Abstract
Anthropogenic eutrophication is one of the consequences of human activities observed from the beginning of urbanization processes and is now a widespread problem around the globe. Mitigation of eutrophication directly corresponds to the achievement of at least three main Sustainable Development Goals. Due to the complexity of eutrophication mechanisms, there are still many issues with managing this process and its consequences, that complicate the achievement of the above-mentioned objectives. So far, there is no universal definition of eutrophication and there is no unified methodological approach to surface water trophic status assessment. In connection with the above, a need arises for the development of scientifically and economically justified methods of reliable trophic state assessment, useful for solving application and engineering tasks. This paper presents a new approach to trophic state assessment based on the innovative universal trophic index (UTI), which can be used for fresh, brackish and saltwater bodies. The proposed UTI index was developed and verified using an extensive database of the Baltic Sea monitoring data for over 100 years. The elaborated method is a reliable tool for the assessment of water trophic status, which also allows for the development of predictive mathematical models as the basis for effective eutrophication management. In addition, the method is simple to use and enables the trophic state assessment at low cost, time and effort. The above-mentioned features make the proposed indicator meet the requirements set for modern ecological indicators and determine the advantage of the proposed methodological approach over other currently used methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intensive exploitation of water resources leads to their contamination and disturbs the natural functions of water ecosystems. The most obvious manifestation of ecological imbalance in surface water bodies is anthropogenic eutrophication, which in the second half of the twentieth century became a global problem, causing the disturbance of all types of water usage: water supply and wastewater disposal, recreation, fishery, hydrotechnical engineering and water transport (Henderson-Sellers 1984; Schiewer 1998; Ansari et al. 2011; Chislock et al. 2013; Ménesguen et al. 2018; Andersen et al. 2020; Korpinen et al. 2021; Preisner et al. 2021).
During water blooms and aquatic biomass decay, the water quality deteriorates with regard to many aesthetic, sanitary and hygienic parameters: smell, taste, colour, turbidity, biochemical oxygen demand (BOD), chemical oxygen demand (COD), etc., with a simultaneous increase of its corrosive and toxic properties and the appearance of favourable conditions for pathogenic microorganisms (Beiras 2018).
Anthropogenic eutrophication, as one of the consequences of human activities, is characterised by complicated mechanisms and influence factors totality which are not fully understood yet (Carlson 1992; Yang et al. 2008; Nixon 2009; Glibert 2017). Within the current literature, there are no universal opinion on the essence of this phenomenon, the only definition of eutrophication, there are no universal indicators of trophic level, nor a unified assessment methodology for surface water trophic state (Carlson and Havens 2005; Cunha et al. 2013; Callisto et al. 2014; Yalçın et al. 2017). The previous contradictions remain and new ones still arise with regard to opinions on the development of eutrophication process, its stages, limiting factors, stability and possible reversibility (Granéli et al. 1990; Kobayashi et al. 2008). All of this hinders the development of scientifically and economically justified methods of prevention and protection of water bodies against anthropogenic eutrophication and its consequences as well as the establishment of scientific grounds for eutrophication control and management.
One of the fundamental, but so far unsolved problems in this respect are: (1) the lack of universal methodology of surface water trophic state assessment and (2) the lack of simple and low-cost numerical indicators, that would allow quick and reliable assessment of trophic state and be suitable for application purposes, especially for forecasting and solving of engineering tasks.
There are approximately 50 criteria used for the assessment of water trophic state (Lee et al. 1978; Henderson-Sellers 1984; Neverova-Dziopak 2007). In the practice of traditional ecological monitoring the following main indicators are used: phytoplankton species structure, phytoplankton abundance and biomass, concentration of chlorophyll “a”, the rate of photosynthesis, water transparency, concentration of biogenic substances, etc. There are also several numerical indexes based on statistical relationships between the factors and indicators of eutrophication. The most widely used numerical indicators are the Index of Trophic State (TSI) developed by R. Carlson (Carlson 1977), the Trophic Index (TRIX) developed by R. Vollenweider (Vollenweider et al. 1998), Eutrophication Index (EI), by I. Primpas (Primpas et al. 2010) and a number of others as shown in Table 1.
The listed above trophic state indexes were successfully applied in many different assessments e.g. for dam reservoirs (Saghi et al. 2015; Neverova-Dziopak and Droździk 2017), estuaries and coastal waters (Seisdedo et al. 2013; O’Boyle et al. 2013), natural lakes (Galvez-Cloutier and Sanchez 2007; Zhang et al. 2018), urban lakes (Chen et al. 2020) and streams (Dodds et al. 1998).
The trophic state assessment systems have been also developed, including the Assessment of Estuarine Trophic Status (ASSETS) which uses the annual chlorophyll-a values combined with the frequency of algal blooms occurrence to determine chlorophyll-a conditions in a specific salinity zone (Borja et al. 2011). A major contribution to the development of water state assessment with eutrophication consideration has been made by the Helsinki Commission (HELCOM), which have introduced common concepts and methods for the Baltic Sea status assessment, and performs assessments at regional scale (HELCOM 2017). One of them has been provided within the HELCOM Initial Holistic Assessment (HOLAS) during 2003–2007 (HELCOM 2010) and HELCOM Second Holistic Assessment of the Ecosystem Health of the Baltic Sea (HOLAS II) during 2014–2018. HOLAS II was based on core indicators for the assessment of environmental status which have been tested during the project. The indicators included the aspects of: (I) the distribution of human activity in the Baltic Sea, (II) key pressures and biodiversity, (III) integrated status assessments for biodiversity, (IV) eutrophication and hazardous substances and (V) economic and social analyses for assessment of the marine environment degradation costs (HELCOM 2017).
Besides the holistic approach for the water state assessment, HELCOM have introduced a tool dedicated for the eutrophication status assessment—HELCOM Eutrophication Assessment Tool (HEAT). The principle of trophic status assessment in the HEAT method is shown in Fig. 1.
The concept of eutrophication assessment according to HEAT method (Andersen et al. 2011)
HEAT has been used in the assessment of the Baltic Sea state by using the Water Framework Directive (WFD) methodology for water status assessment (HEAT 1.0) and the Marine Strategy Framework Directive (MSFD) assessment methodology (HEAT 3.0) (Wesslander 2017). The Baltic Sea integrated eutrophication status assessment for 2011–2016 was done using HEAT based on WFD indicators, such as the level of phytoplankton production (biomass or chlorophyll-a concentration), benthic invertebrate fauna, macrophytes (macroalgae and angiosperms), concentrations of nitrogen and phosphorus and water transparency (HELCOM 2016). In the long term analysis for open seas (without the coastal assessment) and the thematic assessment of the open sea areas of the Baltic Sea for the period 2007–2011 HEAT based on MSFD methodology was used (HELCOM 2013).
The main difference between the methodology applied in the WFD and the MSFD is the method of eutrophication indicators grouping. In the WFD, there are two groups (biological and physico-chemical elements). Biological elements include phytoplankton, macro vegetation and invertebrate fauna, while physico-chemical elements include nutrients, water transparency and dissolved oxygen (DO) content. The MSFD methodology sets indicators for three groups: nutrients, direct effects (phytoplankton, water transparency and abundance of opportunistic macro vegetation) and indirect effects (abundance of perennial seaweed and sea grass and DO). Furthermore, the number of water status classes is different in the WFD and the MSFD methods. The WFD methodology assumes five classes of ecological status (high, good, moderate, poor and bad), while the MSFD sets two classes for environmental status: good environmental status (GES) or bellow good environmental status (subGES) (Wesslander 2017). The basic shortcomings of the existing methods of trophic state assessment have been identified as follows:
-
a)
Labour-intensive water sampling in field conditions and high costs of transport, maintenance and treatment of the samples under laboratory conditions;
-
b)
Traditional indicators commonly used in practice (such as chlorophyll-a, nitrogen and phosphorus content, transparency etc.) allow to characterise the rate of primary production, but it does not provide information on the state of biotic balance (production/decomposition) which determines the trophic state;
-
c)
The boundary values of indicators elaborated by different authors for the same trophic levels are characterized by very large variety due to the natural background variability: for instance, the content of chlorophyll-a for eutrophic conditions could vary between 6 µg/L and 100 µg/L (Lundberg et al. 2005; Neverova-Dziopak 2007; Kowalewski 2012).
-
d)
Reliable assessment of water trophic state based on the entirety of many traditional indicators may be carried out only by highly qualified specialists.
Most indicators and their boundary values for various trophic conditions were defined mainly for fresh lentic waters in different geographical areas (Cunha et al. 2013). There are only few indices specially developed for running waters (rivers and streams) or marine waters (Justić 1991; Schiewer 1998). The boundary values of indicators for each trophic level are extremely difficult to determine, because it depends on the type of water, hydrological, morphological and hydrobiological properties as well as geographical and climatic conditions. Moreover, the establishment of boundary values is not free from subjective preferences of the authors. All this complicates the clear and reliable assessment on the base of the existing methods.
When developing a new trophic state indicator, the authors followed the requirements and recommendations for environmental indicators developed by the Commission on Environmental Policy of Economic Commission for Europe (Economic Commission—Committee on Environmental Policy 2007), working group on Environmental Monitoring and Assessment (UNECE) (Working Group on Environmental Monitoring and Assessment 2008), Intergovernmental Oceanographic Committee (UNESCO) (Belfiore et al. 2003) and EC recommendations (European Commission 1999; Dale and Beyeler 2001).
After analysing the above-mentioned documents, the basic requirements which the eutrophication indicators should meet were formulated. Therefore, eutrophication indicators should have the following features:
-
To be theoretically and technically justified;
-
To reflect the essential function of aquatic ecosystems;
-
To reflect the changes in aquatic ecosystems caused by anthropogenic factors;
-
To integrate information in time and space;
-
To be based on a limited amount of data and characterized by moderate costs;
-
To have the developed reference values;
-
To be suitable for forecasting the changes in ecosystems and for solving application tasks;
-
To characterize phenomena and processes without unnecessary details and be developed in a form available to all users.
The scientific novelty of the presented methodology is revealed by the fact that the developed UTI trophic state index is based on scientifically justified theoretical assumptions regarding the fundamental complex mechanisms of the aquatic ecosystems functioning. UTI is taking into account the main mechanisms, processes and relationships of the studied phenomenon, which was described in the form of correlation relationships. The reliability of the developed index is guaranteed by a unique data bank collected from a 100-year of Baltic Sea monitoring period.
Theoretical knowledge allowed for the development of a universal approach to the assessment of the trophic state in the form of the UTI numerical index, which in fact reflects the integral response of the ecosystem to all eutrophication factors and can be easily and quickly adapted to various aquatic ecosystems. Compared to the other numerous methods and approaches to trophic status assessment, it is the easiest to use, interpret, and cheapest assessment method that allows for continuous tracking and prediction of changes in trophic status and retrospective evaluation of past changes. It is suitable for formulating mathematical models to develop protective measures and evaluate their effectiveness.
Materials and methods
Theoretical assumptions
It is a well-known fact, that the trophic state of surface water is conditioned by the state of biotic balance in water ecosystems (so called balance approach), i.e. the balance of primary organic matter production (photosynthesis rate) and its biochemical decomposition rate (Ohle 1956; Odum 1957; Vinberg 1963, 1965; De Ceballos et al. 1998; Liou and Lo 2005). When the eutrophication process intensifies, the rate of production processes increases, biotic balance shifts towards photosynthesis processes, and decaying algae biomass accumulates in water (Howarth 1988). This in turn deteriorates water quality and leads to the disturbances of all kinds of water use (Neverova-Dziopak and Kowalewski 2014). The knowledge of trophic status of water ecosystems allows resource managers to control the balance of processes of production and decomposition of organic matter produced by primary producers and to prevent negative consequences of ecological imbalance in water ecosystems.
With regard to their trophic state, surface water bodies can be divided into dystrophic, which are characterised by a negative biotic balance; oligotrophic—with sustainable biotic balance and eutrophic—with positive biotic balance (Table 2). There are also transitional states: ultraoligotrophic and mesotrophic.
Therefore, the process of anthropogenic eutrophication can be considered as the process of water ecosystem transformation from dystrophic or oligotrophic to eutrophic state under the influence of anthropogenic activity (Smith and Schindler 2009). Taking into account the above considerations, it should be stated, that there is a need for the development of surface water trophic state indices, which would integrally reflect the biotic balance in water bodies, and would be suitable for application purposes.
The proposal of such indicator is the index entitled the Index of Trophic State (ITS) which was developed in previous studies (Neverova-Dziopak 2010). The ITS is based on a theoretical assumption that the change in the intensity of autotrophic and heterotrophic processes leads to a change in the ratios of oxygen (O2) and carbon dioxide (CO2) concentrations, i.e. the final products of these processes. It means that biotic balance is connected with the gas regime in the water body and the concentrations of oxygen and CO2 allows the estimation of the balance of intensity of the process of primary matter production and its decomposition. When the rate of organic matter production (Vprod) exceeds the rate of its decomposition (Vdecomp), the concentration of CO2 content in the environment decreases and the concentration of oxygen increases (Vinberg 1970; Tsvetkova et al. 1995; Neverova-Dziopak 2010; Wallace et al. 2014). This means, that the ratio of CO2 concentration and O2 concentration in the water environment reflects the ratio of organic matter production and decomposition rates, i.e. the state of the biotic balance. This also follows the photosynthesis balance Eq. (1):
where: n—number of molecules participating in the reaction.
In the professional literature, there can be found the descriptions of the attempts to assess the trophic state on the basis of oxygen regime or CO2 assimilation, as well as pH values (Justić 1991; Tsvetkova et al. 1995; Giordani et al. 2009; Seisdedo et al. 2013; O’Boyle et al. 2013). Thus, for instance, O. Alekin had already calculated in 1952, that an increase in the value of pH by 0.1 corresponds to a reduction in the CO2 concentration by 0.8 mg/L (Alekin 1952).
In water ecosystems, the ratio of CO2 and O2 may be expressed by the ratio of pH value and oxygen saturation %O2 (Neverova-Dziopak 2010), which results from the following assumptions. According to the C.M. Guldberg and P. Waage law of mass-action, which specifies the state of chemical balance depending on the concentrations of substrates and reaction products, the above-mentioned statement can be written in the form of the following Eq. (2) (Alekin 1952; Dojlido 1995; Neverova-Dziopak 2010; Brezonik and Arnold 2011):
whence:
where: k1 and k2—chemical reaction rate constant, respectively, for production and decomposition; n-concentrations of reactants.
According to the mentioned chemical law, concentrations of substances in individual phases are constant and do not affect the rate of chemical reactions. Then:
In surface water bodies with low dissolved salt content the change in CO2 concentrations leads to changes of pH value, because the content of carbon dioxide and hydrogen in the aquatic environment are mutually related through carbonate equilibrium (Dojlido 1995; Lower 1999; Brezonik and Arnold 2011) i.e. the state of specific proportion between hydrocarbonate and bicarbonate ions:
When the biotic balance is distorted during eutrophication (under an increased rate of primary production) the concentration of CO2 decreases, and pH exceeds the values of pHs (pH of carbonate equilibrium) (Alekin 1952; Dojlido 1995; Tsvetkova et al. 1995). This means that the trophic state may be described by the following Eq. (7):
It has been empirically determined that the dependence of pH value on oxygen saturation is well approximated by linear correlation dependence (Neverova-Dziopak and Kowalewski 2014; Neverova-Dziopak 2021). At the same time, the free member of the regression equation consistently increases with the transformation of water ecosystems from dystrophic to eutrophic state. For freshwater bodies, such dependence is presented in Fig. 2.
Interdependence between pH and oxygen saturation in freshwater bodies of different trophic levels and the corresponding ITS index values (Neverova-Dziopak 2010)
From the above relationship, it can be assumed that under identical oxygen saturation pH value will be higher in water bodies with a higher trophic state. This statement allowed to propose the application of pH value at 100% oxygen saturation at equilibrium as an index of trophic state (ITS) for fresh surface water, which can be calculated according to Eq. (8) (Tsvetkova et al. 1995; Neverova-Dziopak 2010):
where: pH and DO-mean values of pH and dissolved oxygen saturation (%) measured simultaneously; 100-100% oxygen saturation; a-empirical coefficient (pH vs oxygen linear regression slope).
The following numeric values of the ITS index for waters of different trophic states have been determined empirically for surface waters with a low degree of mineralization and are shown in Fig. 2: for dystrophic water the ITS value is lower than 5.7 ± 0.3; for ultraoligotrophic water: 6.3 ± 0.3; for oligotrophic water: 7.0 ± 0.3, for mesotrophic water: 7.7 ± 0.3, for eutrophic water it is over 8.3 ± 0.3 (Tsvetkova et al. 1995). The practical possibility of its application and the accuracy of the assessment results has been proven by numerous authors while assessing the trophic status of various types of surface waters with low mineralization level in different countries (Martin and Pedersen 2004; Giordani et al. 2009; Kowalewski 2012; Neverova-Dziopak and Kowalewski 2018; Nasrollahzadeh Saravi et al. 2019; Tugrul et al. 2019; Lin et al. 2020).
As it has been already mentioned, the eutrophication of the water ecosystem leads to the increase of pH value and the level of DO saturation which, in turn depends on the degree of water salinity. Therefore, the authors undertook the task of elaborating a universal trophic criterion, that could be used to assess the trophic status of both fresh, salt and brackish waters on the base of the modified ITS index. The modified index was elaborated based on the established linear dependence between DO saturation and water salinity. The established regression model allowed to modify the basic Eq. (8) and obtain the modified formula for the Universal Trophic Index (UTI) calculation (9).
where: pH and DO-the averaged values of pH and dissolved oxygen saturation (%) measured simultaneously; 100-100% oxygen saturation; a-empirical coefficient (pH vs oxygen linear regression slope); Sal—water salinity; β-empirical coefficient (oxygen vs salinity linear regression slope).
On the basis of the existing regularity and the established character of relationships between pH value, oxygen saturation and the degree of salinity, the new index has been developed for the assessment of trophic state of fresh, salt and brackish waters. The UTI index is the basis of a new methodological approach to the assessment of the trophic status of all types of surface waters. A feature that distinguishes UTI from other assessment methods is that its application is based on the limited number of parameters to measure: there are only three hydrochemical parameters (pH, DO saturation and salinity level) required for the assessment of trophic state based on the UTI index. These parameters are the standard parameters of water quality analysed during any routine monitoring. Therefore, UTI can be easily and quickly adapted to any type of aquatic ecosystem after obtaining a mathematical formula describing the correlation between these three parameters based on the monitoring data of individual water bodies. The more frequent the annual measurements, the higher is the reliability of trophic state assessment as the biotic balance may change during the year.
Research methodology
To develop a universal methodology for the assessment of trophic status of waters with different levels of salinity, a correlation and regression analysis was performed to modify the base Eq. (9) and to adapt it to various salinity conditions by introducing the numerical amendment to salinity. The analysis was carried out based on long-term monitoring data of the Baltic Sea basin obtained from HELCOM and the International Council for the Exploration of the Sea (ICES) information resources dataset (HELCOM 2020; ICES 2020). The reliability of the results obtained by the proposed methodology was verified based on an analysis of their compliance with the results of the assessment provided by HELCOM.
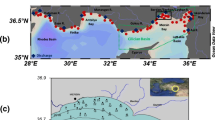
The measurements constituting the analysed dataset were carried out in the period of 1902–2018 at 10,469 monitoring stations within the Baltic Sea region (currently defined as HELCOM stations) in accordance with the ICES station dictionary (ICES 2020). Figure 3 presents a map of the monitoring stations location.
The location of HELCOM stations (ICES 2020)
The analysed raw dataset contained 23 variables and more than 30 million of individual observations set in more than 2 million rows. The key monitoring parameters covered by a statistical analysis were the following: sea depth [m], pH, water oxygen saturation [%] and salinity [psu]. Due to the lack of systematic measurements of the necessary parameters in the years 1902–1977, data from this period were omitted in the research. Only the monitoring data from the period 1978–2018 were taken into account, while the regular international monitoring of the Baltic Sea was started at the end of the 1970s, after signing and the ratification of the Helsinki Convention.
After cleaning and tidying data for statistical analysis purposes the dataset has been reduced to 223 577 rows (Fig. 4). All analysis were performed using R software version 4.0.2 with RStudio version 1.3.959 (Wickham et al. 2019; R Core Team 2020; RStudio Team 2020). Statistical analysis were performed with significance level α = 0.05. The strength of the relationship between the variables was measured by the value of the Pearson correlation coefficient (Pearson 1895). Table 3 presents the basic statistics of the analysed parameters.
The course of the statistical analysis carried out in order to develop the equation for the calculation of the UTI index and verification of its application was the following:
-
1)
Correlation analysis to determine the nature of pH dependence on water saturation with oxygen to confirm the possibility of the ITS index application as the basis for formulating the UTI index,
-
2)
Correlation analysis to determine the dependence of water oxygen saturation on salinity to determine the salinity amendment,
-
3)
Formulating the equation for calculating the UTI value,
-
4)
Verification of the accuracy of the trophic status assessment based on the UTI by the comparison with HELCOM assessment results.
Results and discussion
Elaboration of regression equation for UTI calculation
According to the adopted theoretical assumptions, the first stage of developing the statistical model for the assessment of trophic status based on the UTI was a correlation analysis to determine the nature of the dependence between pH and DO (Figure 5).
This relationship was described by the regression Eq. (10).
The value of the Pearson correlation coefficient describing the relationship between pH and DO is 0.76. The existence of a strong correlation between pH and DO confirmed the possibility of further application of the base Eq. (8) for UTI elaboration. Therefore, the next step was the correlation analysis to determine the relationship between DO and water salinity, which was described by the regression equation (Eq. 11). The value of the Pearson correlation coefficient describing the relationship between DO and water salinity is 0.60.
The performed statistical analysis allowed for the formulation of a regression model, which allows for the calculation of the UTI index value for Baltic Sea conditions (UTIBaltic) as shown in Eq. 12.
The application domain of the Eq. (12) is determined by the range of the parameter values presented in Table 4.
The application of UTI index for trophic state assessment of Baltic Sea
On the basis of the calculated UTI index value obtained from Eq. 12 and its reference values elaborated for different trophic levels and presented in Table 4, it is possible to assess the water trophic status of different water areas.
The results of the trophic state assessment of various areas of the Baltic Sea on the base of UTI index, calculated with Eq. 12 obtained for the 41 years period are presented in Fig. 6.
The trophic status assessment result obtained for the Baltic Sea basin on the basis of the UTI against the HELCOM assessment results (HEAT+) in the period 2006–2016 [based on HELCOM 2013)]
In general, it can be stated that during the analysed period the trophic conditions in the Baltic Sea fluctuated between mesotrophic and eutrophic, with the rare exception of the occurrence of oligotrophic conditions in individual monitoring points, mainly in the northern parts of the sea. Most often the eutrophic conditions occurred in the south-eastern waters of the Baltic Sea, while mesotrophic conditions prevailed in the northern waters of the sea. The most unfavourable were the years 1979, 1981, 1985, 1987 and the period of 2004–2009 when the dominant part of the sea area was characterized by eutrophic conditions. On the other hand, since 2010, a tendency to improve the trophic state can be observed, as the share of the Baltic Sea areas characterized by eutrophic conditions consequently decreases, changing to mesotrophic conditions.
Verification of the assessment results
Verification of the possibility of UTI index application as a Baltic Sea trophic status assessment tool and the accuracy of its results was carried out based on a comparative analysis of the assessment results obtained with UTI and the results obtained using HELCOM methodologies (HEAT, HOLAS I, HOLAS II) in different periods. The results of the comparative assessment are shown in Figs. 7, 8, 9. The results of the assessment based on the UTI index are marked in the form of dots of appropriate colours corresponding to different trophic states against the background of appropriate colour expressing the HELCOM assessment results.
The trophic status assessment result obtained on the basis of the UTI against the HELCOM assessment results (HOLAS I) in the period 2003–2007 [based on (HELCOM 2010)]
The trophic status assessment result obtained on the basis of the UTI against the HELCOM assessment results (HOLAS I) in the period 2007–2011 [based on (HELCOM 2010)]
The trophic status assessment result obtained on the basis of the UTI against the HELCOM assessment results (HOLAS II) in the period 2011–2016 [based on (HELCOM 2010)]
The graphic interpretation of the trophic status assessment results obtained according to the HELCOM methodology follows the principle of interpretation presented in Fig. 1, where only two descriptive categories were distinguished: unaffected by eutrophication (high–good status) and affected by eutrophication (moderate-poor-bad status).
The results of the assessment based on the UTI showed very high compatibility with the assessment results based on the HELCOM methodology (Table 5).
In the analysed period, the status of the Baltic Sea according to HELCOM assessments was below acceptable (Fig. 1) i.e. the sea area was affected by eutrophication:
-
In 2006–2016 (Fig. 6) moderate and poor status dominated, with the occurrence of bad, between how UTI indicated mesotrophic and eutrophic conditions;
-
In 2003–2007 (Fig. 7)-the predominant status was bad and poor, and UTI also indicated eutrophic and mesotrophic conditions;
-
In 2007–2011 (Fig. 8)-poor and moderate status definitely prevailed, and UTI accordingly indicated eutrophic and mesotrophic conditions;
-
In 2011–2016 (Fig. 9)-practically the entire area of the Baltic Sea was characterized by bad conditions, most assessments based on UTI also characterized it as eutrophic.
The trophic state of analysed sea basins has also been determined by other researchers as eutrophic or mesoeutrophic on the basis of a set of traditional indicators (chlorophyll-a, concentrations of biogenic substances and other indicators) (Lundberg et al. 2005; Raateoja et al. 2005; Pitkänen et al. 2007; SKYE 2016).
Application perspectives of UTI index
The assessment methodology used by HELCOM allows only a descriptive assessment of the water status (good, moderate, bad, etc.), which is largely determined by the advancement of eutrophication processes. Therefore, the descriptive water trophic status categories such as “unaffected” or “affected” are not very accurate and do not allow the assessment of the exact trophic level, which is characterized by a specific biotic balance. Moreover, the HELCOM multi-criteria methodology based on the integration of a great number of parameters is very complicated. It requires a wide scope of monitoring, a large number of determinations of water quality parameters and is characterized by complicated interpretation of the results. In addition, the costs of such assessment methods are very high. Therefore, the frequency of its implementation is limited to the frequency of at most once a year.
It should be noted that the analysis of UTI application to assess the trophic status over the period of 41 years showed, that HELCOM tools do not allow the assessment of the state of the sea at the turn of the 1970s and 1980s due to the lack of data on all the necessary parameters, while the UTI allows for a retrospective assessment since the required parameters began to be measured in the early twentieth century. In contrast, the UTI-based assessment methodology is simple to implement (only 3 parameters are needed, regularly measured within the obligatory monitoring). UTI is determined from a simple statistical model and can be adapted to each type of water. Moreover, the result of the assessment is a numerical value, that allows for an accurate assessment of the degree of advancement of the sea eutrophication process and its dynamics and also suitable for the formulation of mathematical prognostic models.
UTI index is a fast, low-cost and reliable tool for assessing the trophic status of marine waters and can serve as an express-monitoring method for the state of the marine environment. Currently, the automated measurements of water quality parameters pH, %DO and water salinity directly in natural conditions (even in non-stop regime) are widely used in the monitoring of aquatic ecosystems. It enables a constant control of the water trophic state at minimum effort, time and costs.
However, to obtain reliable results, the following monitoring limitations should be taken into account:
-
It is necessary to establish the existence of a relationship character (linear correlation) between pH, DO and the degree of salinity (in the case of fresh waters—pH and DO are enough) based on the monitoring data of the individual type of water body;
-
Changes in water oxygen saturation without correlated changes in pH cannot be the basis for the assessment of trophic status based on UTI (e.g. in situations where oxygen from the atmosphere is mechanically introduced into the water) because only the biochemical DO must be taken into account;
-
Water samples should be taken from the euphotic layer, which would require determining its depth;
-
Mixing the upper layers of water with the bottom layers (due to natural causes or e.g. construction works) may lead to the disturbance of the results;
-
It is not recommended to take water samples for the UTI assessment in the vicinity of acidic or alkaline wastewater discharge sites.
The experience with practical application of the proposed method of trophic state assessment based on the previously developed ITS index showed, that in comparison to the traditional existing methods, the time needed for assessment is approximately 50 times shorter and the cost of monitoring is 20–30 times less (Kowalewski 2012).
Conclusion
The proposed methodology is based on a new scientifically justified approach to eutrophication process essence and application of the innovative UTI index, which is suitable for the assessment of trophic state of fresh, salt and brackish water bodies. The universality of the elaborated index consists in the fact that: (1) it reflects the water ecosystems response to eutrophication factors; (2) it is based on basic functional characteristics of the water ecosystem—its biotic balance, which in turn determines the trophic status of surface water; (3) it can be easily adapted to specific conditions of individual water ecosystems; (4) it can be used for the formulation of prognostic mathematical models of eutrophication development; (5) it can be a tool for low-cost instant monitoring of water ecosystems and other application purposes.
The assessment requires measuring only three basic water quality parameters directly under field conditions during routine water monitoring. This also allows, as opposed to the assessment based on the measurements in laboratory conditions, to take into account the emergent properties of the water ecosystem. The UTI index reflects the diversity of mutually related processes of photosynthesis, chemosynthesis and destruction of primary production products in surface water and gives an objective, reliable and sufficiently precise assessment of its trophic state at low labour, time and costs inputs. All the above provides an advantage of the proposed method of assessment when compared with the existing traditional methods. On the background of traditional methods of trophic state assessment, the proposed new methodology may be considered to be an efficient, reliable and easily automated tool for regular trophic state assessment to control the process of anthropogenic eutrophication and prevent its negative ecological, financial and social consequences.
All the above-mentioned characteristics of the new eutrophication indicator UTI confirms that it meets the requirements of numerous international documented recommendations for ecological indicators, which are a tool for achieving the Sustainable Development Goals.
When applying the proposed method to obtain high accuracy results, it is recommended to perform basic statistical analysis to determine the coefficients values of the regression equation adapted to the analysed water body.
In the existing professional literature and patent solutions, there are no releases on the analogous methods of the trophic state assessment. Therefore, the method described in the present study is characterized by a significant scientific novelty and high application value.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Alekin OA (1952) Basics of hydrochemistry (in Russian). Hydrometeoizdat, Sankt Petersburg
Andersen JH, Axe P, Backer H et al (2011) Getting the measure of eutrophication in the Baltic Sea: towards improved assessment principles and methods. Biogeochemistry 106:137–156
Andersen JH, Al-Hamdani Z, Harvey ET et al (2020) Relative impacts of multiple human stressors in estuaries and coastal waters in the North Sea-Baltic Sea transition zone. Sci Total Environ 704:135316. https://doi.org/10.1016/j.scitotenv.2019.135316
Ansari AA, Gill SS, Lanza GR, Rast W (2011) Eutrophication: causes, consequences and control. Springer, Berlin
Baban SMJ (1996) Télédétection appliquée à la classification trophique et au contrôle des écosystèmes lacustres. Hydrol Sci J 41:939–957. https://doi.org/10.1080/02626669609491560
Beiras R (2018) Nonpersistent Inorganic Pollution. In: Pollution M (ed) Beiras RBT-MP. Elsevier, Amsterdam, pp 31–39
Belfiore S, Balgos M, Mclean B et al (2003) A reference guide on the use of indicators for integrated coastal management. UNESCO, Paris
Borja A, Basset A, Bricker S et al (2011) Classifying ecological quality and integrity of estuaries. In: Wolanski E, McLusky DS (eds) Treatise on estuarine and coastal science. Academic Press, Waltham, pp 125–162
Brezonik PL, Arnold WA (2011) Water chemistry an introduction to the chemistry of natural and engineered aquatic systems. Oxford University Press, New York
Callisto M, Molozzi J, Barbosa J (2014) Eutrophication of Lakes. Eutrophication causes, consequences Control, vol 2. Springer, Berlin, pp 1–262
Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22:361–369. https://doi.org/10.4319/lo.1977.22.2.0361
Carlson R (1992) Global freshwater quality: a first assessment. Trans Am Fish Soc 121:141. https://doi.org/10.1577/1548-8659-121.1.141
Carlson RE, Havens KE (2005) Simple graphical methods for the interpretation of relationships between trophic state variables. Lake Reserv Manag 21:107–118. https://doi.org/10.1080/07438140509354418
Chapra S, Dobson H (1981) Quantification of the lake trophic typologies of Naumann (surface quality) and Thienemann (oxygen) with special reference to the Great Lakes. J Gt Lakes Res 7(2):182–193
Chen Q, Huang M, Tang X (2020) Eutrophication assessment of seasonal urban lakes in China Yangtze River Basin using Landsat 8-derived Forel-Ule index: a six-year (2013–2018) observation. Sci Total Environ 745:135392. https://doi.org/10.1016/j.scitotenv.2019.135392
Chislock MF, Doster E, Zitomer RA, Wilson AE (2013) Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat Educ Knowl 4(4):10
Cunha DGF, do Calijuri MC, Lamparelli MC (2013) A trophic state index for tropical/subtropical reservoirs (TSItsr). Ecol Eng 60:126–134. https://doi.org/10.1016/j.ecoleng.2013.07.058
Dale VH, Beyeler SC (2001) Challenges in the development and use of ecological indicators. Ecol Indic 1:3–10. https://doi.org/10.1016/S1470-160X(01)00003-6
De Ceballos BSO, König A, De Oliveira JF (1998) Dam reservoir eutrophication: a simplified technique for a fast diagnosis of environmental degradation. Water Res 32:3477–3483. https://doi.org/10.1016/S0043-1354(98)00095-5
Dillon PJ, Rigler FH (1975) A simple method for predicting the capacity of a lake for development based on lake trophic status. J Fish Res Board Canada 32:1519–1531. https://doi.org/10.1139/f75-178
Dodds WK, Jones JR, Welch EB (1998) Suggested classification of stream trophic state: distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res 32:1455–1462. https://doi.org/10.1016/S0043-1354(97)00370-9
Dojlido JR (1995) Chemistry of Surface Waters (in Polish). Ekonomia i Środowisko, Białystok
Galvez-Cloutier R, Sanchez M (2007) Trophic status evaluation for 154 lakes in Quebec, Canada: monitoring and recommendations. Water Qual Res J Canada 42:252–268. https://doi.org/10.2166/wqrj.2007.028
Giordani G, Zaldívar JM, Viaroli P (2009) Simple tools for assessing water quality and trophic status in transitional water ecosystems. Ecol Indic 9:982–991. https://doi.org/10.1016/j.ecolind.2008.11.007
Glibert PM (2017) Eutrophication, harmful algae and biodiversity—challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull 124:591–606. https://doi.org/10.1016/j.marpolbul.2017.04.027
Granéli E, Wallström K, Larsson U et al (1990) Nutrient limitation of primary production in the Baltic Sea area. Ambio 19:142–151
Howarth R (1988) Nutrient limitation of net primary production in marine ecosystems. Annu Rev Ecol Evol Syst 19:89–110
Justić D (1991) A simple oxygen index for trophic state description. Mar Pollut Bull 22:201–204. https://doi.org/10.1016/0025-326X(91)90471-4
Kobayashi JT, Thomaz SM, Pelicice FM (2008) Phosphorus as a limiting factor for Eichhornia crassipes growth in the upper Paraná River floodplain. Wetlands 28:905–913. https://doi.org/10.1672/07-89.1
Korpinen S, Laamanen L, Bergström L et al (2021) Combined effects of human pressures on Europe’s marine ecosystems. Ambio. https://doi.org/10.1007/s13280-020-01482-x
Lee GF, Rast W, Jones RA (1978) Water Report: Eutrophication of water bodies: insights for an age old problem. Environ Sci Technol 12:900–908. https://doi.org/10.1021/es60144a606
Lin G, Xu X, Wang P et al (2020) Methodology for forecast and control of coastal harmful algal blooms by embedding a compound eutrophication index into the ecological risk index. Sci Total Environ 735:139404. https://doi.org/10.1016/j.scitotenv.2020.139404
Liou YT, Lo SL (2005) A fuzzy index model for trophic status evaluation of reservoir waters. Water Res 39:1415–1423. https://doi.org/10.1016/j.watres.2005.01.014
Lower SK (1999) Environmental chemistry. Simon Fraser University, Burnaby
Lundberg C, Lönnroth M, Von Numers M, Bonsdorff E (2005) A multivariate assessment of coastal eutrophication. Examples from the Gulf of Finland, northern Baltic Sea. Mar Pollut Bull 50:1185–1196. https://doi.org/10.1016/j.marpolbul.2005.04.029
Martin AJ, Pedersen TF (2004) Alteration to lake trophic status as a means to control arsenic mobility in a mine-impacted lake. Water Res 38:4415–4423. https://doi.org/10.1016/j.watres.2004.08.025
Ménesguen A, Desmit X, Dulière V et al (2018) How to avoid eutrophication in coastal seas? A new approach to derive river-specific combined nitrate and phosphate maximum concentrations. Sci Total Environ 628–629:400–414. https://doi.org/10.1016/j.scitotenv.2018.02.025
Nasrollahzadeh Saravi H, Pourang N, Foong SY, Makhlough A (2019) Eutrophication and trophic status using different indices: a study in the Iranian coastal waters of the Caspian Sea. Iran J Fish Sci 18:531–546. https://doi.org/10.22092/ijfs.2018.117717
Neverova-Dziopak E (2007) Environmental Aspects of Surface Water Protection (in Polish). Oficyna Wydawnicza Politechniki Rzeszowskiej, Rzeszów
Neverova-Dziopak E (2010) Fundamentals of anthropogenic eutrophication management (in Polish). Wydawnictwo AGH, Kraków
Neverova-Dziopak E (2021) Surface water eutrophication in poland: assessment and prevention BT - quality of water resources in poland. In: Kubiak-Wójcicka K, Negm AM (eds) Zeleňáková M. Springer International Publishing, Cham, pp 321–346
Neverova-Dziopak E, Droździk A (2017) Analysis of long-term changes of tresna dam reservoir trophic state in terms of its location and the role in reservoirs Cascade (in Polish). Inżynieria Ekol 18:135–148. https://doi.org/10.12912/23920629/67001
Neverova-Dziopak E, Kowalewski Z (2014) Towards methodological problems of trophic state assessment of running waters. Ecol Chem Eng S 21:637–650. https://doi.org/10.1515/eces-2014-0046
Neverova-Dziopak E, Kowalewski Z (2018) The development of the eutrophication process in Dobczyce reservoir. E3S Web Conf 45:1–8. https://doi.org/10.1051/e3sconf/20184500055
Nixon SW (2009) Eutrophication and the macroscope. Hydrobiologia 629:5–19. https://doi.org/10.1007/s10750-009-9759-z
Nürnberg GK (1996) Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake Reserv Manag 12:432–447. https://doi.org/10.1080/07438149609354283
O’Boyle S, McDermott G, Noklegaard T, Wilkes R (2013) A simple index of trophic status in estuaries and coastal bays based on measurements of pH and dissolved oxygen. Estuaries Coasts 36:158–173
Odum HT (1957) Trophic structure and productivity of silver Springs, Florida. Ecol Monogr 27:55–112. https://doi.org/10.2307/1948571
Ohle W (1956) Bioactivity, production, and energy utilization of lakes. Limnol Oceanogr 1:139–149
Pearson K (1895) Notes on regression and inheritance in the case of two parents. Proc R Soc London 58:240–242
Pitkänen H, Kiirikki M, Savchuk OP et al (2007) Searching efficient protection strategies for the eutrophied gulf of finland: the combined use of 1D and 3D modeling in assessing long-term state scenarios with high spatial resolution. Ambio 36:272–279. https://doi.org/10.1579/0044-7447
Preisner M, Neverova-Dziopak E, Kowalewski Z (2021) Mitigation of eutrophication caused by wastewater discharge: a simulation-based approach. Ambio 50:413–424. https://doi.org/10.1007/s13280-020-01346-4
Primpas I, Tsirtsis G, Karydis M, Kokkoris GD (2010) Principal component analysis: development of a multivariate index for assessing eutrophication according to the European water framework directive. Ecol Indic 10:178–183. https://doi.org/10.1016/j.ecolind.2009.04.007
Raateoja M, Seppälä J, Kuosa H, Myrberg K (2005) Recent changes in trophic state of the Baltic Sea along SW coast of Finland. Ambio 34:188–191
Saghi H, Karimi L, Javid AH (2015) Investigation on trophic state index by artificial neural networks (case study: Dez Dam of Iran). Appl Water Sci 5:127–136. https://doi.org/10.1007/s13201-014-0161-2
Schiewer U (1998) 30 years’ eutrophication in shallow brackish waters - lessons to be learned. Hydrobiologia 363:73–79. https://doi.org/10.1007/978-94-017-1493-8
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24:201–207. https://doi.org/10.1016/j.tree.2008.11.009
Tugrul S, Ozhan K, Akcay I (2019) Assessment of trophic status of the northeastern Mediterranean coastal waters: eutrophication classification tools revisited. Environ Sci Pollut Res 26:14742–14754. https://doi.org/10.1007/s11356-018-2529-6
Vinberg GG (1963) The Primary Production of Bodies of Water (in Russian). U.S. Atomic Energy Commission Division of Technical Information, Washington, DC
Vinberg GG (1965) Biotic balance of matter and energy and the biological productivity of water basins (in Russian). Hydrobiol Zhurnal 1:25–32
Vinberg GG (1970) Energy flow in aquatic ecological system. Pol Arch Hydrobiol 17:11–19
Vollenweider RA, Giovanardi F, Montanari G, Rinaldi A (1998) Characterization of the trophic conditions of marine coastal waters with special reference to the NW Adriatic Sea: Proposal for a trophic scale, turbidity and generalized water quality index. Environmetrics 9:329–357
Wallace RB, Baumann H, Grear JS et al (2014) Coastal ocean acidification: the other eutrophication problem. Estuar Coast Shelf Sci 148:1–13. https://doi.org/10.1016/j.ecss.2014.05.027
Welch EB, Lindell T (1992) Ecological effects of wastewater: applied limnology and pollutants effects. Spon, London
Wickham H, Averick M, Bryan J et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Xiao Y, Ferreira JG, Bricker SB et al (2007) Trophic assessment in Chinese coastal systems - review of methods and application to the Changjiang (Yangtze) Estuary and Jiaozhou Bay. Estuaries Coasts 30:901–918. https://doi.org/10.1007/BF02841384
Yalçın B, Artüz ML, Pavlidou A et al (2017) Nutrient dynamics and eutrophication in the Sea of Marmara: data from recent oceanographic research. Sci Total Environ 601–602:405–424. https://doi.org/10.1016/j.scitotenv.2017.05.179
Yang XE, Wu X, Hao HL, He ZL (2008) Mechanisms and assessment of water eutrophication. J Zhejiang Univ Sci B 9:197–209. https://doi.org/10.1631/jzus.B0710626
Zhang Y, Song C, Ji L et al (2018) Cause and effect of N/P ratio decline with eutrophication aggravation in shallow lakes. Sci Total Environ 627:1294–1302. https://doi.org/10.1016/j.scitotenv.2018.01.327
Burns N, Bryers G, Bowman E (2000) Protocol for monitoring lake trophic levels and assessing trends in trophic state, Lakes Consulting Client Report: 99/2 March 2000
California Regional Water Quality Control Board North Coast Region (2006) Resolution No. R1- 2006-0052
Economic Commission - Committee on Environmental Policy (2007) Making Monitoring and Assessment an Effective Tool in Environmental Policy (ECE/CEP/2007/7)
European Commission (1999) Towards Environmental Pressure indicators for the EU: Indicator Definition
HELCOM (2010) Baltic Sea Environment Proceedings No. 122. Ecosystem Health of the Baltic Sea HELCOM Initial Holistic Assessment
HELCOM (2013) HELCOM Thematic assessment Eutrophication status in the Baltic Sea Eutrophication status of the Baltic Sea 2007–2011 A concise thematic assessment
HELCOM (2016) HELCOM thematic assessment of eutrophication 2011–2016. Supplementary report to the ‘State of the Baltic Sea’ report. Eutrophication Supplementary report
HELCOM (2017) State of the Baltic Sea- Second HELCOM holistic assessment, 2011–2016
HELCOM (2020) HELCOM databases, from: https://helcom.fi/baltic-sea-trends/data-maps/databases/. Accessed 5 Feb 2021
Henderson-Sellers B (1984) Engineering limnology, Pitman Advanced Pub. Program, Boston
ICES (2020) Data Centre - Oceanography, from: https://www.ices.dk/data/data-portals/Pages/ocean.aspx Accessed Feb 5 2021
Istvánovics V (2009) Eutrophication of Lakes and Reservoirs. In: Likens GEBT-E of IW (ed). Academic Press, Oxford, pp 157–165
Kowalewski Z (2012) Verification of the possibility of integral criterion application to assess the trophic status of running waters (PhD thesis, in Polish). AGH University of Science and Technology
New Hampshire Departament of Environmental Services (1997) Layman’s Guide for Measuring a Lake’s Trophic State
Oram B (2012) Monitoring the Quality of Surfacewaters. http://www.waterresearch.net/watrqualindex/index.htm. Accessed Oct 2020
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA http://www.rstudio.com/.
Seisdedo M, Herrera R, Arencibia G (2013) Index for Assessing Water Trophic Status in Semi-Enclosed Cuban Bays. Case Study: Cienfuegos Bay. Mar Pollut Bull
SKYE (2016) The Gulf of Finland assessment. Helsinki
Tsvetkova LI, Neverova EW, Kopina GI, Al. E (1995) G.I. FR Patent No. 2050128
Waikato Reginal Council (2011) Water quality glossary, from: www.waikatoregion.govt.nz/Environment/Natural-resources/Water/Lakes/Water-quality-glossary
Wesslander K (2017) Coastal eutrophication status assessment using HEAT 1.0 (WFD methodology) versus HEAT 3.0 (MSFD methodology) and Development of an oxygen consumption indicator. Report Oceanography No. 51
Working Group on Environmental Monitoring and Assessment (2008) A Practical guide. IX session of the Working Group in Geneva
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts were identified.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neverova-Dziopak, E., Kowalewski, Z. & Preisner, M. The universal trophic index: new methodological approach to eutrophication monitoring and control. Aquat Sci 85, 6 (2023). https://doi.org/10.1007/s00027-022-00901-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00901-3