Abstract
The European eel (Anguilla anguilla L.) is a critically endangered catadromous fish. Their inshore and in-river arrival as glass eel and elvers is an important stage of their life cycle, marking the transition from marine to freshwater habitats. Considerable knowledge gaps remain on the temporal and spatial patterns of this transition period to freshwater residency. Stable isotope (SI) analysis (δ13C, δ15N) was used to assess the timing and duration of the marine to freshwater transition among glass eels and elvers migrating upstream of the weirs at, or just upstream of, the tidal limit of four English rivers. (Parrett, Frome, Piddle, Chelmer). Variability in SI was low in the Parrett and Frome, resulting in narrow isotopic niches, but was high in the Piddle and Chelmer, resulting in wider niches. The Parrett and Frome data were then used to train a discriminant function analysis (DFA) model to classify eels as ‘marine’, ‘freshwater-established’ and ‘transitioning’. When applied to the Piddle and Chelmer eel SI data, only a small proportion of eels were classified as marine and transitioning, with most being freshwater established. These results suggest that most eels present in the lower reaches rivers have been present for sufficient time for their SI values to represent feeding on local prey resources, with relatively few eels being newly arrived from the marine environment. The transition of eels from marine to freshwater in this species can therefore be prolonged, with many ascending rivers at least one winter after their initial arrival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species with complex life histories often exhibit ontogenetic distribution shifts and exploit a wide range of habitats across their lifecycle (Hobbs et al. 2019). For many fish species, the ability to select environments that provide the functional habitats necessary at each life stage is important to maximise their fitness (Kristensen et al. 2019). This is particularly important for diadromous species, whose movements between marine and freshwater environments involve trade-offs between their ability to gain greater body mass via accessing new and profitable feeding grounds versus the elevated predation risks and energetic costs of moving to these areas (e.g. Jensen et al. 2019). These risks and costs might be elevated in situations where accessing the new feeding grounds requires movements across complex environments over extended time periods (Arai 2020, 2022).
The European eel (Anguilla anguilla L.) is a facultative catadromous fish with a complex life history involving oceanic migration to continental habitats as larvae, continental migration as juveniles and oceanic migration back to spawning grounds as adults (Arai 2022). Following adult spawning in the Sargasso Sea, leptocephalus larvae migrate to European coastlines where they metamorphose into post-larval, non-pigmented eels, referred to as ‘glass eels’ (< 80 mm) (Tesch 1980; Cresci 2020; ICES 2022). To commence their continental migration, glass eels must cross the continental shelf into coastal waters, where there is high variability in their habitat use as individuals use marine, brackish and/or freshwater (Arai 2022). As individuals move into brackish and then freshwater, they begin to develop a pigmentation and are often referred to as pigmented glass eels (< 80 mm) before developing into elvers (81–120 mm) and then yellow eels (Tesch 1980; Cresci 2020; ICES 2022). They remain as yellow eels until the commencement of their oceanic migration back to the spawning grounds as silver eels (Schmidt 1923; Righton et al. 2016).
Glass eels use currents and passive tidal transport to move upstream into brackish and freshwater environments (Gascuel 1986; Laffaille et al. 2007). The duration of this transition period between saline and freshwater can be variable, as some glass eels move into freshwater relatively quickly, while others remain in estuarine or coastal waters for extended periods (Bardonnet and Riera 2005). Although the feeding ecology and habitat preferences of elver and yellow eel have received a great deal of attention (e.g. Harrod et al. 2005; Yokouchi et al. 2012; Denis et al. 2022), there remains uncertainty in the extent of their movements as they transition between brackish and freshwater environments (Elise et al. 2014). This is made more complex by the lack of available methods to track the movements of these early life stages, with the relatively small size of these life stages inhibiting the use of external or internal tags. However, natural chemical tags, especially stable isotopes (SI), have successfully been applied to eel trophic ecology, where the isotopic values of the eel tissues reflect the isotopic signature of their recent foraging areas, providing temporally integrated information on their resource and habitat use (e.g. Harrod et al. 2005). When bulk stable isotope analysis is used, the 13C isotope is useful for discriminating between marine (enriched values, e.g. –19‰, –20‰) and freshwater habitats (depleted values, e.g. < 27‰) (Nolan et al. 2019). Stable isotope data are also influenced by the tissues analysed, with different tissues having contrasting isotopic turnover rates with, for example, dorsal muscle having a considerably longer isotopic turnover rate than blood and mucus (Vander Zanden et al. 2015; Hobson 2023).
With European eel assessed as critically endangered on the IUCN Red List (Jacoby and Gollock 2014; Pike et al. 2020), understanding their habitat use, feeding ecology and foraging behaviour throughout all life stages and distributions is crucial for their conservation and management (Feunteun 2002). The aim of this study was to assess the timing and duration of the marine to freshwater transition of non-pigmented (glass eel hereafter) and pigmented glass eels/pigmented eels (elver hereafter) in four rivers in England through an approach based on bulk stable isotope analysis (δ13C, δ15N).
Using eel traps located on structures either at or just upstream of the tidal limit of each river, individuals migrating upstream of the structures in 2021 and 2022 were sampled, with δ13C and δ15N used to determine and predict their recent habitat use (i.e. marine versus freshwater), including the extent of variation within and between rivers.
Materials and methods
Sample sites
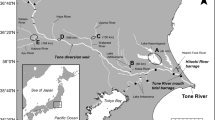
The movements of glass eels and elvers were assessed in four rivers located in eastern, southern and southwest England (Fig. 1). The River Frome (50.688533, –2.081004) is a lowland chalk stream located in southern England, which rises in central Dorset near Evershot and flows for approximately 70 km (Fig. 1). Eels were collected at a side-stream at East Stoke (50.679936, –2.185172), about 8 km upstream from the tidal limit at Wareham, where this tidal limit is ‘soft’ (i.e. there is not a hard barrier between the saltwater—freshwater zones, which vary according to tidal state and river flow). The River Piddle (50.688096, –2.124328) flows south-eastwards, roughly parallel to the Frome, before emptying into Poole Harbour, through which both rivers reach the English Channel (Fig. 1). As with the Frome, there is no hard structure marking the tidal limit. Eels were captured monthly from May to September 2021 by back-mounted electric fishing (LR-24, Smith-Root, Vancouver, WA, USA) in the River Frome and weekly by using a trap operated over 24 h periods on an existing elver pass on the River Piddle.
Location map of study sites with general location (a), the River Chelmer catchment (b) with the points representing the location of Beeleigh Weir (purple dot), the River Parrett catchment (c) with the points representing the location of Huntspill Sluice (red dot) and Oath Weir (green dot) and Poole Harbour catchment (d) with the points representing the location of Frome (black dot) and Piddle (orange dot)
The River Parrett (51.047146, –2.883918) is located in southwest England and flows northwest through Somerset, before reaching its confluence with the Bristol Channel at Burnham-on-Sea, where it flows into Bridgwater Bay. Eels (Fig. 1) were captured once a month by installing monitoring traps over 24 h periods from March to June throughout 2021 and 2022 at Oath Lock (51.047094, –2.881561) and Huntspill Sluice (51.204015, –2.988507) (located on different channels), which are approximately 30 km and 5 km, respectively, from the Bristol Channel confluence. In both channels, the sluices represented a hard barrier between the upstream freshwater habitat and the tidal, brackish habitat downstream.
The River Chelmer (51.736099, 0.479800) is located in eastern England and flows for approximately 48 km from its source near Thaxted to Springfield Basin in Chelmsford, where it joins a number of other significant tributaries and rivers (Fig. 1). The Chelmer combines with Blackwater at Beeleigh Weir, near Maldon, discharging into the North Sea via the Blackwater Estuary (Fig. 1). Eels were captured once a month between March and September in 2021 and 2022 using monitoring traps over 24 h periods at Beeleigh Weir (51.743006, 0.662116), which is the tidal limit, with the weir separating the lower freshwater river from the upper estuary.
Data collection
After capture, the eels were counted and a subsample taken (maximum 30 per sampling occasion), which were euthanised (overdose of anaesthetic; MS-222). Individuals were transferred to small sample bags and transported to the laboratory on ice (except the River Chelmer, where samples were preserved in ethanol). Ethanol preservation does not significantly alter the δ13C and δ15N values of eel dorsal muscle (Boardman et al. 2022), and so no corrections to the SI data were required. Sampling permissions were through the Environment Agency (permit reference EP/EW027-C-042/19919/01), with eel sampling and euthanasia completed after an ethical review by the UK Home Office Project licence (P47216841).
In the laboratory, each eel was defrosted individually and measured [total length (TL), nearest mm], before a sample was taken for stable isotope analysis (SIA). For all eels except those from the River Chelmer, SIA was completed using epidermal mucus. The rationale for using mucus was that it generally provides insight into shorter-term dietary changes than dorsal muscle and fin (Church et al. 2009; Winter et al. 2019b), so is appropriate for assessing whether there have been recent changes in habitat use (i.e. marine to freshwater). As the River Chelmer samples had been preserved in ethanol, mucus samples were unable to be taken effectively, so dorsal muscle had to be used instead. Epidermal mucus was collected from individual eels by running a sterile coverslip along the length of one side of the eel before being transferred to a sample tube with no further treatment (Winter et al. 2019a; Winter and Britton 2021). For muscle, a sample of tissue was excised from the dorsal area. All samples were then dried to constant weight (60 °C for 48 h), before being bulk analysed for δ13C and δ15N in a Thermo Delta V isotope ratio mass spectrometer (Thermo Scientific, Waltham, MA, USA) interfaced to an NC2500 elemental analyser (CE Elantach Inc, Lake-wood, NJ, USA). Analytical precision of the δ13C and δ15N sample runs was estimated against an internal standard sample of animal material every ten samples, with the overall standard deviation estimated at 0.08 and 0.04‰, respectively. With some C:N ratios exceeding 4.0, mathematical lipid correction was required (Post 2002), using the equation of Kiljunen et al. (2006). Non-normalised SI data summary statistics are provided in Supplementary Information: Table S1.
Data and statistical analyses
Eel length and SI data were similar between years (2021–2022) at each site, with no significant differences in δ13C. Although δ15N was significantly different this was likely due to the size of individual eel and not a reflection of temporal differences and so were combined for all subsequent analyses (Table S2). Inter-site differences in δ13C and δ15N of eels were evaluated using ANOVA, with Tukey post hoc tests identifying the site differences. The effect of sampling month and eel length on δ13C and δ15N were tested using generalised linear models (GLM; Gaussian distribution). Initially, the full model included the intercept, eel length and sampling month, with a backward stepwise approach used to select the best fitting model based on the lowest Akaike Information Criterion (AICc) value (corrected for small sample size), with model fit also assessed through visual examination of Pearson residuals plotted against fitted values and model covariates. To then assess the spatial and temporal variability in eel δ13C and δ15N, site-specific data were grouped by month and the isotopic niche size estimated using standard ellipse areas (SEA) in the R package SIBER (Jackson et al. 2011), where each ellipse enclosed the core 90% of the SI data (Jackson et al. 2011). A Bayesian estimate of SEA (SEAB) tested the differences in niche size between each site and month.
Predictions of the recent migration history were made for the rivers Piddle and Chelmer (which had the most extensive monthly sampling and where the eel samples were highly variable in their stable isotope values; cf. Results). These predictions were developed from the SI data and eel length data from the rivers Parrett and Frome. The glass eel life stage indicates a relatively recent metamorphosis from leptocephali and arrival into inshore areas (Miller et al. 2015), with only glass eels sampled from the River Parrett. Thus, δ13C data from these eels were considered as representative of eels newly arrived from the marine environment (‘marine’; –19.00 to –21.00‰; Table 1). The δ13C data of some Parrett eels were also considered to represent eels transitioning from marine to freshwater (–21.00 to –24.00‰; Table 1). These values are typical of European eel in estuarine habitats, i.e. eels transitioning between the marine and freshwater environment (Bardonnet and Riera 2005). Conversely, all eels sampled from the River Frome were elvers, and compared with the other sites, relatively large (Table 1), plus were sampled in freshwater approximately 8 km from the tidal limit. Thus, their δ13C data were considered as representing eels that had established in freshwater (< –25.00‰; Table 1). These δ13C data were used as the basis of a discriminant function analysis model (DFA) developed in the MASS and klaR R package, where the DFA was trained using the rivers Parrett and Frome δ13C, δ15N and eel length data to classify between newly arrived (i.e. marine), transitioning and freshwater-established eels. The model was then applied to classifying the individual eels from the Piddle and Chelmer into these three groups. The performance of the classification groups was assessed through cross-validation in which one individual is removed from the original matrix (jack-knife classification). All data analyses were conducted in R (2023) and prior to analyses, data were tested for normality (Shapiro–Wilks) and homogeneity of variance (Levene’s test).
Results
Variation in δ13C and δ15N by site
Across both sampling years, the River Parrett eels were strongly and significantly enriched in 13C and depleted in 15N compared with other sites (13C ANOVA: F3,625 = 318.21, P < 0.001; 13N ANOVA: F3,625 = 93.17, P < 0.001) (Table 1; Fig. 2). The most depleted 13C values were in elvers from the River Frome, where the maximum value was –27.71‰. The highest δ13C range was in the River Piddle (12.16‰) but was also relatively high for the River Chelmer (9.01‰) (Table 1). For 15N, the most depleted mean values were in River Parrett eels (6.91‰). Although mean δ15N values were similar between the other three rivers, a proportion of the Piddle and Chelmer eels had δ15N values similar to Parrett eels, but this was not evident in the Frome (Table 1; Fig. 2).
Eel lengths versus temporal SI data
Eel TL ranged from 59 to 129 mm (mean 77 ± 0.83 mm) and differed significantly across all sites (ANOVA: F3,625 = 120.01, P < 0.001; Fig. 3). The smallest TLs were glass eels in the Parrett and largest for elvers in the Frome (Table 1). The best-fitting GLMs indicated that δ13C and δ15N values were significantly influenced by eel TL and sample month in the River Piddle (Table 2). Month and TL had no significant influence on δ13C at the rivers Frome and Parrett, while δ15N values were significantly influenced by TL in the River Frome, and month in the Parrett (Table 2, Table S3). For the River Chelmer, δ13C values were influenced by TL and δ15N by month (Table 2, S3).
Temporal patterns in eel isotopic niche
Stable isotope biplots of eels from the rivers Piddle and Chelmer indicated distinct temporal patterns. Across both sampling years, Piddle eels had a wide range of δ15N and δ13C values in May but with the isotope range narrowing by month up to September, whereas the opposite pattern was generally apparent in the Chelmer (Fig. S1). The overall isotopic niche size (as SEAB) was largest for the River Chelmer, followed by the Piddle and was smallest for the River Frome (Fig. 4; Table 3), with the 95% posterior draws of SEAB indicating that the isotopic niches by month differ significantly for each river (Fig. 4; Table S4). River Piddle eels had a relatively large isotopic niche early on in the sampling season (May), with this niche then becoming significantly smaller in samples collected by September. Conversely, the smallest isotopic niche in the River Chelmer was in samples collected in May, with niche size being significantly larger in other months (Figs. 4; S4). The reduction in isotopic niche size over time in the Piddle was through the loss of 13C-enriched fish from samples collected throughout the summer, whereas in the Chelmer, the increase in isotopic niche size over time was through an increase in 13C-depleted fish (Fig. 4).
Predicting recent eel movements
In the trained DFA based on Parrett (marine, transitioning) and Frome (freshwater established) eels, the first discriminant function (δ13C) explained most of the variation between the groups (98%) (Wilk’s lambda λ = 0.21, P < 0.001) (Fig. S7). The observed versus predicted group classification of these eels had 79% agreement for the Parrett and 100% in the Frome (cross-validated) (Table 4). When the trained model was applied to the Piddle and Chelmer eels, a total of 65% were classified as freshwater established, 23% were in transition and only 11% were recently arrived from the marine environment (Table 4). The proportion of eels classified as from the marine environment and transitioning was higher in the Chelmer than the Piddle (Table 4).
In the River Piddle, eels classified as marine ranged from 61 to 76 mm (mean: 70 ± 4.19 mm) and were only present in samples between May and July (Table 5). The lengths of classified Piddle eels differed significantly between marine, transitioning, and freshwater established (ANOVA: F2,314 = 11.19, P < 0.001), with marine and transitioning eels being smaller than freshwater established (Tukey’s post hoc tests: P < 0.01) but were not different between the marine and transitioning eels (P = 0.99) (Table 5). In the River Chelmer, eels classified as marine were 63–77 mm (mean: 70 ± 0.98 mm) and although these fish were present in samples collected between March and September, the majority were sampled in May (67%) (Table 5). Length also differed between the classified groups (ANOVA: F2,196 = 15.78, P < 0.001), with marine classified being significantly smaller than transitioning and freshwater established (P < 0.01), but with no difference between transitioning and freshwater (P = 0.93).
Discussion
Our study revealed significant differences in the δ13C and δ15N values of the eels both within and between sites, suggesting considerable individual variability in the timing and duration of their transition into freshwater. The DFA based on eel length, δ13C and δ15N accurately classified eels between the rivers Parrett (marine, transitioning) and Frome (freshwater established), with most of this variation explained by the δ13C isotope. When applied to the Piddle and Chelmer eels, the model classified the majority of eels as freshwater established, followed by transitioning and then as recently arrived from the marine environment.
Eels captured from the River Parrett were strongly enriched in 13C, indicating recent arrival from the marine environment, given their δ13C values were similar to those reported for eels feeding on marine particulate organic matter (Bardonnet and Riera 2005). The Parrett eels also exhibited low isotopic variability, suggesting minimal foraging on freshwater resources. Although the δ13C data suggested that ‘marine’ individuals were present in all collected samples, no samples were collected after June due to low catches, which is consistent with other studies that suggest the peak arrival period of glass eels into Northern Europe is during May and June (Naismith and Knights 1988; Cresci et al. 2020).
The duration of the transition period between marine and freshwater habitats of glass eels and elvers can be from a few weeks to several years (Jellyman 1979; Sorensen and Bianchini 1986; Moriarty and Dekker 1997). In the River Parrett, eel movements from the marine to freshwater environment were considered as relatively rapid, given their enriched 13C values, which suggested most had newly arrived from the marine environment. This contrasted to the eels in the Frome, whose relatively depleted 13C values were similar to other freshwater fishes in that reach of river (Warren et al. 2023), indicating these eels had all been in freshwater for a considerable period. Conversely, both the Chelmer and Piddle samples comprised of eels with a wider range of lengths and SI values that were predicted by DFA as comprising of some marine and transitioning eels, but with most predicted as freshwater established, especially those sampled after June.
The DFA results suggested that most eels moving upstream into the rivers Piddle and Chelmer have already spent some time at upper estuary/freshwater boundary where they foraged on local prey resources that were relatively depleted in 13C and enriched in 15N versus their prey in the marine environment (Bardonnet and Riera 2005). As glass eels arrive into estuarine habitats, they undergo physiological and morphological changes, including development of pigmentation, jaws and teeth, which facilitates adaptation to their new environment and enables their exploitation of the novel prey resources (Tesch 1977; Cresci et al. 2020). Our data support the suggestion that the freshwater areas of tidal rivers are important foraging areas during this continental settlement period in the eel lifecycle (Bardonnet and Riera 2005). This period of residency in the lower reaches of rivers could enable eels to increase their energy reserves through foraging on locally abundant prey resources, which might then facilitate their subsequent upstream movements (Bureau Du Colombier et al. 2007).
Larger eels (> 415 mm) in the lower River Frome and in Piddle that were implanted with acoustic transmitters moved regularly between the freshwater and tidal reaches of the two rivers via Poole Harbour, with the movements being across a considerable salinity gradient and occurring over 24 h periods (Walker et al. 2013). Eels that settled initially into coastal lagoons in the Mediterranean remained in lagoons for 1–2 years before moving into freshwater (Panfili et al. 2012). Studies based on otolith microchemistry suggest that some eels settle into estuarine environments and remain there until they metamorphose into silver eels, with no use of freshwater environments at all (Tzeng et al. 1997; Daverat et al. 2006; Jessop et al. 2008; Bureau Du Colombier et al. 2011), while others make frequent movement back and forth between freshwater and marine systems (Tsukamoto and Arai 2001). Consequently, the patterns detected in the SI data of the smaller eels in the Piddle and Chelmer, where the majority of eels had values that were already based on freshwater prey resources, could have been making frequent small-scale movements in these lower river reaches prior to their capture. However, the small-scale movement ecology of these eels must remain speculative in the absence of any data on their actual movements.
Individuals classified as ‘marine’ in the River Piddle were only present in May and June, which suggests a relatively short period of eel immigration into Poole Harbour, with many of these eels considered as likely remaining in the lower, freshwater part of the River Piddle for their first winter before moving upstream during the following spring as water temperatures increase (and then being sampled). In contrast, ‘marine’ eels in the River Chelmer were present in samples—albeit in low proportions—throughout the summer. A range of factors have been shown to attract and direct eels to upstream freshwater habitats, including salinity gradients (Edeline et al. 2005b), tidal periodicity (Daverat and Tomás 2006), developmental stage (Crean et al. 2005) energetic status (Edeline et al. 2005a, 2006) and water temperature, with eels commencing upstream movements when water temperatures reach 15 °C (August and Hicks 2008; Overton and Rulifson 2008). These temporal patterns highlight the dynamic nature of the habitat use of these eel life stages and suggest potential shifts in foraging strategies and resource availability throughout the season that have high context dependency on local conditions and habitat structure.
Although stable isotopes are considered a reliable tool to reconstruct animal movements at broad spatial scales (Hobson 2023), it is important to consider the issue of differences in isotopic turnover between different tissues, especially when inferring the movements of the Chelmer eels based on muscle. Tissues that exhibit a rapid response, such as mucus and blood plasma, provide insights into more recent feeding habits compared with bone and muscle, which have a slower turnover rate (Church et al. 2009; Ziegler et al. 2023). For instance, in rainbow trout (Oncorhynchus mykiss), the half-lives of δ13C and δ15N in mucus were 30 and 36 days, respectively, whereas, in muscle, they were 136 and 94 days (Church et al. 2009). In the American eel (Anguilla rostrata), the half-lives of δ15N were 67 days in mucus and 191 days in muscle (Eberhardt 2019). Although measuring the residence times of eels was unable to be completed in our study, the mucus SI data for the Piddle, Frome and Parrett were considered to represent their diet in recent weeks, whereas for the Chelmer, the muscle SI data was likely to provide a longer temporal perspective. The use of different tissues between these two rivers is thus a study limitation, but one imposed by logistics that meant the Chelmer eels required preserving in ethanol that then prevented the effective collection of mucus samples. Nevertheless, in future studies, it is recommended that mucus is preferably used wherever possible for the SIA of eels due to its non-lethal application and ability to highlight relatively recent shifts in diet compared with dorsal muscle.
Our results provide valuable insights into the duration of eel transition from marine to freshwater environments, suggesting considerable variability between how individuals move through these habitats, with some individuals moving relatively quickly into freshwater at lengths up to 70 mm, while others at this size already have a strong freshwater SI signal. For eels that migrate up the River Piddle, they must first move through Poole Harbour, a complex environment of approximately 38 km2 comprised mainly of shallow waters and extensive mud flats that are exposed during low tide, with a tidal cycle of high-water periods separated by a short period of slack water, followed by a single low-water phase (Walker et al. 2013). This complex environment might mean it take a considerable time for some newly arrived eels to move through the harbour, with them having to regularly seek refuge during low-water periods, such as in any remaining flooded sections (given adult eels tend to move into the deepest sections of tidal creek systems during daytime low tides Helfman et al. 1983). Remaining in the lower freshwater reach of the River Piddle for considerable periods before moving upstream might be thus advantageous for these eels, given there is a high diversity of habitats available that provide both refugia and foraging areas, with the tidal reaches of lower rivers often being highly productive for the foraging of juvenile fishes (Denis et al. 2022).
In contrast to the River Piddle, eels that moved into the River Parrett were entering a less complex system, where flood sluices represented the tidal limits of rivers with straightened channels, with such hard barriers known to significantly impact the migratory behaviour of fish through blocking their upstream movements (Piper et al. 2013; Wright et al. 2015). Given all Parrett eels moving upstream of the tidal sluices were glass eels and largely with marine SI values, we suggest these eels moved relatively quickly through the upper estuary, most likely using passive tidal transport given that the Bristol Channel has a relatively large tidal range, before facing a binary decision of remaining in the estuary or ascending the eel pass on the sluice to move upstream. SI values in both the Chelmer and Piddle exhibited greater heterogeneity, which suggests that the more complex environments in these rivers provided the newly arriving eels with a greater range of habitats to exploit than the Parrett, resulting in a high proportion taking advantage by settling (and foraging) there. While engineering lower rivers and regulating their tidal flows is advantageous for society, the resulting simplification of the freshwater–estuarine transitional zone reduces habitat complexity, likely resulting in the relatively swift movement of eels through what should otherwise be important habitats for settlement and early life history. Given that these tidal structures remain important for flood control, options to increase the habitat complexity of these rivers remain limited. Consequently, future management plans should ensure that barriers are fitted with eel passes to reduce the number of migrating eels that are facing delays in accessing freshwater habitats upstream that could provide higher habitat complexity and more profitable foraging areas. Estuarine habitats can provide diverse and productive foraging areas (Harrod et al. 2005) and thus many eels remain in these areas throughout their continental life stage (Arai 2022). This research highlights the significance of providing diverse and complex habitats for eels during their transition from marine to freshwater environments. Where possible, eel conservation management plans should also prioritise the protection and restoration of freshwater habitats, where the removal of migration barriers enable the free ranging of eels through the river system.
In summary, this study demonstrates considerable individual variability in the movements of glass eels and elvers into the lower reaches of four rivers in England. Where there was some habitat complexity in downstream areas, the eels migrating upstream were a mix of newly arrived, transitioning and freshwater established, whereas in the heavily engineered River Parrett, they all had marine SI values. These results suggest that the upstream migration of eels into tidal and freshwater habitats is not uniform, highlighting the importance to provide eels with a wide range of settlement and over-wintering habitats wherever possible.
Data availability
Data are available from the corresponding author on reasonable request.
References
Arai T (2020) Ecology and evolution of migration in the freshwater eels of the genus Anguilla Schrank, 1798. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e05176
Arai T (2022) Migration ecology in the freshwater eels of the genus Anguilla Schrank, 1798. J Trop Ecol 63:155–170. https://doi.org/10.1007/s42965-021-00217-7
August SM, Hicks BJ (2008) Water temperature and upstream migration of glass eels in New Zealand Implications of climate change. Enviro Biol Fishes 8:195–205. https://doi.org/10.1007/s10641-007-9191-z
Bardonnet A, Riera P (2005) Feeding of glass eels (Anguilla anguilla) in the course of their estuarine migration: new insights from stable isotope analysis. Estuar Coast Shelf Sci 63:201–209. https://doi.org/10.1016/j.ecss.2004.11.009
Boardman RM, Pinder AC, Piper AT, Gutmann Roberts C, Wright RM, Britton JR (2022) Effects of preservation by ethanol on δ13C and δ15N of three tissues of the critically endangered European eel Anguilla anguilla. J Fish Biol 103:179–182. https://doi.org/10.1111/jfb.15394
Bureau Du Colombier S, Bolliet V, Lambert P, Bardonnet A (2007) Energy and migratory behavior in glass eels (Anguilla anguilla). Physiol Behav 92:684–690. https://doi.org/10.1016/j.physbeh.2007.05.013
Bureau Du Colombier S, Bolliet V, Lambert P, Bardonnet A (2011) Metabolic loss of mass in glass eels at different salinities according to their propensity to migrate. Estuar Coast Shelf Sci 93:1–6. https://doi.org/10.1016/j.ecss.2011.02.021
Church MR, Ebersole JL, Rensmeyer KM, Couture RB, Barrows FT, Noakes DL (2009) Mucus: a new tissue fraction for rapid determination of fish diet switching using stable isotope analysis. Can J Fish Aquat Sci 66:1–5. https://doi.org/10.1139/F08-206
Crean SR, Dick JTA, Evans DW, Rosell RS, Elwood RW (2005) Survival of juvenile European eels (Anguilla anguilla), transferred among salinities, and developmental shifts in their salinity preference. J Zool 266:11–14. https://doi.org/10.1017/S0952836905006539
Cresci A (2020) A comprehensive hypothesis on the migration of European glass eels (Anguilla anguilla). Biol Rev. https://doi.org/10.1111/brv.12609
Cresci A, Sandvik AD, Sævik PN, Ådlandsvik B, Olascoaga MJ, Miron P, Durif CMF, Skiftesvik AB, Browman HI, Vikebø F (2020) The lunar compass of European glass eels (Anguilla anguilla) increases the probability that they recruit to North Sea Coasts. Fish Oceanogr 30:315–330. https://doi.org/10.1111/fog.12521
Daverat F, Tomás J (2006) Tactics and demographic attributes in the European eel Anguilla anguilla in the Gironde watershed, SW France. Mar Ecol Prog Ser 307:247–257. https://doi.org/10.3354/meps307247
Daverat F, Limburg KE, Thibault I, Shiao JC, Dodson JJ, Caron F, Tzeng WN, Wickström H (2006) Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar Ecol Prog Ser 308:231–241. https://doi.org/10.3354/meps308231
Denis J, Rabhi K, Le loc’h F, Lasram F, Boutin K, Kazour M, Diop M, Gruselle MC, Amara R (2022) Role of estuarine habitats for the feeding ecology of the European eel (Anguilla anguilla). PLoS ONE. https://doi.org/10.1371/journal.pone.0270348
Eberhardt A (2019) Evaluation of New England Salt Marsh Support of the American Eel, Anguilla rostrata, and the Impacts of Hydrologic Restriction. Dissertation, University of New Hampshire.
Edeline E, Bardonnet A, Bolliet V, Dufour S, Elie P (2005a) Endocrine control of Anguilla anguilla glass eel dispersal: effect of thyroid hormones on locomotor activity and rheotactic behavior. Horm Behav 48:53–63. https://doi.org/10.1016/j.yhbeh.2005.02.001
Edeline E, Dufour S, Elie P (2005b) Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla Anguilla. Mar Ecol Prog Ser 304:191–199. https://doi.org/10.3354/meps304191
Edeline E, Lambert P, Rigaud C, Elie P (2006) Effects of body condition and water temperature on Anguilla anguilla glass eel migratory behaviour. J Exp Mar Biol Ecol 331:217–225. https://doi.org/10.1016/j.jembe.2005.10.011
Elise B, Lasne E, Acou A, Guillaudeau J, Bertier C, Feunteun E (2014) Migration behaviour of silver eels (Anguilla anguilla) in a large estuary of Western Europe inferred from acoustic telemetry. Estuar Coast Shelf Sci 137:22–31. https://doi.org/10.1016/j.ecss.2013.11.023
Feunteun E (2002) Management and restoration of European eel population (Anguilla anguilla): an impossible bargain. Ecol Eng 18:575–591. https://doi.org/10.1016/S0925-8574(02)00021-6
Gascuel D (1986) Flow-carried and active swimming migration of the glass eel (Anguilla anguilla) in the tidal area of a small estuary on the French Atlantic coast. Helgol Mar Res 40:321–326. https://doi.org/10.1007/BF01983739
Harrod C, Grey J, McCarthy TK, Morrissey M (2005) Stable isotope analyses provide new insights into ecological plasticity in a mixohaline population of European eel. Oecologia 144:673–683. https://doi.org/10.1007/s00442-005-0161-x
Helfman GS, Stoneburner DL, Bozeman EL, Christian PA, Whalen R (1983) Ultrasonic telemetry of American eel movements in a tidal creek. Trans Am Fish Soc 112:105–110. https://doi.org/10.1577/1548-8659
Hobbs J, Lewis L, Willmes M, Denney C, Bush E (2019) Complex life histories discovered in a critically endangered fish. Sci Rep. https://doi.org/10.1038/s41598-019-52273-8
Hobson KA (2023) Stable isotopes and a changing world. Oecologia. https://doi.org/10.1007/s00442-023-05387-w
ICES (2022) Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL). ICES Sci Rep. https://doi.org/10.17895/ices.pub.20418840
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R: Bayesian isotopic niche metrics. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jacoby D, Gollock M (2014) Anguilla anguilla. The IUCN red list of threatened species 20. https://doi.org/10.2305/IUCN.UK.20141.RLTS.T60344A45833138.en.
Jellyman DJ (1979) Upstream migration of glass-eels (Anguilla spp.) in the Waikato River. N Z J Mar Freshw Res 13:13–22. https://doi.org/10.1080/00288330.1979.9515776
Jensen AJ, Finstad B, Fiske P (2019) The cost of anadromy: marine and freshwater mortality rates in anadromous Arctic char and brown trout in the Arctic region of Norway. Can J Fish Aquat Sci 76:2408–2417. https://doi.org/10.1139/cjfas-2018-0428
Jessop BM, Cairns DK, Thibault I, Tzeng WN (2008) Life history of American eel Anguilla rostrata: new insights from otolith microchemistry. Aquat Bio 1:205–216. https://doi.org/10.3354/ab00018
Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI (2006) A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J Appl Ecol 43:1213–1222. https://doi.org/10.1111/j.1365-2664.2006.01224.x
Kristensen ML, Birnie-Gauvin K, Aarestrup K (2019) Behaviour of veteran sea trout Salmo trutta in a dangerous fjord system. Mar Ecol Prog 616:141–153. https://doi.org/10.3354/ab00018
Laffaille P, Caraguel JM, Legault A (2007) Temporal patterns in the upstream migration of European glass eels (Anguilla anguilla) at the Couesnon estuarine dam. Estuar Coast Shelf Sci 73:81–90. https://doi.org/10.1016/j.ecss.2006.12.011
Miller MJ, Bonhommeau S, Munk P, Castonguay M, Hanel R, McCleave JD (2015) A century of research on the larval distributions of the Atlantic eels: a re-examination of the data. Biol Rev 90:1035–1064. https://doi.org/10.1111/brv.12144
Moriarty C, Dekker W (1997) Management of European eel fisheries. Iris Fish Bull 15:1–110
Naismith IA, Knights B (1988) Migrations of elvers and Juvenile European eels, Anguilla Anguilla L., in the river Thames. J Fish Biol 33:161–175. https://doi.org/10.1111/j.1095-8649.1988.tb05570.x
Nolan ET, Gutmann Roberts C, Britton JR (2019) Predicting the contributions of novel marine prey resources from angling and anadromy to the diet of a freshwater apex predator. Freshw Biol 64:1542–1554. https://doi.org/10.1111/fwb.13326
Overton AS, Rulifson RA (2008) Annual variability in upstream migration of glass eels in a Southern USA coastal watershed. Environ Biol Fishes 84:29–37. https://doi.org/10.1007/s10641-008-9386-y
Panfili J, Darnuade A, Lin Y, Chevalley M, Lizuka Y, Tzeng WN, Alain C (2012) Habitat residence during continental life of the European eel Anguilla Anguilla investigated using linear discriminant analysis applied to otolith SR:ca ratios. Aquat Biol 15:175–185. https://doi.org/10.3354/ab00414
Pike C, Crook V, Gollock M (2020) Anguilla anguilla. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T60344A152845178.en
Piper AT, Wright RM, Walker AM, Kemp, (2013) Escapement, route choice, barrier passage and entrainment of seaward migrating European eel, Anguilla anguilla, within a highly regulated lowland river. Ecol Eng 57:88–96. https://doi.org/10.1016/j.ecoleng.2013.04.030
Post DM (2002) Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecol 83:703–718. https://doi.org/10.2307/3071875
Righton D, Westerberg H, Feunteun E, Okland F, Gargan P, Amilhat E, Metcalfe M, Lobon-Cervia J, Sjöberg N, Simon J, Acou A, Vedor M, Walker A, Trancart T, Brämick U, Aarestrup K (2016) Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci Adv. https://doi.org/10.1126/sciadv.1501694
Schmidt J (1923) The breeding places of the eel. Philos Trans R Soc 211:382–390. https://doi.org/10.1098/rstb.1923.0004
Sorensen PW, Bianchini ML (1986) Environmental correlates of the freshwater migration of elvers of the American eel in a Rhode Island Brook. Trans Am Fish Soc 115:258–268. https://doi.org/10.1577/1548-8659
Tesch FW (1977) The eel. Blackwell, London
Tesch FW (1980) Occurrence of eel Anguilla Anguilla larvae west of the European continental shelf 1971–1977. Environ Biol Fishes 5:185–190. https://doi.org/10.1007/BF00005354
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar Ecol Prog Ser 220:265–276. https://doi.org/10.3354/meps220265
Tzeng WN, Severin KP, Wickstrom H (1997) Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Mar Ecol Prog Ser 149:73–81. https://doi.org/10.3354/meps149073
Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC (2015) Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE. https://doi.org/10.1371/journal.pone.0116182
Walker A, Godard M, Davison P (2013) The home range and behaviour of yellow-stage European eel Anguilla anguilla in an estuarine environment. Aquat Conserv Mar 4:155–165. https://doi.org/10.1002/aqc.2380
Warren BIC, Pinder AC, Parker B, Tarkan AS, Britton JR (2023) Trophic relationships of translocated and indigenous chub Squalius cephalus populations with trophically analogous fishes. Hydrobiologia. https://doi.org/10.1007/s10750-023-05389-y
Winter ER, Britton JR (2021) Individual variability in stable isotope turnover rates of epidermal mucus according to body size in an omnivorous fish. Hydrobiologia 848:363–370. https://doi.org/10.1007/s10750-020-04444-2
Winter ER, Nolan ET, Busst GM, Britton JR (2019a) Estimating stable isotope turnover rates of epidermal mucus and dorsal muscle for an omnivorous fish using a diet-switch experiment. Hydrobiologia 828:245–258. https://doi.org/10.1007/s10750-018-3816-4
Winter ER, Nyqvist M, Britton JR (2019b) Non-lethal sampling for stable isotope analysis of pike Esox lucius: how mucus, scale and fin tissue compare to muscle. J Fish Biol 95:956–958. https://doi.org/10.1111/jfb.14059
Wright GV, Wright RM, Kemp PS (2015) Impact of tide gates on the migration of adult European Eels, Anguilla anguilla. Estuarine Coasts 38:2031–2043. https://doi.org/10.1007/s12237-014-9931-1
Yokouchi K, Fukuda N, Miller M, Aoyama J, Daverat F, Tsukamoto K (2012) Influences of early habitat use on the migratory plasticity and demography of Japanese eels in Central Japan. Estuar Coast Shelf Sci 107:132–140. https://doi.org/10.1016/j.ecss.2012.05.009
Ziegler A, Bluhm B, Renaud P, Jorgensen L (2023) Isotopic turnover in polar cod (Boreogadus saida) muscle determined through a controlled feeding experiment. J Fish Biol 102:1332–1454. https://doi.org/10.1111/jfb.15389
Acknowledgements
We thank all Environment Agency staff for their assistance in collecting the raw data for this study. RB was supported by a studentship funded by the Environment Agency and Bournemouth University.
Funding
RMB was supported by a PhD studentship funded by the Environment Agency and Bournemouth University.
Author information
Authors and Affiliations
Contributions
Conceptualisation: RMB, ACP, AP, CGR, RW, JRB. Developing methods: RMB, ACP, AP, CGR, RW, JRB. Data analysis: RMB. Preparation of figures and tables: RMB. Data interpretation: RMB. Writing: RMB, ACP, AP, CGR, RW, JRB.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Ethical statement
The study was completed following the gaining of all relevant ethical and legislative approvals (UK Home Office Project Licence P47216841; Environment Agency permit reference EP/EW027-C-042/19919/01.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boardman, R.M., Pinder, A.C., Piper, A.T. et al. Variability in the duration and timing of the estuarine to freshwater transition of critically endangered European eel Anguilla anguilla. Aquat Sci 86, 18 (2024). https://doi.org/10.1007/s00027-023-01033-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01033-y