Abstract
We compared structural and functional changes in macrophyte species composition in softwater lakes with isoetids located along the southern shore of the Baltic Sea (NW Poland) in two time periods (1955–1959 and 2015–2020). The research aimed to determine the trend of changes in macrophyte composition influenced by fields and/or urban fabric, as land use. The land-use pressure measure referred to the volume of land occupied around the lake. In the second time period, the number of plant species in the lakes increased twofold (20 vs. 39), compared to the first period. The average values of species richness were statistically higher (p < 0.001) in the second period (15.7 vs. 8.6). The functional diversity of plants in the lakes revealed statistically significant differences in both periods compared. The FD Rao values calculated for plant life span, growth forms, and FD multi-traits were statistically higher in the second period (p < 0.001). Our findings revealed that the anthropogenic pressure on lakes over a period of 60 years caused a decrease in the share of sensitive species in macrophyte species composition (isoetids and mosses), but an increase in common plants with a completely different set of species functional traits. This is related to the environmental changes that occurred between the two periods studied. First of all, we noticed significant changes in the transparency (visibility) of the water. In the second period, the value of this trait is used in each lake, which uses the transmission of photosynthetic active radiation (PAR) light transmittance to the plant and can affect the species composition. These findings show that an increase in biodiversity can relate to a decrease in freshwater ecosystem function, mainly via lost function of evergreen isoetid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The northern European softwater lakes are defined by physicochemical conditions given by the Organisation for Economic Co-operation and Development (OECD 1982) and Moss et al. (1996). These are lakes with Ca2+ < 3 mg/l (Murphy 2002) and very low alkalinity (Pulido et al. 2011). The lakes are also very prone to acidification because of their low buffering capacity, while the vegetation is often dominated by isoetids (e.g., Isoetes lacustris, Lobelia dortmanna, and Littorella uniflora), with morphological and physiological adaptations to overcome limitations in inorganic carbon availability (Wium-Andersen 1971; Søndergaard and Sand-Jensen 1979; Pedersen et al. 2013). Isoetids are evergreen aquatic plants, well adapted to clear-water acidic lakes and low inorganic nutrients and carbon. Moreover, they create clusters in the littoral zone (Szmeja 1994a, 1994b, 1994c); as a result, the gaps between them can also be occupied by other plant species groups, especially when softwater lakes are affected by eutrophication, acidification, brownification (i.e., under the influence of humic substances), or other human impacts (Iversen et al. 2019).
One of the effects of human pressure on lakes includes an extinction of native aquatic plant species and invasion of alien plants or expansion of species not specific to the local habitats. Changes in the vegetation of such lakes may stem from disturbances in the acquisition of inorganic carbon for photosynthesis or from water and/or sediment (Riis and Sand-Jensen 1997; Baastrup-Spohr et al. 2015; Maberly and Gontero 2018).
In the lakes that are subject to human pressure, interspecies interactions are probably stronger than the impact resulting in dispersion or extinction of species (Chmara et al. 2013). At that point, significant changes can be observed in the species composition and relationships between plant species with different life histories, which may lead to the development of a new zonation of vegetation in the lake (Chmara et al. 2018).
The impact of land use surrounding lakes on the physical and chemical characteristics of lake water is fairly well understood. Similar to other ecosystems, softwater lakes with isoetids are subject to natural and anthropogenic transformations, which may be caused by (1) eutrophication, alkalinization, acidification, and hydro-regulation in the catchment areas (Szmeja 2006; Banaś 2016), (2) land use in the catchments (mainly agricultural in the twentieth century, now predominantly for regular or seasonal residential purposes, with compact or scattered building patterns), (3) surface runoff from anthropogenically transformed catchments, and (4) destruction of the filtration zone around lakes (vegetation cover, especially meadow, forest, and grassland areas) (Ecke 2009; Lindholm et al. 2020).
The aim of this study is to determine changes in environmental conditions and vegetation structure in 10 softwater lakes based on a comparison of water characteristics, structural characteristics of vegetation, and functional traits of macrophytes in the mid-twentieth century with those at the end of the second decade of the twenty-first century. We formulate a hypothesis providing that fields and urban fabric, as land use, contribute to the increase in the share of common aquatic plants in the lakes and the decrease in sensitive species, which may have led to remodeling of the structural and functional composition of macrophytes in lakes over the last 60 years of use.
Material and methods
Study sites
The study area is located in northern Poland, southwest of Gdańsk, in the Kashubian Lake District (Fig. 1). According to Solon et al. (2018), this region, which covers about 3000 km2, is situated in the area of the last glaciation (Baltic), with diverse hypsometry (maximum elevation 329 m ASL) and more than 500 lakes, most of them located between 149 and 216 m ASL. The mean annual air temperature is 7.8 °C, while the precipitation amounts to 612–754 mm/year. The softwater lakes in this region are well oxygenated, with low pH and conductivity (oligotrophic), as well as low calcium content and good visibility in the water (Szmeja et al. 1997).
Location of studied lakes (1–10): 1 = Stacino (54° 16.8 N, 17° 50.7 E), 2 = Karlikowskie (54° 19.5 N, 18° 18.7 E), 3 = Sitno (54° 20.1 N, 18° 18.0 E), 4 = Morzycz (54° 27.3 N, 17° 52.7 E); 5 = Brzeżonko (54° 27.5 N, 18° 15.9 E), 6 = Ostrowieckie (54° 17.8 N, 17° 43.6 E), 7 = Jelonek (54° 26.8 N, 18° 15.0 E), 8 = Czarne (54° 22.0 N, 17° 48.7 E), 9 = Miemino (54° 21.6 N, 17° 49.6 E), 10 = Księże (54° 23.1 N, 18° 17.9 E)
Vegetation and water chemistry sampling
The study was conducted in 10 softwater lakes with isoetids. All the lakes constitute the same small but very specific group of shallow-water oligotrophic and mesotrophic (post-glacial) reservoirs, highly susceptible to anthropogenic changes. Their average depth is similar, not great (in individual cases there is a very small surface depression even up to 14 m), with the dominant mode of supply being rainwater, while in all the studied lakes the vegetation is shallow, mainly because the dominant group of plants are isoetids. The morphometric characteristics of these lakes are presented in Online Resource 1. The lakes were sampled during the vegetation season in the same months (June, July, and August) in two periods: (I) 1955–1959 and (II) 2015–2020. The data regarding environmental conditions in lakes for the first period were determined based on Szmal and Szmal (1965), Dąmbska (1965), and our archive data (data for publication were collected between 1955 and 1959, while the publication itself appeared in 1965), whereas the second set was based on research we conducted. In both periods, sampling was based on the same methodology (samples were collected from the same depths and transects). Between 1955 and 1959, the collection of plant samples was performed by taking noninvasive phytosociological relevés in the form of researchers’ notes (exactly as it was done these days) containing information about all species of aquatic plants occurring in a given area and their cover in strips parallel to the shore every 1 m of depth (from each depth zone—1.0 m interval). These samples were recorded along the previously designated transect of 250–500 m (one for a given lake). The transect was led along the shore, with a specific form of use of the catchment, such as fields. Samples were collected using a special visor to perform a thorough inspection of the lake bottom. This was performed only from the surface of the water, without the help of a diver, because of the availability of equipment and methods applied at that time. These two methods of examining macrophytes are (at the depths explored) equally accurate and give the same effect (the use of a diver is easier, due to the faster identification of macrophyte species).
In the second period, i.e., between 2015 and 2020, during field research, we applied the same methodology as in the first period (as close as possible to the same level of accuracy) but using the diving equipment. The same transect that was selected in the first research period was examined (one in each lake): perpendicular to the shoreline (with or without a specific form of catchment use, depending on whether after 60 years there was still human pressure or natural plant communities), delineated in each of the selected lakes, then divided in intervals into strips parallel to the shore every 1 m of depth. They are referred to as depth zones throughout this study. From each depth zone (1.0 m interval), 100 plant samples were collected (100 plant samples per depth zone in each lake) by a diver using a noninvasive process in the form of notes on a plastic plate regarding all plant species and the plant cover of each observed species (expressed as % of a sample) in randomly selected bottom areas of 0.1 m2 (according to the scheme provided in the work by Ronowski et al. 2020) (Fig. 2). In both periods, the data were collected in the same way and in the same places. The method for sample collection entailed taking phytosociological relevés in the transect (in both periods, the transect ran along the shore of the lake with a specific form of use). During field research, an almost identical surface of the lake bottom was examined. Between 1955 and 1959, it amounted to 2458 m2, and between 2015 and 2020, it comprised 2700 m2. The samples from the first period (phytosociological relevés in vegetation patches) were larger in terms of surface than our samples. This resulted from the methodological approach applied more than 60 years ago. Our samples were collected in accordance with the methodology applied today (with the help of a diver). The exact same area of the lakes was examined, only with the use of smaller phytosociological images (2740 were collected), with accuracy levels as close as possible to the those of the samples collected 60 years ago (samples were collected from the same depths).
In each depth zone just above the plants, the diver took three water samples, a total of 1.5 l each, which were used as material for the physical and chemical analyses of water properties (some parameters were determined directly in the field). The water samples were transported to a cold store and later analyzed in the laboratory, according to the methods proposed by Hermanowicz et al. (1999), Wetzel (2001) and Eaton et al. (2005). In the water samples, the following properties were recorded: in the lab: (1) calcium concentration (Ca2+/l; complexometric titration with calconcarboxylic acid as an indicator); in the field (from the boat): (2) oxygenation (%) (WTW Oxi 197 oxygen meter with an EOT 196 electrode); (3) pH; pH meter 320/SET1 with a SenTix 97T measuring electrode; (4) visibility (m; Secchi disk); (5) depth (m; using Nexus Depth or Eagle TriFinder depth finders). However, different methods and equipment for chemical analysis available at the time were applied. They yielded the same results; however, they differed in the performance method. For example, water samples (also transported to the laboratory and analyzed there) from each depth zone were not collected by a diver but by a Ruttner sampler. In the water samples, the following properties were recorded: in the lab: (1) pH (colorimetrically with the use of appropriate standard solutions); (2) oxygen (mg O2/l; Winkler method without bromination); (3) oxidizability (Kubler and Tiemann method); (4) Ca2+, titration using appropriate reagents; in the field: (5) visibility (m; Secchi disk); (6) depth (m) (Szmal and Szmal 1965).
Land-use evaluation
The data on the share of land use types around the lakes for the first period (1955–1959) were determined based on Szmal and Szmal (1965), Dąmbska (1965), and our archive data. The descriptions of land use provided in these works were confirmed in precise topographical maps from the mid-twentieth century. In the second period (2015–2020), the land-use assessment was based on our findings. Using the maps in Geoportal (www.geoportal.gov.pl) and CORINE Land Cover with the data from 2018, as well as the ArcGIS program, a 100-m-wide strip (filtration zone, direct catchment) of land was marked out around the lakes (all possible doubts and generalizations were detailed and corrected in ArcGIS, working directly on the orthophoto map). The land use around study lakes was separated into forests, fields, urban fabric, peat bogs, heatlands, and grasslands, and then we calculated their relative share around the lakes (in %). Based on these data, we analyzed their impact on the macrophyte functional traits.
Data treatment
We calculated species diversity for each plant sample, based on Simpson’s Diversity (D) index (Simpson 1949), according to the formula:
where S is the number of species, N is the number of individuals, and ni is the number of individuals of the ith species. The diversity index data were normalized, and mean values were compared between the first and second periods using Student’s t test.
In addition, for each lake, we assessed the changes in macrophyte functional traits after 60 years of land use around the lakes. We used “soft” traits; the calculation of Rao’s Q formula FDQ takes into account four traits of species, namely (1) plant life span (perennial, perennial-evergreen, annual), (2) leaf type (tubular, capillary, flat-leaf), (3) growth forms (charophytes, bryophytes, isoetids, potamids, myriophyllids, ceratophyllids, vallissnerids, nymphaeids, and herbids), and (4) macrophyte group (submerged plants, emerged plants, floating plants) under Rao’s Q formula (Ricotta 2005; Lepš et al. 2006):
where FDQ is the sum of trait dissimilarity among all pairs of species weighted by species relative abundance, and dij is the functional distance equal to the squared Gower distance between species i and j. FDQ calculations were based on four functional traits as a multi-trait index, but we also calculated this functional index for each trait separately, including plant life span, leaf type, growth form, and macrophyte group, and the following indices were calculated (see Online Resource 2). To evaluate the turnover of plant species groups, we calculated the floating/submerged ratio index (FS index), expressed as the ratio of the number of floating-leaved species and submerged species.
PCA was used to evaluate changes in land use over time. The two periods were compared using PCA based on the proportion of land use (relative share in percentage of land use). PCA was performed using Canoco version 4.5 software (Ter Braak 2008). All variables were log-transformed to normal distribution. The correlations between the eigenvector scores of the land-use variables on the PCA1 axis and the functional traits of plants were evaluated using ordinary least squares (OLS) regression. For each diversity index, data were normalized, and mean values were compared between the first and second periods using Student’s t test.
Results
Land use
The land-use analysis (Table 1) shows that in the first period (1955–1959), forests dominated around three lakes (no. 1, 2, 6), and in the second period (2015–2020) they were dominant around four (1, 2, 4, 5), whereas fields were dominant around six (4, 5, 7–10) and five lakes (6–10), respectively. It is worth noting that in the second period, the share of urban fabric increased around as many as five lakes (no. 2, 3, 6, 7, 10). The relative share of urban fabric around these lakes is actually much larger, since a significant share of newly built houses, used year-round or seasonally, are located more than 100 m from the lakes, outside the mapped land-use areas. The increase in the share of urban fabric may affect the environmental conditions, submerged vegetation, and functional diversity of macrophytes. It must be noted that natural units of land cover such as peat bogs, heatlands, or grasslands have a rather small share.
Changes in land use around the lakes between the first and second periods are significant. PCA based on the relative share of land use showed a clear distinction between the groups assigned to respective periods of land use (Fig. 3). In this study, the environmental land-use gradient is represented by the first axis (PCA1), which alone explained 90.4% of total variance, with the second axis explaining 3.7 of total variance. The first group, widely distributed in the ordination space, represents land use in the first period. The dominant form of land use at that time included forests and peat bogs. The second group represents land use between 2015 and 2020. In this period, we observed shifts in land use, with an increase in the share of urban fabric and a reduction in peat bog area.
The ordination diagram of principal component analysis (PCA) representing the first two axes for land use in the first period (1955–1959; Red Cross) and the second period (2015–2020 purple square) of study lakes. The proportion of land use (relative share in % of land use) included in the PCA analysis. The first axis was used as proxy environmental land-use gradient
Changes in water conditions
A comparison of water characteristics (pH, Ca2+ concentration, and clarity/visibility) between the two periods reveals that lakes without urban fabric are more acidic in the second period than in the first period (lakes no. 1, 4, 5; see Table 2). Slight changes in pH (< 0.5 pH unit) were detected from the first to the second period in four lakes (lakes 1, 6, 7, 10), whereas more significant changes (> 1.0 pH unit) were seen in another four (lakes 2, 4, 5, 9). Of note is the increase in acidification in lakes with a significant share of forests in the second period (lakes 4, 5) and a decrease in lake acidification with the then largest increase in the share of urban fabric (lake 2). However, the scale span of the pH of water in the first period (6.1–8.3) is similar to that of the second period (5.8–8.2). Moreover, we observed significant changes in water clarity (visibility). In the second period, the value of this feature decreased in every lake, which resulted in a reduction in photosynthetic active radiation (PAR) light transmission to plants and may have had a negative impact on some macrophyte species.
Replacement of plant species groups
A comparison of the number of isoetids, submerged non-isoetid vascular plants, mosses, and charophyte species in the first and the second periods (Table 3) shows a mean of 2.6 isoetid species in the samples in the first period, which is greater than that of 2.0 in the second period. This difference is clearly visible in lakes with a significant share of fields (lakes no. 8–10, Table 1), where the number of isoetid species is always equal to or greater than the number of species in the samples collected in the second period. Moreover, these species were completely eliminated from lakes no. 5 and 10. In lakes 8 and 10, Isoetes lacustris and Lobelia dortmanna were completely eliminated, while in lake 8, instead of these two species, Littorella uniflora appeared, the coverage of which is now 44.7%. In lake no. 9, the coverage of all three isoetid species decreased: Isoetes lacustris from 52.5 to 11.3%, Lobelia dortmanna from 47.5 to 12.3%, and Littorella uniflora from 55 to 25% (Online Resource 3).
It is worth noting the number of submerged non-isoetid vascular plants in both analyzed periods (Table 3). In the first period, the number of species in lakes is low (≤ 5/0.1 m2, average 2.2, no occurrence at all in lakes no. 4 and 8). Moreover, in the second period, especially in lakes with urban fabric and fields (lakes no. 2, 3, 6–10; Table 1), the number of non-isoetid vascular plant species is twice as large, amounting to 9 species/0.1 m2 (lakes no. 3 and 10). In lake no. 3, where the greatest change occurred between periods in submerged non-isoetid vascular plants, only Myriophyllum alterniflorum was present in the first period, reaching 82% coverage, while in the second study period as many as nine new species appeared, of which the highest coverage was achieved by Stratiotes aloides (55.6%) and Nitella flexilis (44.7%), while the coverage of Myriophyllum alterniflorum drastically decreased to only 5% (Online Resource 3). In the second period, the arithmetic mean of the number of such species in samples from all lakes is lower (6.9; p < 0.05, t test).
As for the share of mosses and charophytes in the samples for the two analyzed periods, it should be noted that a slight nonsignificant decrease was seen in the number of species.
Structural and functional diversity of macrophytes
Species richness varied from 3 to 14 species per lake in the first period and 12 to 20 in the second period (Online Resource 3). After 60 years of land use around the lakes, the number of plant species in lakes had doubled (20 vs. 39). The mean values of species richness were statistically higher (p < 0.001; Table 4) in the second period (15.7 vs. 8.6). Some species that were characteristic of the lakes in the first period became extinct, while in the second period new ones appeared, such as Elodea canadensis, Ceratophyllum demersum, and Myriophyllum spicatum. The analysis of plant functional traits shows that the Simpson index increased in the second period as well as the share of perennial species, but above all, the share of evergreen species decreased, especially isoetids and mosses. Some species, including Isoetes lacustris and Lobelia dortmanna, showed lower occurrence during the second land-use period. Conversely, the occurrence of eutrophic submerged plant species including Elodea canadensis, Ceratophyllum demersum, and Myriophyllum spicatum increased during the second period (from 0 to 16.1%, 19.9%, and 24% respectively; Online Resource 3).
It is worth noting that the Simpson index values are higher for lakes in the second period than the first period, which means that the species diversity in lakes increased (p < 0.01; Table 4). We explain this by the fact that environmental traits have changed (Table 2), and as a result, new plant species typical for eutrophic waters have emerged (Table 3). A significant increase in the Simpson index value is visible primarily in lakes with agricultural usage (lakes 8–10). The greatest changes in this index were found in lakes influenced by fields and urban fabric as the main types of land use (Table 5, see lakes no. 3 and 4).
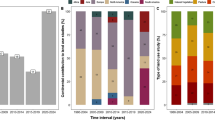
The functional diversity of plants in the investigated lakes showed statistically significant differences between periods. Values of FD Rao calculated for growth forms and multi-traits were statistically higher (p < 0.001 Table 4) in the second period (Fig. 4). All lakes demonstrated higher values of FD Rao for plant life in the second period, with the greatest increase in lakes no. 3, 2, and 4 (Online Resource 3 and 4). Similarly, higher values for FD Rao growth forms were observed in all lakes in the second period, with the greatest increase in lake no. 3 (from 0.22 in period I to 0.73 in period II).
The results of analysis using the floating/submerged ratio index (FS index) showed temporal changes in the turnover of plant species groups. We found significant differences in the FS index between the first and second periods (t = −2.80, p = 0.01, t test), with the ratio of floating to submerged plants increasing over time (Table 5), indicating that in most lakes in the second period, we observe the colonization of phytolittoral zones by floating-leaved species.
To assess the relationship between land use and functional diversity environmental conditions, we used the PCA axis score in the first and second periods as proxy environmental and land-use gradients (Fig. 5). Analysis of the relationship between species and functional traits, and PCA1 axis scores revealed significant positive correlations: FD Rao growth forms and FD_multi-traits (Fig. 5A,B), PCA1—water oxygenation, respectively (Fig. 5D). Furthermore, PCA analysis showed a strong negative correlation between PCA1 scores and water oxygenation (r = 0.68; p = 0.01) (Fig. 5D).
Relationships between PCA1 axis, as land-use scores, and FDQ_GF, FDQ_multi, clarity, and oxygenation (panels A–D) in the first period (blue squares) and the second period (red points). Environmental land-use gradient is represented by PCA1 axis score. Each point for panels A–D represents a single lake; r = Pearson correlations, ordinary least squares model (OLS)
Discussion
In support of our main hypothesis, we found a significant increase in species richness and moderate functional diversity shifts over time (Fig. 4). FD Rao for plant life span, growth forms, and FD multi-traits was generally higher in the first period (1955–1959) than the second period (2015–2020). The moderate increase in the functional diversity over time may indicate functional differentiation of macrophyte composition. Similar results were reported by Zhang et al. (2018) for temporal (1970s compared to after 2000s) changes in macrophyte assemblages across the floodplain lakes of the Yangtze River in China and by Lindholm et al. (2020) in species composition located in the boreal zone. They found functional differentiation of macrophyte assemblages instead of homogenization. Functional homogenization is part of biotic homogenization and means a loss of specialized species or functional groups (Olden et al. 2004). On the other hand, studies on species composition along the acidity gradient in softwater lakes in northwestern Poland showed relative functional differentiation and no signs of functional homogenization (Chmara et al. 2015). Similarly, our work, although it shows the loss of specialized groups of plant species (some isoetids and mosses), does not yet substantiate the concept of functional homogenization of softwater lake ecosystems. This can be explained by the replacement of highly specialized plant species during this period by numerous other, non-specialized species.
Even in lakes where there is a significant share of natural forms of land use (forests, peat bogs, heaths, and grasslands; Table 1) but in the catchment there are also fields and urban fabric, structural and functional changes in vegetation can be observed (Fig. 3). The main reason for the reduction in surface runoff from fields and urban fabric is the efficient filtration zone. The concentration of calcium ions in the water of such lakes is still low, and the water is acidic and relatively transparent (Table 2), which is important primarily for isoetids and mosses. However, the vegetation of such lakes gradually converges with that occurring in mesotrophic lakes with non-isoetids, as shown by Alahuhta et al. (2012) and Kanninen et al. (2013), mainly due to the increase in the concentration of nutrients and calcium and the decrease in light intensity in the water (Akasaka et al. 2010; Alahuhta et al. 2013; Banaś 2016). According to Akasaka and Takamura (2011) and Chmara et al. (2013, 2015, 2019), such changes may be responsible for a new distribution pattern of the functional traits of macrophytes in lakes that differs from the pattern in softwater lakes with isoetids.
There is no doubt that agricultural use of land around lakes has a negative impact on the macrophyte population, which is confirmed by numerous studies. Many of them concern the effects of fertilizers, heavy metals, and other toxins on freshwater bodies and water quality (e.g., Mesters 1995; Akasaka et al. 2010; Haris et al. 2021). Furthermore, water bodies are under much wider influence of agricultural land use (Johnson and Gage 1997; Alahuhta 2015), i.e., plant species distribution and their structural and functional diversity. As shown by Murphy et al. (2019), agricultural land use may even be the second most important factor, after geographical latitude, determining the global distribution of macrophytes.
The reason for the extinction or reduction of isoetids and mosses and the expansion of non-isoetid vascular plants that use HCO3− as a source of inorganic carbon for photosynthesis is the additional transmission of bicarbonate from fields to lakes. For our study we chose small and shallow lakes so that the impact of farmland on macrophytes would be more visible after 60 years. It is worth noting that one of the essential disturbances of environmental conditions for macrophytes involves liming soils in the fields. Since the soil around our lakes is acidic, farmers supply calcium to the fields. As a result, additional bicarbonates from the catchment flow into the lakes throughout this time. One of the effects includes the increase in the alkalinity of lakes and decrease in free CO2 concentration in water. According to Wium-Andersen (1971), Søndergaard and Sand-Jensen (1979), and Maberly et al. (2015), such a situation may lead to disturbances in isoetid photosynthesis and, as shown by Riis and Sand-Jensen (1997),in moss photosynthesis.
We found the largest replacement of species in those lakes which have been situated among the fields for the last 60 years (lakes no. 8, 9 and 10, Table 1). In these lakes, the isoetids and mosses have been replaced by functional growth forms such as myriophyllids (Myriophyllum alterniflorum, M. spicatum), potamids (e.g., Potamogeton crispus), and others (Online Resources 3) such as aquatic plants using bicarbonate as a source of inorganic carbon for photosynthesis, as reported by Iversen et al. (2019). Therefore, our studies on the functional diversity of macrophytes in softwater lakes are compatible with the impact of buffer zone properties on photosynthetic trait composition of freshwater species noted by Iversen et al. (2019), and our results agree with the global aquatic macrophyte distribution model presented by Murphy et al. (2019).
The quantitative and qualitative relationships between the functional traits of macrophytes in lakes have also changed under the influence of urban fabric. In the study area, this is a relatively new type of land use around the lakes which has become increasingly common since the 1980s, especially near the cities. There is no doubt that this type of land use around lakes causes changes such as the increase in the trophic levels and water alkalinity, decrease in water clarity, and damage to the aquatic vegetation (lakes no. 2, 3, and 7; Tables 1 and 2). These are common effects of urban fabric on macrophytes. The turnover of plant species and changes in functional traits in such lakes are similar to those we found in lakes with a relatively large share of fields.
There is no doubt that urban fabric is a very complex and multidimensional environmental factor. This is evidenced by numerous published works over 150 years (Lindholm et al. 2020; Boston 1986; Arbuckle and Downing 2001; Elo et al. 2018, Vöge 1992). Intuitively, the influence of urban fabric on the functional diversity of macrophytes should differ from that of fields. On the contrary, our research shows that fields and urban fabric have similar effects on the vegetation of softwater lakes with isoetids.
The temporal land-use changes noted were related to environmental conditions (Table 2). We observed decreased clarity and increased water oxygenation over time. These environmental drivers are significantly correlated with land-use changes, expressed as the PCA1 axis (Fig. 5C, D). The decrease in clarity in all lakes degrades the light conditions in water, and consequently causes the replacement of macrophyte functional groups. In these lakes, the submerged macrophytes were replaced by floating-leaved species, expressed as the FS index (Table 5). A similar finding was reported in studies of temporal changes in aquatic plants in Japan (Kim and Nishihiro 2020).
Conclusions
Our results indicate that some species that were typical of the lakes in the first period have become extinct, while new ones appeared, above all eutrophic plant species. This is related to the change in environmental conditions that occurred between the two study periods. First, we observed significant changes in the transparency (visibility) of the water. In the second period, transparency decreased in each lake, which resulted in reduced PAR light transmission to plants and may have had a negative impact on species composition. Furthermore, we found a significant increase in species richness and moderate functional diversity shifts over time. Our work, although it shows the loss of specialized groups of plant species (some isoetids and mosses), does not yet substantiate the concept of functional homogenization of softwater lake ecosystems after 60 years of changes in land use. This can be explained by the replacement of highly specialized plant species during this period by numerous other, non-specialized species. We found a shift from an isoetid-dominated community to an emergent and free-floating plant community. These findings show that an increase in biodiversity can relate to a decrease in freshwater ecosystem function, mainly via lost function of evergreen isoetid species.
Data availability
Most data generated or analyzed during this study are included in this article. The rest of the included data are available from the authors on reasonable request.
References
Akasaka M, Takamura N (2011) The relative importance of dispersal and the local environment for species richness in two aquatic plant growth forms. Oikos 120:38–46. https://doi.org/10.1111/j.1600-0706.2010.18497.x
Akasaka M, Takamura N, Mitsuhashi H, Kadono Y (2010) Effects of land use on aquatic macrophyte diversity and water quality of ponds. Fresh Biol 55:909–922. https://doi.org/10.1111/j.1365-2427.2009.02334.x
Alahuhta J (2015) Geographic patterns of lake macrophyte communities and species richness at regional scale. J Veg Sci 26(3):564–575. https://doi.org/10.1111/jvs.12261
Alahuhta J, Kanninen A, Vuori KM (2012) Response of macrophyte communities and status metrics to natural gradients and land use in boreal lakes. Aquat Bot 103:106–114. https://doi.org/10.1016/j.aquabot.2012.07.003
Alahuhta J, Kanninen A, Hellsten S, Vuori KM, Kuoppala M, Hämäläinen H (2013) Environmental and spatial correlates of community composition, richness and status of boreal lake macrophytes. Ecol Indic 32(1):172–181. https://doi.org/10.1016/j.ecolind.2013.03.031
Arbuckle KE, Downing JA (2001) The influence of watershed land use on lake N: P in a predominantly agricultural landscape. Limnol Oceanogr 46:970–975. https://doi.org/10.4319/lo.2001.46.4.0970
Baastrup-Spohr L, Iversen LL, Borum J, Sand-Jensen K (2015) Niche specialization and functional traits regulate the rarity of charophytes in the Nordic countries. Aquat Conserv 25(5):609–621. https://doi.org/10.1002/aqc.2544
Banaś K (2016) The principal regulators of vegetation structure in lakes of north-west Poland. A new approach to the assembly of macrophyte communities. Wydawnictwo Uniwersytetu Gdańskiego, Gdańsk
Boston HL (1986) A discussion of the adaptations for carbon acquisition in relation to the growth strategy of aquatic isoetids. Aquat Bot 26:259–270. https://doi.org/10.1016/0304-3770(86)90026-4
Chmara R, Szmeja J, Ulrich W (2013) Patterns of abundance and co-occurrence in submerged plants communities. Ecol Res 28(3):387–395. https://doi.org/10.1007/s11284-013-1028-y
Chmara R, Banaś K, Szmeja J (2015) Changes in the structural and functional diversity of macrophyte communities along an acidity gradient in softwater lakes. Flora 216:57–64. https://doi.org/10.1016/j.flora.2015.09.002
Chmara R, Szmeja J, Banaś K (2018) The relationships between structural and functional diversity within and among macrophyte communities in lakes. J Limnol 77:100–108. https://doi.org/10.4081/jlimnol.2017.1630
Chmara R, Szmeja J, Robionek A (2019) Leaf traits of macrophytes in lakes: Interspecific, plant group and community patterns. Limnologica. https://doi.org/10.1016/j.limno.2019.125691
Corine Land Cover (2018) https://clc.gios.gov.pl/index.php/clc-2018/o-projekcie. Accessed 10 Feb 2023
Dąmbska I (1965) The litoral vegetation of the Lobelia lakes in the Kartuzy Lake District. Prace Kom Biol PTPN 30(3):1–53 (In Polish)
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association American Water Works Association and Water Environment Federation, Washington
Ecke F (2009) Drainage ditching at the catchment scale affects water quality and macrophyte occurrence in Swedish lakes. Fresh Biol 54:119–126. https://doi.org/10.1111/j.1365-2427.2008.02097.x
Elo M, Alahuhta J, Kanninen A, Meissner KK, Seppälä K, Mönkkönen M (2018) Environmental characteristics and anthropogenic impact jointly modify aquatic macrophyte species diversity. Front Plant Sci 9:1001. https://doi.org/10.3389/fpls.2018.01001
Haris M, Shakeel A, Hussain T, Gufran A, Khan AA (2021) New trends in removing heavy metals from industrial wastewater through microbes. In: Shah MP (ed) Removal of emerging contaminants through microbial processes. Springer, pp 183–205
Hermanowicz W, Dojlido J, Dożańska W (1999) Fizyczno-chemiczne badanie wody i ścieków. Wydawnictwo Arkady, Warszawa (In Polish)
Iversen LL, Winkel A, Baastrup-Spohr L, Hinke AB, Alahuhta J, Baattrup-Pedersen A, Birk S, Broderson P, Chambers PA, Ecke F, Feldmann T, Gebler D, Heino J, Jespersen TS, Moe SJ, Riis T, Sass L, Vestergaard O, Maberly SC, Sand-Jensen K, Pedersen O (2019) Catchment properties and the photosynthetic trait composition of freshwater plant communities. Science 366(6467):878–881. https://doi.org/10.1126/science.aay5945
Johnson LB, Gage SH (1997) A landscape approach to analysing aquatic ecosystems. Fresh Biol 37:113–132. https://doi.org/10.1046/j.1365-2427.1997.00156.x
Kanninen A, Hellsten S, Hämäläinen H (2013) Comparing stressor-specific indices and general measures of taxonomic composition for assessing the status of boreal lacustrine macrophyte communities. Ecol Indic 27:29–43. https://doi.org/10.1016/j.ecolind.2012.11.012
Kim JY, Nishihiro J (2020) Responses of lake macrophyte species and functional traits to climate and land use changes. Sci Total Environ 736:139628. https://doi.org/10.1016/j.scitotenv.2020.139628
Lepš J, de Bello F, Lavorel S, Berman S (2006) Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Presilia 78:481–501
Lindholm M, Alahuhta J, Heino J, Hjort J, Toivonen H (2020) Changes in the functional features of macrophyte communities and driving factors across a 70-year period. Hydrobiologia 847:3811–3827. https://doi.org/10.1007/s10750-019-04165-1
Maberly SC, Gontero B (2018) Trade-offs and synergies in the structural and functional characteristics of leaves photosynthesizing in aquatic environments. In: Adams WW, Terashima I (eds) The leaf: a platform for performing photosynthesis. Springer, Cham, pp 307–343
Maberly SC, Berthelot SA, Stott AW, Gontero BJ (2015) Adaptation by macrophytes to inorganic carbon down a river with naturally variable concentrations of CO2. J Plant Physiol 172:120–127. https://doi.org/10.1016/j.jplph.2014.07.025
Mesters CML (1995) Shifts in macrophyte species composition as a result of eutrophication and pollution in Dutch transboundary streams over the past decades. J Aquat Ecosys Health 4:295–305. https://doi.org/10.1007/BF00118010
Moss B, Johnes P, Phillips G (1996) The monitoring of ecological quality and the classification of standing waters in temperate regions, a review and proposal based on a worked scheme for British waters. Biol Rev 71:301–339. https://doi.org/10.1111/j.1469-185X.1996.tb00750.x
Murphy KJ (2002) Plant communities and plant diversity in softwater lakes of northern Europe. Aquat Bot 73:287–324. https://doi.org/10.1016/S0304-3770(02)00028-1
Murphy K, Carvalho P, Efremov A, Grimaldo JP, Molina-Navarro E, Davidson TA, Thomaz SM (2019) Latitudinal variation in global range-size of aquatic macrophyte species shows evidence for a Rapoport effect. Fresh Biol 65(9):1622–1640. https://doi.org/10.1111/fwb.13528
OECD (1982) Eutrophication of waters, monitoring, assessment and control. Organisation for Economic Co-operation and Development, Paris
Olden JD, LeRoy PN, Douglas MR, Douglas ME, Fausch KD (2004) Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol 19(1):18–24. https://doi.org/10.1016/j.tree.2003.09.010
Pedersen O, Colmer TD, Sand-Jensen K (2013) Underwater photosynthesis of submerged plants—recent advances and methods. Front Plant Sci 4:140. https://doi.org/10.3389/fpls.2013.00140
Pulido C, Sand-Jensen K, Lucassen ECHET, Roelofs JGM, Brodersen KP, Pedersen O (2011) Improved prediction of vegetation composition in NW European softwater lakes by combining location, water and sediment chemistry. Aquat Sci 74:351–360. https://doi.org/10.1007/s00027-011-0226-3
Ricotta C (2005) A note on functional diversity measures. Basic Appl Ecol 6:479–486. https://doi.org/10.1016/j.baae.2005.02.008
Riis T, Sand-Jensen K (1997) Growth reconstruction and photosynthesis of aquatic mosses: influence of light, temperature and carbon dioxide at depth. J Ecol 85:359–372. https://doi.org/10.2307/2960508
Ronowski R, Banaś K, Szmeja J, Merdalski M (2020) Plant replacement trend in soft-water lakes with isoetids. Oceanol Hydrobiol Stud 49:157–167. https://doi.org/10.1515/ohs-2020-0015
Simpson E (1949) Measurement of diversity. Nature 163:688. https://doi.org/10.1038/163688a0
Solon J, Borzyszkowski J, Bidłasik M, Richling A, Badora K, Balon J, Brzezińska-Wójcik T, Chabudziński Ł, Dobrowolski R, Grzegorczyk I, Jodłowski M, Kistowski M, Kot R, Krąż P, Lechnio J, Macias A, Majchrowska A, Malinowska E, Migoń P, Myga-Piątek U, Nita J, Papińska E, Rodzik J, Strzyż M, Terpiłowski S, Ziaja W (2018) Physico-geographical mesoregions of Poland: verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr Pol 91(2):143–170. https://doi.org/10.7163/GPol.0115
Søndergaard M, Sand-Jensen K (1979) Carbon uptake by leaves and roots of Littorella uniflora (L.) Aschers. Aquat Bot 6:1–12. https://doi.org/10.1016/0304-3770(79)90047-0
Szmal Z, Szmal B (1965) Hydrochemical investigation of 02 lakes of the Gdansk and Koszalin provinces. PraceKomis Biol PTPN 30(1):1–55 (In Polish)
Szmeja J (1994a) An individual’s status in populations of isoetids species. Aquat Bot 48:203–224. https://doi.org/10.1016/0304-3770(94)90016-7
Szmeja J (1994b) Effect of disturbances and interspecific competition in isoetid populations. Aquat Bot 48:225–238. https://doi.org/10.1016/0304-3770(94)90017-5
Szmeja J (1994c) Dynamics of the abundance and spatial organisation of isoetid populations in an oligotrophic lake. Aquat Bot 49:19–32. https://doi.org/10.1016/0304-3770(94)90003-5
Szmeja J (2006) A guide to the study of aquatic plants. Wydawnictwo Uniwersytetu Gdańskiego, Gdańsk
Szmeja J, Banaś K, Bociąg K (1997) Ecological conditions and tolerance limits of isoetids along the southern Baltic coast. Ekol Pol 45:343–359
Ter Braak CJF (2008) CANOCO release 4.5. Microcomputer Power, Ithaca
Vöge M (1992) Tauchuntersuchungen an der submersen Vegetation in 13 Seen Deutschlands unterbesondener Berücksichtigung der Isoetiden-Vegetation. Limnologica 22:82–96
Wetzel RG (2001) Limnology: lake and river ecosystems. Academic Press, San Diego/San Francisco/New York/Boston/London/Sydney/Tokyo
Wium-Andersen S (1971) Photosynthetic uptake of free CO2 by the roots of Lobelia dortmanna. Physiol Plant 73:245–248. https://doi.org/10.1111/j.13993054.1971.tb01436.x
Zhang M, García Molinos J, Zhang X, Xu J (2018) Functional and taxonomic differentiation of macrophyte assemblages across the Yangtze River floodplain under human impacts. Front Plant Sci 9:1–15. https://doi.org/10.3389/fpls.2018.00387
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RR and RC. The first draft of the manuscript was written by RR, RC and professor JS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This declaration is not applicable to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ronowski, R., Chmara, R. & Szmeja, J. Structural and functional changes in macrophyte species composition in softwater lakes after 60 years of land use. Aquat Sci 85, 104 (2023). https://doi.org/10.1007/s00027-023-00999-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-00999-z