Abstract
During the past decades, major anthropogenic environmental changes occurred in Lake Victoria, including increased predation pressure due to Nile perch introduction, and decreases in water transparency and dissolved oxygen concentrations due to eutrophication. This resulted in a collapse of the haplochromine cichlids in the sub-littoral waters of the Mwanza Gulf in 1986–1990, followed by a recovery of some species in the 1990s and 2000s, when Nile perch densities declined. We studied two data sets: (1) haplochromines from sand and mud bottoms in the pre-collapse period; (2) haplochromines from sub-littoral areas during the pre-collapse, collapse and recovery periods. Water over mud is murkier and poorer in oxygen than water over sand, and differences in haplochromine communities in these natural habitats during the pre-collapse period may predict the effects of anthropogenic eutrophication during the collapse and recovery periods. In the pre-collapse period, haplochromine densities over sand and mud did not differ, but species richness over sand was 1.6 times higher than over mud bottoms. Orange- and white-blotched colour morphs were most common at the shallowest sand station. More specifically, insectivores and mollusc-shellers had higher numbers of species over sand than over mud, whereas for mollusc-crushers no difference was found. Laboratory experiments revealed that mollusc shelling was more affected by decreased light intensities than mollusc crushing. During the pre-collapse period, spawning occurred year-round in shallow areas with hard substrates and relatively clear water. In deeper areas with mud bottoms, spawning mainly occurred during months in which water clarity was high. No effects of hypoxia on spawning periods were found. It follows that clearer water seems to support differentiation in feeding techniques as well as year-round spawning, and both may facilitate species coexistence. Water clarity is also known to be important for mate choice. These observations may explain why, since the decline of Nile perch, haplochromine densities have recovered, the numbers of hybrids increased and species diversity in the current eutrophic sub-littoral waters has remained 70 % lower than before the environmental changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Lake Victoria, more than 500 haplochromine cichlid species, 99 % of which are endemic, made up some 80 % of the bottom dwelling fish mass until the beginning of the 1980s (Greenwood 1974; Kudhongania and Cordone 1974; Witte et al. 2007a). During the 1980s, the majority of the haplochromine species in the sub-littoral (6–20 m deep) and offshore areas disappeared (Ogutu-Ohwayo 1990; Witte et al. 1992). This disappearance coincided with the upsurge of the introduced predator, Nile perch, Lates niloticus (L.) (Goudswaard et al. 2008), and with strong eutrophication of the lake. The latter resulted in increased algal blooms and decreases in both water transparency and dissolved oxygen concentrations (Kaufman 1992; Hecky et al. 1994; Seehausen et al. 1997a; Wanink et al. 2001). The process of eutrophication had already started decades earlier, with the strong growth of human populations along the lake’s shores (Verschuren et al. 2002; Hecky et al. 2010). Disruption of the original food web and increased deforestation for Nile perch smoking, may have additionally contributed to eutrophication and algal blooms. Beginning in the first half of the 1990s, after the decline of Nile perch probably as a result of over-fishing (Matsuishi et al. 2006; Kayanda et al. 2009; Witte et al. 2009), a resurgence of some haplochromine species was observed (Seehausen et al. 1997b; Witte et al. 2007a, b). However, the initial diversity of haplochromines has not fully recovered.
During the past years, a lively debate has been ongoing regarding impacts of Nile perch predation, over-fishing and eutrophication on the diversity and abundance of haplochromine cichlid species in Lake Victoria. It appears that over-fishing may have had a negative impact on haplochromines locally, but this is unlikely to be a major driver of the lake-wide loss of cichlid diversity (Witte et al. 2007b). Discriminating between the effects of Nile perch predation and eutrophication is more difficult, as they occurred in the same period (Seehausen et al. 1997a; Verschuren et al. 2002; Witte et al. 2007a, b; Kolding et al. 2008; Hecky et al. 2010). Moreover, there are two major aspects of eutrophication that may affect haplochromine communities: decreases in water transparency and decreases in dissolved oxygen (DO) concentrations.
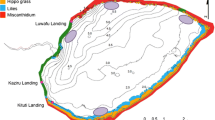
Before the environmental changes in the lake, we sampled relatively pristine sand and mud habitats that differed in light conditions and in DO concentrations close to the sediment. Mud bottoms are covered with a thick layer of organic debris that easily stirs up, making the water near the sediment hypoxic and murky. Thus, comparison of densities and species richness of haplochromine cichlids in pristine sand and mud stations offers a unique possibility to identify potential impacts of decreased water transparency and DO concentrations that result from lake-wide anthropogenic nutrient pollution. Therefore, we analyzed haplochromine catches from littoral sand and mud bottoms in Butimba Bay. These catches were made in 1979 and 1980, before the major ecological changes. We compared differences in densities and species diversity of haplochromines between these stations, with the differences in densities and diversity before and after the environmental changes in the sub-littoral waters of the Mwanza Gulf.
Methods
Study period and areas
In the present study, three periods are recognized: pre-collapse (1973–1984), collapse (1986–1990), recovery (2001–2006) (Table 1). The division in these three periods is based on the following observations in the Mwanza Gulf: (1) dominance by weight, and great diversity, of haplochromine cichlids in bottom trawl catches in sub-littoral waters of the Mwanza Gulf until 1984 (Witte et al. 1992); (2) the almost complete collapse of the haplochromine populations in sub-littoral waters of the Mwanza Gulf (except for those in rocky areas with clear water) in the period between 1986 and 1990 (Witte et al. 1992; Seehausen et al. 1997a), together with algal blooms, and low DO concentrations and Secchi values since 1986 (Wanink et al. 2001; Witte et al. 2005); (3) recovery of some haplochromine species in sub-littoral waters since the 1990s (Witte et al. 2007b).
The main study area comprised ten sampling stations (A to J) across the Mwanza Gulf (Fig. 1). Stations A to D with approximately the same length (each about 150 m) are located in Butimba Bay, which has a size of less than 0.5 km2 (Fig. 1). Stations A and B have sand bottoms, stations C and D mud bottoms. The depth range of stations A and C is 2–4 m; that of stations B and D 4–6 m. The sub-littoral stations E to J with mud bottoms and depths ranging from 7 to 14 m were pooled in the present study and further referred to as stations E–J.
Environmental parameters
As the original study in the pre-collapse period was not aimed at relating species diversity with environmental parameters, the latter were only collected occasionally (Table 1). To establish the distribution of sand and mud bottoms in Butimba Bay, substrate samples were collected with an Ekman dredge (Wildlife Supply Company, model 196). A van Dorn water sampler was used to get an impression of the thickness of the flocculent mud layer.
Secchi measurements at stations A to D were made in March and April 1980 (pre-collapse period) and in February and April 2002 (recovery period). In the latter period and in March 2006 a few light measurements were made at the bottoms of stations A to D. Secchi and light measurements in the recovery period were made to confirm the relative differences in light conditions over sand and mud bottoms, irrespective of the general light conditions. In 2002 a light sensor connected to an ampere meter was used, in 2006 we used a Voltacrast Lx 1108 light meter. By using both meters at the same time in 2006, we established a factor to convert milliamperes of the home-made meter into Lux.
Seasonality in water transparency in the Mwanza Gulf during the pre-collapse period was derived from a graph of monthly Secchi values measured by Akiyama et al. (1977) at an 8 m deep sub-littoral station, about 7 km south of station G, from April 1973 till January 1975.
Oxygen profiles were measured with a DO meter (Yellow Springs Instruments model 57) during the pre-collapse period (Table 1). At stations A to D, DO concentrations were measured in November 1979 and in March, April and July 1980. At station G, with a depth of 14 m, DO was measured on 12 days between November 1979 and May 1980, and on 17 days between February 1983 and February 1984. Except for the months June, July, August and October one to seven DO measurements were available per month. Average monthly DO concentrations at the bottom and at 50 cm above the bottom, from where haplochromines used to be collected, were calculated.
Species abundance and diversity in Butimba Bay during the pre-collapse period
Catches were made with a small bottom-trawler (20 or 25 hp, trawl head rope 4.6 m, codend 5 or 15 mm). Stations A to D were sampled monthly in the pre-collapse period from March 1979 until July 1980, except in July 1979 and February 1980. Trawl shots at each station lasted about 2 min and the four stations were always sampled on the same day. If catches were large, a sub-sample of about 2.5 kg was taken to establish species composition.
The total species richness (S tot ) at stations A to D was determined by counting all species caught per station during the study period. Mean number of fish per haul (mean catch rate) and mean species richness (S m ) per sample were calculated. To take into account richness and equitability of abundances, we calculated the Shannon–Wiener index (H = −∑ (P i ln[P i ]); in which P i is the number of individuals of a given fish species divided by the total number of fish) and the mean Shannon–Wiener index per sample (H m ). S m per trophic group and the contribution of each group to H m were also calculated. As they were rare in our catches, paedophages and scale-scrapers were added to piscivores for these calculations. Species belonging to other rare trophic groups and those of which the diet was unknown were assigned to a group called “rest”. White-blotched (WB or piebald) and orange-blotched (OB) individuals that represent colour morphs, were scored as percentage of the total number of individuals. Because the numbers of OB and WB individuals per species were low, OB and WB individuals of all species were pooled.
Species abundance and diversity in the Mwanza Gulf during three periods
For the pooled stations E–J, we collected data on haplochromine densities and total species richness in 1979–1982 (pre-collapse), in 1987–1990 (collapse) and in 2001–2005 (recovery).
The same gear was used as at stations A to D, but trawl shots lasted 10 min (for further details see Witte et al. 1992, 2007b). The methods for the three sampling periods were the same, except that in the pre-collapse period all fish >5 cm SL were considered as adults, and in the last two periods all fish >4 cm SL because size at first maturity had decreased strongly in the course of the 1980s (Witte et al. 1995).
Spawning periods in Butimba Bay and Mwanza Gulf during the pre-collapse period
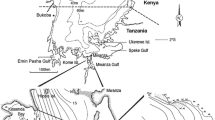
To establish patterns in monthly spawning activity in relation to seasonal changes in light conditions and DO concentrations during the pre-collapse period, the number of females carrying eggs in their mouth (brooding females) in 1979–1980 at stations A to D and E–J was expressed as percentage of the total number of adult females per catch. Data on brooding females of the same months in the two subsequent years were averaged, thus giving a general picture of months with high and low spawning activity. Additionally, we investigated the presence of spawning seasonality in haplochromine species living exclusively over shallow rocky substrates at a clear water island with no pronounced seasonality in clarity (Seehausen et al. 1998). Here the fish were collected with gillnets and by angling; developmental stages of gonads were studied, as it is not possible to collect brooding females by angling, and because the brood is spit out in gillnets. We used a 12-month series of three rock-dwelling species that were numerically dominant at Makobe Island (Speke Gulf). Samples were taken monthly between June 1990 and July 1991. Note that the haplochromine communities at these rocky shores did not collapse, probably due to the low effects of Nile perch and eutrophication in this habitat (Witte et al. 1992; Seehausen et al. 1997a). The developmental stages of gonads were recorded after preservation in formalin. Scores between 1 and 6 were given (gonads not visible; immature; early ripening or mature but inactive; late ripening; ripe; spent).
Feeding experiments under different light intensities
Laboratory raised offspring of the oral shelling molluscivores Platytaeniodes degeni Boulenger and Haplochromis (Ptyochromis) fischeri Seegers (named H. (P.) sauvagei in all previous publications) and of the pharyngeal mollusc crusher Haplochromis (Labrochromis) ishmaeli Boulenger, were tested for their feeding efficiency on snails under high (≫10 Lux) and low (<1 Lux) light intensities. Oral-shellers target the foot of larger snails and, after grasping it, shake the snail forcefully until a part of the body is removed from the shell. Pharyngeal-crushers eat smaller snails by swallowing them entirely and crushing the shell between their pharyngeal jaws. In captivity, oral-shellers also crush small snails, provided the shell is not too strong (Slootweg 1987). In each experiment a group of four fish of the same species was kept in a tank of 50 × 50 × 50 cm. The fish were offered ten snails to feed on during 40 min (once per day), under standardized low light or high light intensities. The snails were introduced approximately in the centre of the tank, but soon spread as they started crawling around. Feeding efficiency was scored as the number of snails eaten in 40 min. While they were raised, the fish were mainly fed on flake food, frozen midge larvae and mixtures of minced shrimps and vegetables. When available, once or twice a week some snails (planorbids and melanoids) harvested from the filters were given. In the shelling experiments, four groups (replicates) of P. degeni and one of H. fischeri were offered planorbid snails (7–10 mm cross section). In the crushing experiments, two groups of P. degeni, one of H. fischeri and one of H. ishmaeli were offered melanoid snails with a cross section of about 1 mm. In both, shelling and crushing experiments, each group was tested two to five times (pseudo-replicates) on low and high light intensities.
Data analysis and statistics
Deviations from normal distribution were tested with the Shapiro–Wilk test. Because they were collected in periods with different overall water clarity and because of small sample sizes, a Wilcoxon Signed Ranks test was run to compare Secchi values and light concentrations at mud and sand bottoms of similar depths on the same day. The same test was used for DO concentrations at sand and mud bottoms.
ANOVAs were performed to compare mean catch rates and mean haplochromine species richness at stations A to D. Bonferroni post hoc multiple comparisons were used to test for differences between individual stations. When data were not normally distributed, the Kruskal–Wallis test was used and when significant, data were further analyzed with Mann–Whitney tests to trace the differences between individual stations. Runs tests for above and below the median were used to identify spawning periodicity. As data on brooding females, transparency and DO concentrations at stations E–J were not collected during the same years, we did not calculate correlations between the environmental conditions and the frequency of brooding females. Instead, Mann–Whitney tests were used to test for differences in mean percentages of brooding females during months with expected high and low transparency and expected high and low DO concentrations. To test if patterns in monthly spawning frequencies at each of the stations A to D were similar to those at the combined sub-littoral stations E–J in the same years, Pearson correlation tests were used.
To test for spawning seasonality in rock-dwelling cichlids, we first plotted the gonadal stage for each species separately against standard length and determined the size at 50 % maturity (defined as stage 4 or higher). Since runs tests for seasonality in size were not significant, only individuals larger than size at 50 % maturity were considered in further analysis. Runs tests for above and below the median were performed on monthly mean gonad stages and on the monthly proportion of ripe individuals.
To test for differences in shelling or crushing under low and high light intensities, ANOVAs were used for single groups, and nested ANOVAs when replicates were involved. In the latter case, groups per species were nested in the low and high light treatments. Residuals were tested for normality.
In case of multiple comparisons, a sequential Bonferroni procedure was used to adjust the significance levels. Tests were done in SPSS 16 and 17 for Windows.
Results
Environmental parameters in Butimba Bay
Water samples from the bottom at the mud stations C and D revealed a layer of flocculent mud of ca. 20 cm. This easily stirred up, resulting in water samples that were murky compared to those collected from the sandy stations A and B. Secchi values at stations A to D measured on 13 March and 15 April 1980 (pre-collapse period) ranged from 1.73 to 2.17 m and measurements on 28 February and 1 April 2002 (recovery period) ranged from 1.23 to 1.53 m (Table 2). On the same day, Secchi values at sand stations were slightly, but significantly higher than at mud stations of comparable depth (mean difference 9 cm; Table 2). In 2002, Secchi values at the same stations were 40–70 cm lower than in 1980 (Table 2), which agrees with the overall decline of transparency in the Mwanza Gulf since the end of the 1980s (e.g. Seehausen et al. 1997a). Light measurements at the bottom, in 2002 and 2006 (recovery period), were 134–499 Lux at sand stations A and B and less than 0.5 Lux at mud stations C and D (Table 2). Light measurements at the deeper sand station B (4–6 m) were lower than at sand station A (2–4 m; Table 2).
Dissolved oxygen concentrations 50 cm above the bottom at stations A to D in 1979/80 (pre-collapse period) were >6 mg L−1 and did not differ significantly. However, at the bottom, mud stations had significantly lower DO concentrations than sand stations (Table 2). Of station pairs with the same substrate, the deeper ones had slightly lower DO concentrations at the bottom than the shallow ones, but the differences were not significant (Wilcoxon Signed Ranks Test, exact 1-tailed: for station A and B P = 0.188, n = 4; for station C and D P = 0.062, n = 4).
Environmental parameters in the Mwanza Gulf during the pre-collapse period
Secchi values in 1973 and 1974 (Akiyama et al. 1977) were between 1.6 and 1.9 m from April to September, which we considered a clear water period. The values ranged from 1.1 to 1.5 m from October to March and this we considered as a period with low transparency. Measurements that were made in 1974 at a 12-m deep station, close to station G (Fig. 1), revealed a very similar pattern (Toshio Akiyama, unpublished report Freshwater Fisheries Institute Nyegezi, Mwanza), though the values were about 20 cm higher than at the 8-m deep station.
Seasonality in DO concentrations was observed at station G (13–14 m) during 1979/1980 and 1983/1984. At the bottom of this station, DO concentrations ranged from 0.3 to 0.6 mg L−1 throughout the year. However, at 50 cm above the bottom of station G, mean DO concentrations were 1.5–2.7 mg L−1during the months February, March and April, and 5.9–7.4 mg L−1 in the remaining months. The low DO concentrations in the period February to April were the result of stratification that occurred in the Mwanza Gulf at depths of about 8 m in the main rainy season during the pre-collapse period (Wanink et al. 2001). During the dry season (May/June to September/October), strong winds resulted in complete mixing of the water column. When low DO concentrations were measured in the lower half of the column at the deeper (7–14 m) stations E to J, such stratification was not found at the shallow (2–6 m) stations A to D.
Haplochromine densities and species diversity in Butimba Bay during the pre-collapse period
The mean number of haplochromines caught at the stations A, B and D during the pre-collapse period did not differ significantly. However, at station C the catch rate was more than twice as high as at the other three stations (Table 2). This was mainly due to high numbers of small juveniles of the zooplanktivore Haplochromis piceatus Greenwood and Gee and of the detritivore H. (Enterochromis) coprologus Niemantsverdriet and Witte at station C (e.g. >1,000 in September 1979). The adults of these common species lived in sub-littoral waters, but station C apparently was a nursery for their juveniles.
In total, we found 82 species of haplochromine cichlids in the bottom trawls at stations A to D in Butimba Bay. The mean number of species per catch (S m ) differed significantly among the stations and showed a decline from the shallow sand station A to the deeper mud station D (Table 2). Bonferroni post hoc multiple comparisons revealed that S m differed significantly between any two stations except between C and D (Table 2). The ratios of mean species diversity at sand and mud stations of the same depth (S m A/S m C and S m B/S m D) were both 1.6. The pattern of the mean Shannon–Wiener index (H m ) was similar to that of S m (Table 2).
White-blotched and OB colour morphs were significantly more common at station A than at the three other stations (Table 2; Kruskal–Wallis P < 0.001; post hoc Mann–Whitney tests P < 0.003), but did not differ significantly at stations B, C and D (Table 2; post hoc Mann–Whitney tests P > 0.367). The WB and OB individuals belonged to the oral-shellers H. (Ptyochromis) fischeri, Macropleurodus bicolor (Boulenger), Hoplotilapia retrodens Hilgendorf, the insectivores H. (Paralabidochromis) “thick skin” and H. (P.) chromogynos Greenwood and to some unknown species.
At trophic group level, S m and H m over sand bottoms were higher than over mud bottoms for insectivores, oral shelling molluscivores and for the “rest” group. Over mud bottoms, S m and H m were higher for detritivores (Table 3). Multiple comparisons between pairs of stations for these significantly differing groups showed that for all combinations of mud and sand stations (A vs. C, A vs. D, B vs. C and B vs. D) the differences in S m were significant (all Mann–Whitney tests P < 0.005). The same held for differences in H m (P ≤ 0.015), except for that of mollusc-shellers between stations B and C, which was not significant after Bonferroni correction (P = 0.033; corrected level of significance = 0.025). Further, S m of insectivores and the “rest” group was significantly higher at station A than at station B (Table 3; Mann–Whitney test: insectivores, P = 0.001; “rest” P = 0.005), however, this was not the case for H m (Table 3; Mann–Whitney test: insectivores P = 0.074; “rest” P = 0.106). For the oral mollusc-shellers, S m seemed also higher at station A than at station B, but after sequential Bonferroni correction this was not significant (P = 0.037; corrected level of significance = 0.025), though H m was significant in this case (P = 0.011). There were no significant differences in S m or H m for any of the groups between the mud stations C and D (Table 3).
Haplochromine densities and species diversity in the Mwanza Gulf during three periods
The number of haplochromines that was caught at stations E–J showed a dramatic decline from an average of more than 1,000 individuals per haul in the pre-collapse period to an average of less than one individual during the collapse period (Table 4). In the same period, the total number of species caught at these stations decreased from more than 72 to five, and the number of identifiable trophic groups from 12 to two. In the recovery period, the mean number of individuals per haul had approached the level of that in the pre-collapse period again, but the total number of species caught in 75 catches in 2001 and 10 catches in 2005 was only about 22, arranged in six identifiable trophic groups (Table 4). Apart from individuals that could be identified to the species level, there were many others that seemed to be hybrids, especially among the closely related ‘curved head’ detritivores that mainly differ in male colouration (de Zeeuw et al. 2010). It should also be noted that the resurgent fish were smaller (both in maximum size and size at first maturity) than those caught in the pre-collapse period.
Patterns in reproductive periodicity during the pre-collapse period
Runs tests for above and below the median revealed seasonality in the occurrence of brooding females at stations B, C, D and E–J (P < 0.05), but not at station A (P = 0.372; Fig. 2). At the rocky shores, there was no evidence for seasonality in the occurrence of ripe females. All runs tests for mean monthly gonad stage, monthly proportions of stage 5 fishes, and of stage 4 + 5 + 6 fishes pooled yielded P values above 0.1.
Seasonality in reproduction of haplochromine cichlids in different habitats expressed as the percentage ripe or brooding females of the total numbers of females (n). Percentages of ripe females, collected in 1990/1991, were used for the rocky habitat and percentages of brooding females, collected in 1979/1980, for the other stations. a Rocks, n = 206; b station A (sand 2–4 m), n = 1,883; c station B (sand 4–6 m), n = 1,681; d station C (mud 2–4 m), n = 2,153; e station D (mud 4–6 m), n = 1,404; f station E–J (mud 4–17 m), n = 16,098. Question marks indicate months for which no catch data were available. Shaded areas indicate estimated periods with high water turbidity. Dotted lines at the bottom indicate the estimated period with low DO concentrations
The average percentages of brooding females at the sub-littoral stations E–J were significantly higher during months with high water transparency (April–September, 8.5 ± 5.3 %) than during months with low transparency (October–March, 1.0 ± 1.3 %; Mann–Whitney test P = 0.002, n = 12; Fig. 2). No significant difference was found for percentages of brooding females between months with high DO concentrations (May–January, 5.2 ± 6.0 %) and months with low DO concentrations (February–April, 3.3 ± 3.4 %; Mann–Whitney test P = 1.000, n = 12).
In 1979/1980 the monthly frequencies of brooding females at stations B and D were significantly correlated with those at stations E–J (station B, R = 0.859, P = 0.001, n = 11; station D, R = 0.881, P < 0.001, n = 11), but this was not the case for the shallow stations A and C (station A, R = 0.078, P = 0.821, n = 11; station C, R = 0.109, P = 0.750, n = 11).
Light intensities and feeding efficiency in laboratory experiments
The average decline in snail shelling efficiency at low light intensities was 88 % for P. degeni, but there was a difference between the replicates (Fig. 3a; Nested ANOVA P = 0.006 for light treatment; P = 0.027 for four replicates). For H. fischeri, the decline in shelling efficiency was 76 % (ANOVA P = < 0.001). However, when small snails that could be swallowed entirely were offered, the decline in number of snails eaten was 24 % for H. fischeri (Fig. 3b; ANOVA P = 0.050) and only 16 % for P. degeni (Fig. 3b; Nested ANOVA P = 0.039 for light treatment; P = 0.850 for two replicates). In the case of the pharyngeal-crusher H. ishmaeli, the decline was 15 % (ANOVA P = 0.008). Though these results were partly based on pseudo-replicates, the results for different species and groups within species were similar and in the predicted direction. Further, shelling efficiency increased with light-intensity treatment (MR and FW unpublished data). Therefore, it is unlikely that the differences were due to other (unknown) factors than the difference in light intensity.
Mean numbers and standard deviation of snails eaten in 40 min under low (<1 Lux) or high (≫10 Lux) light conditions by four individuals per species. a Shelling of larger snails (7–10 mm cross section) by the mollusc-shellers Platytaeniodus degeni (n = 4 × 2; four groups, each tested twice for each light condition) and Haplochromis (Ptyochromis) fischeri (n = 1 × 5); b Crushing of smaller snails (cross section about 1 mm) by the mollusc-shellers P. degeni (n = 2 × 5) and H. (P.) fischeri (n = 1 × 5) and the pharyngeal mollusc-crusher Haplochromis (Labrochromis) ishmaeli (n = 1 × 5)
Discussion
Species richness over sand and mud during the pre-collapse period
Greenwood (1974) monitored haplochromine species at an exposed beach and in a sheltered, papyrus fringed bay near Jinja (Fig. 1). Exposed beaches normally have sand bottoms and sheltered bays mud bottoms. Over a period of 6 years, 35 haplochromine species were caught in the beach habitat and 21 in the bay habitat; resulting in a ratio of 1.7, which is similar to the ratio of 1.6 obtained in the present study. Results of a trawl survey in June 1985 in the south-west corner of the lake (from Kome Island up to Emin Pasha Gulf; depth range 1–10 m), showed that the mean number of species per trawl-shot over sand was significantly higher than over mud (S m sand = 24.8 ± 5.5, n = 5; S m mud = 15.7 ± 5.9, n = 6; t test P = .027; FW unpublished data). Just like in the Butimba Bay, a ratio of 1.6 was found for S m over sand and mud bottoms. So, higher species diversity over sand than over mud bottoms seems a lake-wide phenomenon, but the question remains as to what causes these differences in diversity. In Butimba Bay, apart from physical differences in substrate type, DO concentrations at the bottom were lower over mud than over sand, though 50 cm off the bottom this difference had disappeared. Lower water clarity over mud than over sand was measured from the bottom to several meters above it. In the following paragraphs, possible effects of these differences are discussed.
Substrate type and food distribution Obviously, substrate type will influence the distribution of food types of benthic feeding haplochromine cichlids, and thus the distribution of different trophic groups. The lower number of detritivorous haplochromines over sand may be explained by scarcity of detritus in this habitat, whereas it is abundant over mud bottoms. However, the higher numbers of insectivorous and oral mollusc shelling species we found over sand are not likely explained by higher densities of insects and molluscs on this substrate type, as densities of insects and molluscs were generally higher over mud in Lake Victoria (Mothersill et al. 1980; Okedi 1990).
Dissolved oxygen concentrations In general, Lake Victoria haplochromines, including those of the shallow sandy and rocky habitats, are tolerant to low DO regimes (Chapman et al. 1995). Verheyen et al. (1986) measured relatively high haemoglobin-oxygen affinities (P50 = 1.7–2.3 mmHg) in three typically sand dwelling haplochromine species from Lake Victoria. H. ishmaeli, which had the lowest haemoglobin-oxygen affinity (P50 = 2.3 mmHg) of the three, has been raised successfully in our laboratory from juvenile to adulthood at 0.8 mg L−1 DO, which is similar to the DO concentrations we measured at mud bottoms in the lake. The individuals raised under hypoxia produced new haemoglobins with higher haemoglobin-oxygen affinities (Rutjes et al. 2007). Moreover, haplochromines raised under hypoxia increased their gill surface area (Chapman et al. 2000; Rutjes et al. 2009). These observations suggest that low DO concentrations are unlikely to be the main cause of lower species richness over mud than over sand bottoms. This is further supported by the lower S m and H m at the deepest (station B) of the two sand stations (A and B) in spite of their similar DO concentrations.
Transparency and reproduction Seehausen et al. (1997a) found that the clearer the water and the broader the spectrum of transmitted light, the more colour morphs of a species and the more species of a genus coexisted at rocky islands. This was ascribed to ecological speciation in clear waters, but to incomplete speciation or reversal of speciation—due to increased hybridisation frequencies under poor light conditions—in turbid waters (Seehausen et al. 1997a, 2008; Maan and Seehausen 2010). Laboratory experiments have demonstrated that females, while preferring conspecific to heterospecific males of different coloration under broad-spectrum illumination, do not express this preference under monochromatic light (Seehausen and van Alphen 1998).
A study of WB and OB colour morphs in a rock dwelling species suggest that these morphs represent incompletely assortatively mating incipient species that can only occur in clear water because selective mate choice based on visual cues is required (Seehausen et al. 1999). This is corroborated by the significantly higher frequency of WB and OB morphs at station A, compared to those at the other three stations in Butimba Bay, as at this shallow sand station most light penetrates to the bottom. Also in the trawl survey in June 1985, in the area west of the Mwanza Gulf, WB and OB individuals were encountered more often in catches over sand bottoms. From a total of 19 individuals (representing five species), 18 came from three out of five hauls over sand and only one individual was caught over mud (six hauls; FW unpublished data).
Our observations on spawning periodicity seem to support the hypothesis of a need for fairly clear water for the coexistence of multiple species of Lake Victoria haplochromines on the same spawning ground. Spawning seasonality at the sub-littoral mud stations E–J during the pre-collapse period, correlated well with water transparency, but not with DO concentrations. During the same period, at station A, the clearest habitat in the Butimba Bay, spawning occurred year-round. However, seasonality became apparent at station B, which is deeper than A and where light thus has to travel a longer distance to reach the sediment. Year-round reproductive activity was also observed in the shallow rocky habitat at Makobe Island, where water was clear. Temporal fluctuations in reproductive activity were somewhat more pronounced in species that occupy greater depths in rocky habitats, where less light penetrates (Seehausen et al. 1998). Though we combined reproductive seasonality here across all species in a certain habitat, it should be noted that there was some interspecific variation. For instance, Haplochromis (Yssichromis) pyrrhocephalus Witte and Witte-Maas was spawning throughout the year at stations E–J, while spawning of the closely related H. (Y.) heusinkveldi Witte and Witte-Maas was restricted to the period with clear waters (Witte et al. 2000). This concurs with the observation that, based on characteristics of their photoreceptor cells, Haplochromis pyrrhocephalus seemed better adapted to murky water than H. heusinkveldi (van der Meer et al. 1995). The former appeared one of the most successfully recovering species and the latter seems to have disappeared (Witte et al. 2000, 2007a). From the foregoing, we infer that narrower spawning periods at lower light intensities will result in increased competition for spawning grounds and thus negatively affect species coexistence.
In the same way as under hypoxia, haplochromines that are raised under poor light conditions reveal phenotypic adaptive responses. Experimentally induced shifts in relative expression of different opsin genes, which seem adaptive, have been shown in rock-dwelling cichlids from Lake Malawi (Hofmann et al. 2010). Enlargement of red-green sensitive double cones and reduction in size and number of blue sensitive single cones in response to rearing in darkness was found in laboratory experiments with H. fischeri from Lake Victoria (van der Meer 1993). A recent study showed that the eyes of the resurgent H. tanaos van Oijen and Witte became smaller in the recovery period, possibly to make space for other structures that increased in size (e.g. Witte et al. 2008). In spite of this, the size, and thus photon catching ability, of double cones increased in relation to lens radius. At the same time, there was a reduction in single cone size, and thus short wave sensitivity (van der Meer et al. 2012).
Transparency and feeding A decline in visual range decreases the ‘encounter rates’ of visual predators with prey. Seehausen et al. (2003) suggested that this will negatively affect prey selectivity, and thus the degree of predator specialization for food. Loss of specialization in turn will have a negative impact on species coexistence, because niche overlap, and consequently competition, will increase. Apart from this, decreased water transparency will decrease the bandwidth of underwater light and thus of colour vision, which can negatively affect prey detection (Seehausen et al. 2003).
Some feeding habits may depend more on light than others. Experiments with the oral mollusc-shellers P. degeni and H. fischeri revealed a strong decline in shelling efficiency at low light intensities, whereas snail crushing was hardly affected. Though oral mollusc-shellers target larger snails than pharyngeal-crushers, their attacks are only successful if they can grasp the foot of the snail. Tank observations revealed that this requires careful aiming, which seems impossible if light intensities are low. In contrast to shelling, careful aiming may not be necessary for crushing, where snail-prey are captured and taken whole into the buccal cavity before being crushed. This difference may explain why oral-shellers were rare and not species rich over mud bottoms before the environmental changes in the lake. It may also explain why the trophic group of oral shelling molluscivores among African cichlids is restricted to large lakes with relatively clear waters, whereas crushing molluscivorous cichlids are widespread also in seasonally turbid rivers and swamps (Joyce et al. 2005).
From the foregoing paragraphs, we conclude that water transparency has a negative impact on species coexistence through effects on both reproduction and feeding behaviour.
Can the study of sand and mud communities predict potential effects of eutrophication?
Based on the differences in densities and trophic composition of haplochromines over sand and mud habitats—that reflect different light and DO conditions—during the pre-collapse period, some prudent predictions can be made about the potential effects of increased eutrophication in sub-littoral areas of the Mwanza Gulf during the past decades. In these areas, water transparency and DO concentrations decreased due to sediment influx and eutrophication (Kaufman 1992; Hecky et al. 1994; Seehausen et al. 1997a; Wanink et al. 2001).
The results of haplochromine distributions over sand and mud suggest that with increasing eutrophication: (1) the number of coexisting species will decrease, but (2) the total number of individuals may be less affected; (3) trophic groups which particularly depend on vision, and thus light, for feeding, like oral mollusc-shellers, will be more affected than those that are less dependent on light, like pharyngeal-crushers. Additionally, the data on colour morphs and species diversity in clear water along rocky shores (Seehausen et al. 1997a, 1999) and over shallow sand bottoms suggest that (4) due to effects of sexual selection on male colours, communities containing multiple sympatric closely related species—differentiated by differences in male breeding dress—will lose a larger fraction of their species diversity than communities composed only of distantly related species.
It should be kept in mind, however, that these sub-littoral areas already had a mud bottom during the pre-collapse period. So while the consequences of eutrophication will be further declines of water transparency and DO concentrations, the effects on species richness may not be linear (e.g. Seehausen et al. 1997a).
The catch rates and species richness of the recovered haplochromines (Table 4) agree with predictions 1 and 2. The initial decline in haplochromine densities may have been caused to a large extent by Nile perch predation, followed by resurgence after the decline of the Nile perch. However, the very incomplete recovery in species richness could be due at least partly to the changed environment, especially the reduced water transparency. This is consistent with observations of Mrosso et al. (2004), who found a positive correlation between water transparency and species richness for surviving haplochromines in southern Lake Victoria in the recovery period. In this study, species numbers at muddy, rocky, sandy and vegetated habitats near 20 rocky islands in the entire Mwanza Gulf and the western part of the Speke Gulf were combined.
That our catches in the recovery period contained more hybrids than in the past among the closely related “curved head” detritivores (Seehausen et al. 1997b; de Zeeuw et al. 2010), confirms the impact of reduced water transparency on genetic differentiation, consistent with prediction 4. Mzighani et al. (2010) found genetic evidence for a negative impact of turbidity on reproductive isolation of two zooplanktivorous haplochromines in the sub-littoral of the Mwanza Gulf. Research of Seehausen et al. (1997a, 2008) and Konijnendijk et al. (2011), has shown that also in communities of rock dwelling cichlids of Lake Victoria the frequency of interspecific hybrids is positively correlated with increasing water turbidity. In the same way as in the present study, the catch per unit effort seemed unrelated to turbidity (Seehausen et al. 1997a). Hybridization-induced genetic collapse of a species-pair, associated with among other factors an increase in turbidity, has also been described for a benthic and limnetic stickleback pair in Enos Lake (Taylor et al. 2006). Further, speciation reversal through introgressive hybridization due to eutrophication has been observed in whitefish radiations from 17 pre-alpine European lakes (Vonlanthen et al. 2012).
At the level of trophic groups, we would expect oral mollusc-shellers to be more heavily affected by the worsened light conditions than pharyngeal mollusc-crushers. However, the trends are in the direction opposite to our prediction 3; the crushers, albeit less dependent on vision for feeding, were more affected by the environmental changes than the highly visual shellers (Table 4). In fact, the oral-shellers P. degeni and H. (Ptyochromis) xenognathus Greenwood seem to be more abundant now than in the past (MK-M and FW unpublished data). It should be noted, however, that in the pre-collapse period P. degeni used to be common both over shallow sand bottoms as well as over mud bottoms up to a depth of 10 m (Witte et al. 1992). Moreover, its diet comprised a considerable amount of detritus and fewer molluscs (6–32 % of food volume) than for instance the diet of H. fischeri (51–82 %; Katunzi 1980).
Ecological and morphological changes in the recovering species that we studied may explain deviations in the expectations of resurgence at the level of trophic groups or species. Shifts in habitat and diet were observed in zooplanktivorous, molluscivorous and detritivorous species. Some species in these groups shifted from sand-bottom to mud-bottom habitats (Seehausen et al. 1997b) and/or included more macro-invertebrates in their diet after recovery (Katunzi et al. 2003; Kishe-Machumu et al. 2008; E van Reenen, MJP van Oijen and F Witte, unpublished). Morphological changes, consistent with adaptations to changed environmental conditions, were also observed in several of the resurgent species (Kishe-Machumu et al. 2008; Witte et al. 2008; van der Meer et al. 2012). These rapid morphological changes may reflect environmentally induced plasticity, heritable responses to recently changed natural selection, genetic introgression through hybridisation or, most likely, a combination of several of these factors. Recent studies of contemporary evolution in natural populations, especially in response to environmental changes caused by human activity, yielded estimates of potential rates of evolution many orders of magnitude greater than rates inferred from the fossil record (e.g. Reznick and Ghalambor 2001; Hendry et al. 2008). Interestingly, interspecific hybridization is involved in many of these cases (Grant and Grant 2008). The sometimes rapid adaptive responses and effects on gene flow between species make predicting effects of anthropogenic environmental changes on species diversity in species rich systems such as the African Great Lakes challenging.
Conclusion
However impressive the ability of some populations to cope with changing environments through phenotypic plasticity and/or rapid evolution may be, the sad conclusion that we have to draw from our multi-decadal work on Lake Victoria is that human impacts on the environment have led to a roughly 70 % loss of species and ecological diversity in the sub-littoral waters in just three decades. Also, whereas the upsurge of the Nile perch strongly affected haplochromine densities, and likely species diversity, our results suggest that reduced water transparency mainly affects species diversity. This may explain why, since the decline of the Nile perch, haplochromine densities recovered, while species diversity remained low in the current eutrophic lake. It also implies a serious warning for the other East African lakes with endemic cichlid flocks, as they are all prone to human induced eutrophication.
References
Akiyama T, Kajumulo AA, Olsen, S (1977) Seasonal variations of plankton and physicochemical condition in Mwanza Gulf, Lake Victoria. Bull Freshw Fisheries Res Lab 27 (2):49–61
Chapman LJ, Kaufman LS, Chapman CA, McKenzie FE (1995) Hypoxia tolerance in twelve species of East African cichlids: potential for low oxygen refugia in Lake Victoria. Conserv Biol 9:1274–1288
Chapman LJ, Galis F, Shinn J (2000) Phenotypic plasticity and the possible role of genetic assimilation: hypoxia-induced trade-offs in the morphological traits of an African cichlid. Ecol Lett 3:387–393
de Zeeuw MP, Mietes M, Niemantsverdriet P, Ter Huurne S, Witte F (2010) Seven new species of detritivorous and phytoplanktivorous haplochromines from Lake Victoria. Zool Med 84:201–250
Goudswaard K (PC), Witte F, Katunzi EFB (2008) The invasion of an introduced predator, Nile perch (Lates niloticus, L.) in Lake Victoria (East Africa): chronology and causes. Environ Biol Fish 81:127–139
Grant BR, Grant PR (2008) Fission and fusion of Darwin’s finches populations. Phil Trans Roy Soc B 363:2821–2829
Greenwood PH (1974) The cichlid fishes of Lake Victoria, East Africa: the biology and evolution of a species flock. Bull Br Mus Nat Hist (Zool) Suppl 6:1–134
Hecky RE, Bugenyi FWB, Ochumba P, Talling JF, Mugidde R, Gophen M, Kaufman L (1994) Deoxygenation of the deep water of Lake Victoria, East Africa. Limnol Oceanogr 39:1476–1481
Hecky RE, Mugidde R, Ramlal PS, Talbot MR, Kling GW (2010) Multiple stressors cause rapid ecosystem change in Lake Victoria. Freshw Biol 55 (Suppl 1):19–42
Hendry AP, Farrugia TJ, Kinnison MT (2008) Human influences on rates of phenotypic change in wild animal populations. Mol Ecol 17:20–29
Hofmann CM, O’Quin KE, Smith AR, Carleton KL (2010) Plasticity of opsin expression in cichlids from Lake Malawi. Mol Ecol 19:2064–2074
Joyce DA, Lunt DH, Bills R, Turner GF, Katongo C, Duftner N, Sturmbauer C, Seehausen O (2005) An extant cichlid fish radiation emerged in an extinct Pleistocene lake. Nature 435:90–95
Katunzi EFB (1980) Food preferences of four haplochromine cichlids from Mwanza Gulf in Lake Victoria. MSc thesis University of Dar Es Salaam, Tanzania
Katunzi EFB, Zoutendijk J, Goldschmidt T, Wanink, JH, Witte F (2003) Lost zooplanktivorous cichlid from Lake Victoria reappears with a new trade. Ecol Freshw Fish 12:237–240
Kaufman L (1992) Catastrophic change in species-rich freshwater ecosystems: the lessons of Lake Victoria. BioScience 42:846–858
Kayanda R, Taabu AM, Tumwebaze R, Muhoozi L, Jembe T, Mlaponi E, Nzungi P (2009) Status of the major commercial fish stocks and proposed species-specific management plans for Lake Victoria. Afr J Trop Hydrobiol Fish 12:15–21
Kishe-Machumu MA, Witte F, Wanink JH (2008) Dietary shift in benthivorous cichlids after the ecological changes in Lake Victoria. Anim Biol 58:401–417
Kolding J, van Zwieten P, Mkumbo O, Silsbe G, Hecky RE (2008) Are the Lake Victoria fisheries threatened by exploitation or eutrophication? Towards an ecosystem based approach to management. In: Bianchi G, Skjodal HR (eds) The ecosystem approach to fisheries. CAB International, Wallingford, pp 309–354
Konijnendijk N, Joyce DA, Mrosso HDJ, Egas M, Seehausen O (2011) Community genetics reveal elevated levels of sympatric gene flow among morphologically similar but not among morphologically dissimilar species of Lake Victoria cichlid fish. Int J Evol Biol. doi:10.4061/2011/616320
Kudhongania AW, Cordone AJ (1974) Batho-spatial distribution pattern and biomass estimate of the major demersal fishes in Lake Victoria. Afr J Trop Hydrobiol Fish 3:15–31
Maan EM, Seehausen O (2010) Mechanisms of species divergence through visual adaptation and sexual selection: perspectives from a cichlid model system. Current Zool 56:285–299
Matsuishi T, Muhoozi L, Mkumbo O, Budeba Y, Njiru M, Asila A, Othina A, Cowx IG (2006) Are the exploitation pressures on the Nile perch fisheries resources of Lake Victoria a cause for concern? Fisheries Manag Ecol 13:53–71
Mothersill JS, Freitag R, Barnes B (1980) Benthic macroinvertebrates of north western Lake Victoria, East Africa: abundance, distribution, intraphyletic relationships between taxa and slected concentrations in lake-bottom sediments. Hydrobiologia, 74:215–224
Mrosso HDJ, Msuku BS, Seehausen O (2004) Relationship between water transparency and species richness of surviving haplochromines in selected habitats in Mwanza Gulf—Lake Victoria. Tanz J Sci 30:101–108
Mzighani SI, Nikaido M, Takeda M, Seehausen O, Budeba YL, Ngatunga BP, Katunzi EFB, Aibara M, Mizoiri S, Sato T, Tachida H, Okada N (2010) Genetic variation and demographic history of the Haplochromis laparogramma group of Lake Victoria—an analysis based on SINEs and mitochondrial DNA. Gene 450:39–47
Ogutu-Ohwayo R (1990) The decline of the native fishes of Lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus and the Nile tilapia, Oreochromis niloticus. Environ Biol Fish 27:81–96
Okedi JY (1990) Observations on the benthos of Murchinson Bay, Lake Victoria, East Africa. Afr J Ecol 28:111–122
Reznick DN, Ghalambor CK (2001) The population ecology of contemporary adaptations: what empirical studies reveal about conditions that promote adaptive evolution. Genetica 112–113:183–198
Rutjes HA, Nieveen MC, Weber R.E, Witte F, van den Thillart, GEEJM (2007) Multiple strategies of Lake Victoria cichlids to cope with lifelong hypoxia include hemoglobin switching. Am J Physiol 293:1376–1383
Rutjes HA, de Zeeuw MP, van den Thillart GEEJM, Witte F (2009) Changes in ventral head width, a discriminating shape factor among African cichlids, can be induced by chronic hypoxia. Biol J Linn Soc 98:608–619
Seehausen O, van Alphen JJM (1998) The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav Ecol Sociobiol 42:1–8
Seehausen O, van Alphen JJM, Witte F (1997a) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811
Seehausen O, Witte F, Katunzi EFB, Smits J, Bouton N (1997b) Patterns of the remnant cichlid fauna in southern Lake Victoria. Conserv Biol 11:890–904
Seehausen O, Witte F, van Alphen JJM, Bouton N (1998) Direct mate choice is the mechanism that maintains diversity among sympatric cichlids in Lake Victoria. J Fish Biol 53:37–55
Seehausen O, van Alphen JJM, Lande R (1999) Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol Lett 2:367–378
Seehausen O, van Alphen JJM, Witte F (2003) Implications of eutrophication for fish vision, behavioral ecology and species coexistence. In: Crisman TL, Chapman LJ, Chapman CA, Kaufman LS (eds) Conservation, ecology, and management of African freshwaters, University Press of Florida, Florida. pp 268–287
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626
Slootweg R (1987) Prey selection by molluscivorous cichlids foraging on a schistomiasis vector snail, Biomphalaria glabrata. Oecologia 74:193–202
Taylor EB, Boughman JW, Groenenboom M, Sniatynski M, Schluter D, Gow JL (2006) Speciation in reverse: morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Mol Ecol 15:343–355
van der Meer HJ (1993) Light-induced modulation of retinal development in the cichlid fish Haplochromis sauvagei (Pfeffer, 1896). Zool J Linn Soc-Lond 108:271–285
van der Meer HJ, Anker GC, Barel CDN (1995) Ecomorphology of retinal structures in zooplanktivorous haplochromine cichlids (Pisces) from Lake Victoria. Environ Biol Fish 44:115–132
van der Meer HJ, van Rijssel JC, Wagenaar LC, Witte F (2012) Photopic adaptations to a changing environment in two Lake Victoria cichlids. Biol J Linn Soc 106:328–341
Verheyen E, Blust R, Decleir W (1986) Hemoglobin heterogeneity and the oxygen affinity of hemosylate of some Victoria cichlids. Comp Biochem Phys A 84:315–318
Verschuren D, Johnson TC, Kling HJ, Edgington DN, Leavitt PR, Brown ET, Talbot MR, Hecky RE (2002) History and timing of human impact on Lake Victoria, East Africa. P Roy Soc Lond B 269:289–294
Vonlanthen P, Bittner D, Hudson A.G., Young KA, Müller R, Lundsgaard-Hansen B, Roy D, Di Piazza S, Largiader CR, Seehausen O (2012) Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 482:357–362
Wanink JH, Kashindye JJ, Goudswaard PC, Witte F (2001) Dwelling at the oxycline: does increased stratification provide a predation refugium for the Lake Victoria sardine Rastrineobola argentea? Freshw Biol 46:75–85
Witte F, Goldschmidt T, Wanink J, van Oijen M, Goudswaard K, Witte-Maas E, Bouton N (1992) The destruction of an endemic species flock: quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environ Biol Fish 34:1–28
Witte F, Goldschmidt T, Wanink J (1995) Dynamics of the haplochromine cichlid fauna and other ecological changes in the Mwanza Gulf of Lake Victoria. In: Pitcher TJ, Hart PJB (eds) The impact of species changes in African lakes, vol 18. Fish Fisheries. Chapman & Hall, London, pp 83–110
Witte F, Msuku BS, Wanink JH, Seehausen O, Katunzi EFB, Goudswaard PC, Goldschmidt T (2000) Recovery of cichlid species in Lake Victoria: an examination of factors leading to differential extinction. Rev Fish Biol Fish 10:233–241
Witte F, Wanink JH, Rutjes HA, van der Meer HJ, van den Thillart GEEJM (2005) Eutrophication and its influences on the fish fauna of Lake Victoria. In: Reddy MV (ed) Restoration and management of tropical eutrophic lakes. Science Publishers Inc., Enfield, NH, pp 301-338
Witte F, Wanink JH, Kishe-Machumu M (2007a) Species distinction and the biodiversity crisis in Lake Victoria. Trans Am Fish Soc 136:1146–1159
Witte F, Wanink JH, Kishe-Machumu M, Mkumbo OC, Goudswaard PC, Seehausen, O (2007b) Differential decline and recovery of haplochromine trophic groups in the Mwanza Gulf of Lake Victoria. Aquat Ecosyst Health Manage 10:416–433
Witte F, Welten M, Heemskerk M, van der Stap I, Ham L, Rutjes H, Wanink J (2008) Major morphological changes in a Lake Victoria cichlid fish within two decades. Biol J Linn Soc 94:41–52
Witte F, de Graaf M, Mkumbo O, El-Moghraby AI, Sibbing FA (2009) Fisheries in the Nile system. In: Dumont HJ (ed) The Nile: origin environments, limnology and human use. Springer Science + Business Media B.V., pp 725–749
Acknowledgments
We acknowledge our colleagues from the Haplochromis Ecology Survey Team (HEST) and the Tanzania Fisheries Research Institute (TAFIRI) for support and co-operation during fieldwork. We thank the crews of the trawlers for their skilful labour and Patrick Niemantsverdriet for taking care of the fish during the feeding experiments. We are indebted to Andrea Downing, Martine Maan, Jacco van Rijssel and an anonymous reviewer for comments on earlier drafts of the paper and to Martin Brittijn and Jacco van Rijssel for making figures. The research of HEST was financially supported by The Netherlands Foundation for the Advancement of Tropical Research (WOTRO; grants W87-129, W87-161, W87-189, W84-282, W84-488, WB84-587), the Netherlands Organization for International Cooperation in Higher Education (NUFFIC), the International Foundation for Science (IFS), the Schure Beijerinck-Popping Fonds, the van Tienhoven Stichting, and Yellow Springs Instruments. OS was additionally supported by the Swiss National Science Foundation (grants 31003A-106573 and 31003A-118293).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Witte, F., Seehausen, O., Wanink, J.H. et al. Cichlid species diversity in naturally and anthropogenically turbid habitats of Lake Victoria, East Africa. Aquat Sci 75, 169–183 (2013). https://doi.org/10.1007/s00027-012-0265-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-012-0265-4