introduced mosquitofish: ontogenic diet shift and prey selection. J Fish Biol 55:135–147
García-Berthou E, Moreno-Amich RR (2000) Food of introduced pumpkinseed sunfish: ontogenic diet shift and seasonal variation. J Fish Biol 57:29–40
Hanson MA, Butler MG (1994) Responses of plankton, turbidity, and macrophytes to biomanipulation in a shallow Prairie Lake. Can J Fish Aquat Sci 51:1180–1188
Hembre LK, Megard RO (2003) Seasonal and diel patchiness of a Daphnia population: an acoustic analysis. Limnol Oceanogr 48:2221–2233
Heuschele J, Ekvall MT, Bianco G, Hylander S, Hansson LA (2017) Context-dependent individual behavioral consistency in Daphnia. Ecosphere 8:e01679
INAG (2009) Critérios para a classificação do estado das massas de água superficiais - rios e albufeiras. Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional. Instituto da água I.Pl.
Jacobsen L, Perrow MR, Landkildehus F, Hjorne M, Lauridsen TL, Berg S (1997) Interactions between piscivores, zooplanktivores and zooplankton in submerged macrophytes: preliminary observations from enclosure and pond experiments. Hydrobiologia 342:197–205
Jensen E, Brucet S, Meerhoff M, Nathansen L, Jeppesen E (2010) Community structure and diel migration of zooplankton in shallow brackish lakes: role of salinity and predators. Hydrobiologia 646:215–229. https://doi.org/10.1007/s10750-010-0172-4
Jeppesen E (1998) The ecology of shallow lakes—trophic interactions in the Pelagial. National Environmental Research Institute, Silkeborg
Jeppesen E, Lauridsen TL, Kairesalo T, Perrow MR (1998) Impact of submerged macrophytes on fish-zooplankton interactions in lakes. In: Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K (eds) The structuring role of submerged macrophytes in lakes. Ecological Studies, vol 131. Springer, New York, NY. https://doi.org/10.1007/978-1-4612-0695-8_5
Jiang H, Strickler JR (2007) Copepod flow modes and modulation: a modelling study of the water currents produced by an unsteadily swimming copepod. Philos Trans R Soc B Biolog Sci 362:1959–1971
Jiang H, Meneveau C, Osborn TR (2002a) The flow field around a freely swimming copepod in steady motion. Part II: numerical simulation. J Plankton Res 24:191–213
Jiang H, Osborn TR, Meneveau C (2002b) The flow field around a freely swimming copepod in steady motion. Part I: theoretical analysis. J Plankton Res 24:167–189
Kairesalo T (1980) Diurnal fluctuations within a littoral plankton community in oligotrophic Lake Pääjärvi, southern Finland. Freshw Biol 10:533–537
Kiørboe T (2011) How zooplankton feed: mechanisms, traits and trade-offs. Biol Rev 86:311–339
Kjellerup S, Kiørboe T (2012) Prey detection in a cruising copepod. Biol Let 8:438–441
Klemetsen A, Aase BM, Amundsen PA (2020) Diversity, abundance, and life histories of littoral chydorids (Cladocera: Chydoridae) in a subarctic European lake. J Crustac Biol 40:534–543
Laforsch C, Beccara L, Tollrian R (2006) Inducible defenses: the relevance of chemical alarm cues in Daphnia. Limnol Oceanogr 51:1466–1472
Lauridsen TL, Lodge DM (1996) Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat. Limnol Oceanogr 41:794–798
Lauridsen TL, Jeppesen E, Mitchell SF, Lodge DM, Burks RL (1996) Diel variation in horizontal distribution of Daphnia and Ceriodaphnia in oligotrophic and mesotrophic lakes with contrasting fish densities. Hydrobiologia 408:241–250
Lauridsen TL, Jeppesen E, Sondergaard M, Lodge DM (1998) Horizontal migration of Zooplankton:predator-mediated us of macrophyte habitat. In: Jeppesen E, Sondergaard M, Christoffersen K (eds) The structuring role of submerged macrophytes in lakes. Ecological studies (Analysis and Synthesis). Springer, New York
Lévesque S, Beisner BE, Peres-Neto PR (2010) Meso-scale distributions of lake zooplankton reveal spatially and temporally varying trophic cascades. J Plankton Res 32:1369–1384
Lightbody A, Avener M, Nepf H (2008) Observations of short-circuiting flow paths within a free-surface wetland in Augusta, Georgia, U.S.A. Limnol Oceanogr 53:1040–1053
Lind OT (1979) Handbook of common methods in limnology. Mosby
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
Lovstedt CB, Bengtsson L (2008) Density-driven current between reed belts and open water in a shallow lake. Water Resour Res 44:W10413
Mortimer CH (1974) Lake Hydrodynamics. Mitteilungen Internationale Vereingung fur Theoretische und Angewandte Limnologie.
Manatunge J, Asaeda T, Priyadarshana T (2000) The Influence of Structural Complexity on Fish–zooplankton Interactions: A Study Using Artificial Submerged Macrophytes. Environ Biol Fishes 58:425–438
Mao Y, Lei C, Patterson JC (2019) Natural convection in a reservoir induced by sinusoidally varying temperature at the water surface. Int J Heat Mass Transfer 134:610–627
McCloud CL, Ismail HN, Seuront L (2018) Cue hierarchy in the foraging behaviour of the brackish cladoceran Daphniopsis australis. J Oceanol Limnol 36:2050–2060
Meerhoff M, Fosalba C, Bruzzone C, Mazzeo N, Noordoven W, Jeppesen E (2006) An experimental study of habitat choice by Daphnia: plants signal danger more than refuge in subtropical lakes. Freshw Biol 51:1320–1330. https://doi.org/10.1111/j.1365-2427.2006.01574.x
Montiel-Martínez A, Ciros-Pérez J, Corkidi G (2015) Littoral zooplankton–water hyacinth interactions: habitat or refuge? Hydrobiologia 755:173–182
Naghib A, Patterson J, Lei C (2018) Natural convection induced by absorption of solar radiation in the near shore region of lakes and reservoirs: experimental results. Exp Therm Fluid Sci 90:101–114
Nevalainen L (2010) Evaluation of microcrustacean (Cladocera, Chydoridae) biodiversity based on sweep net and surface sediment samples. Ecoscience 17:356–364
Nurminen LKL, Horppila JA (2002) A diurnal study on the distribution of filter feeding zooplankton: effect of emergent macrophytes, pH and lake trophy. Aquat Sci 64:198–206
Nurminen L, Horppila J, Pekcan-Hekim Z (2007) Effect of light and predator abundance on the habitat choice of plant-attached zooplankton. Freshw Biol 52:539–548
O’Brien WJ (1979) The Predator-Prey Interaction of Planktivorous Fish and Zooplankton: Recent research with planktivorous fish and their zooplankton prey shows the evolutionary thrust and parry of the predator-prey relationship. American Scientist Sigma Xi Sci Res Soc 67: 572–581. http://www.jstor.org/stable/27849438. Accessed 28 Dec 2022
Okely P, Imberger J (2007) Horizontal transport induced by upwelling in a canyon-shaped reservoir. Hydrobiologia 586:343–355
Ortega JCG, Figueiredo BRS, da Graça WJ, Agostinho AA, Bini LM (2020) Negative effect of turbidity on prey capture for both visual and non-visual aquatic predators. J Anim Ecol 89:2427–2439
Padial AA, Thomaz SM, Agostinho AA (2009) Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia 624:161–170
Pálmarsson SÓ, Schladow SG (2008) Exchange flow in a shallow lake embayment. Ecol Appl 18:A89–A106
Pijanowska J, Dawidowicz P, Weider LJ (2006) Predator-induced escape response in Daphnia. Arch Hydrobiol 167:77–87
Pinel-Alloul B (1995) Spatial heterogeneity as a multiscale characteristic of zooplankton community. Hydrobiologia 300:17–42
Pinel-Alloul B, Méthot G, Malinsky-Rushansky NZ (2004) A short-term study of vertical and horizontal distribution of zooplankton during thermal stratification in Lake Kinneret, Israel. Hydrobiologia 526:85–98. https://doi.org/10.1023/B:HYDR.0000041611.71680.fc
Podsetchine V, Schernewski G (1999) The influence of spatial wind inhomogeneity on flow patterns in a small lake. Water Res 33:3348–3356
Ringelberg J (2009) Diel vertical migration of zooplankton in lakes and oceans: causal explanations and adaptive significances. Springer Science & Business Media
Rizo E, Xu S, Tang Q, Papa R, Dumont H, Qian S, Han B-P (2019) A global analysis of cladoceran body size and its variation linking to habitat, distribution and taxonomy. Zool J Linn Soc 187:1119–1130
Rollwagen-Bollens G, Bollens S, Dexter E, Cordell J (2020) Biotic vs. abiotic forcing on plankton assemblages varies with season and size class in a large temperate estuary. J Plankton Res 42:221–237
Sakwińska O (1998) Plasticity of Daphnia magna life history traits in response to temperature and information about a predator. Freshw Biol 39:681–687
Sandercock GA, Scudder GGE (1996) Key to the species of freshwater calanoid copepods (Crustacea) of British Columbia. North. University of British Columbia, Vancouver
Šorf M, Devetter M (2011) Coupling of seasonal variations in the zooplankton community within the limnetic and littoral zones of a shallow pond. Annales De Limnologie-Int J Limnol EDP Sci 47:259–268
Špoljar M, Dražina T, Habdija I, Meseljević M, Grčić Z (2011) Contrasting zooplankton assemblages in two oxbow lakes with low transparencies and narrow emergent macrophyte belts (Krapina River, Croatia). Int Rev Hydrobiol 96:175–190
Stansfield J, Perrow M, Tench L, Jowitt A, Taylor A (1997) Submerged macrophytes as refuges for grazing Cladocera against fish predation: observations on seasonal changes in relation to macrophyte cover and predation pressure. Hydrobiologia 342:229–240
Thackeray SJ, George DG, Jones RI, Winfield IJ (2004) Quantitative analysis of the importance of wind-induced circulation for the spatial structuring of planktonic populations. Freshw Biol 49:1091–1102
Thomas R, Maybeck M, Beim A (1996) Lakes. In: Chapman D (ed) Water quality assessments - a guide to use of biota, sediments and water in environmental monitoring. UNESCO/WHO/UNEP
Thorp JH, Covich AP (2001) An overview of freshwater habitats. In: Thorp JH, Covish AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press, pp 19–41
Tiselius P, Jonsson PR (1997) Effects of copepod foraging behavior on predation risk: An experimental study of the predatory copepod Pareuchaeta norvegica feeding on Acartia clausi and A. tonsa (Copepoda). Limnol Oceanogr 42:164–170
Tremel B, Frey SE, Yan ND, Somers KM, Pawson TW (2000) Habitat specificity of littoral Chydoridae (Crustacea, Branchiopoda, Anomopoda) in Plastic Lake, Ontario, Canada. Hydrobiologia 432:195–205
Tsydenov BO, Kay A, Starchenko AV (2016) Numerical modeling of the spring thermal bar and pollutant transport in a large lake. Ocean Model 104:73–83
USEPA (2000) USEPA Nutrient Criteria Technical Guidance Manual, Lakes and Reservoirs. Breeam Communities. United States Environmental Protection Agency, Washington DC
van Someren Gréve H, Almeda R, Kiørboe T (2017) Motile behavior and predation risk in planktonic copepods. Limnol Oceanogr 62:1810–1824
Viljanen M, Karjalainen J (1993) Horizontal distribution of zooplankton in two large lakes in Eastern Finland. SIL Proceedings - Verhandlungen Des Internationalen Verein Limnologie 25:548–551
Visser AW, Thygesen UH (2003) Random motility of plankton: diffusive and aggregative contributions. J Plankton Res 25:1157–1168
Williamson CE, Morris DP, Pace ML, Olson OG (1999) Dissolved organic carbon and nutrients as regulators of lake ecosystems: Resurrection of a more integrated paradigm. Limnol Oceanogr 44:795–803
Wojtal A, Frankiewicz P, Izydorczyk K, Zalewski M (2003) Horizontal migration of zooplankton in a littoral zone of the lowland Sulejow Reservoir (Central Poland). Hydrobiologia 506–509:339–346
Zhang X, Nepf HM (2009) Thermally driven exchange flow between open water and an aquatic canopy. J Fluid Mech Camb Univ Press 632:227–243
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by the project WinTherface (PTDC/CTA-OHR/30561/2017) funded by FEDER, through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES. Thanks are due to FCT/MCTES for the financial support to CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020) and to CERIS (UIDB/04625/2020), through national funds. Tânia Vidal and Ana M. Ricardo are funded by national funds (OE), through FCT, I.P., in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of the article 23, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19.
Author information
Authors and Affiliations
Contributions
The study was designed by AMR, JP, NA, FG and RF. The field data were collected by JP, ASL, JS, TV, NA, MB, DS, FG, RF and AMR. Field samples were analysed by ASL, JP, TV and JP. Material preparation and analysis were performed by JP, ASL and AMR. The first draft of the manuscript was written by ASL, JP, AMR and RF, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, J.L., Lopes, A.S., Silva, J. et al. Horizontal migration of zooplankton in lake–wetland interfaces. Can temperature-driven surface exchange flows modulate its patterns?. Aquat Sci 86, 29 (2024). https://doi.org/10.1007/s00027-024-01046-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01046-1