Abstract
Current medical therapies for treating acute myeloid leukemia (AML) remain unmet, and AML patients may benefit from targeted immunotherapy approaches that focus on specific tumor antigens. GRP78, which is upregulated in various malignant tumors such as AML, is partially expressed as cell surface GRP78 (csGRP78) on the cell membrane, making it an ideal target for redirecting T cells, including T-cell engagers. However, considering the conventional approach of using two scFv segments to construct a bispecific T-cell engager (BiTE), we have undertaken the development of a novel BiTE that utilizes a cyclic peptide ligand to specifically target csGRP78, which we refer to as GRP78-CD3/BiTE. We studied the effects of GRP78-CD3/BiTE on treatments for AML in vitro and in vivo and assessed the pharmacokinetics of this engager. Our findings demonstrated that GRP78-CD3/BiTE could not only effectively mediate the cytotoxicity of T cells against csGRP78-expressing AML cells but also specifically eliminate primary AML tumor cells in vitro. Furthermore, GRP78-CD3/BiTE exhibited a longer half-life despite having a lower molecular weight than CD19-CD3/BiTE. In a xenograft mouse model of AML, treatment with GRP78-CD3/BiTE prolonged the survival time of the mice. Our findings demonstrate that GRP78-CD3/BiTE is effective and selective for eliminating csGRP78-expressing AML cells and suggest that this approach to targeted immunotherapy could lead to effective new treatments for AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is the most prevalent form of acute leukemia in adults and is a major cause of mortality. The main therapeutic approaches for AML include induction chemotherapy, postremission therapy, and allogeneic stem cell transplantation. Despite the proactive implementation of these treatment methods, achieving a clinical cure remains challenging, and relapse is common. To date, only approximately 30% of adult patients with AML are long-term survivors, while relapse and refractory disease represent the major causes of treatment failure [1]. In the prognostic evaluation of young patients with AML, the 5-year overall survival (OS) rate is only 20–30% [2].
In the current era, many researchers and clinicians are dedicated to the development of novel immunotherapeutic drugs targeting the cellular antigens of AML. For example, bispecific T-cell engagers (BiTEs) are typically designed to bind to a selected tumor-associated antigen (TAA) and to the invariant component of the T-cell receptor (TCR) complex, which is the CD3 chain with signaling capacity [3]. It functions by redirecting the patient’s T cells through T-cell-mediated cytotoxicity to eliminate tumors expressing specific tumor-associated antigens [4, 5].
Relevant studies have shown that glucose-regulated protein 78 (GRP78), also known as immunoglobulin heavy chain binding protein (BiP), is upregulated in multiple malignant tumors such as AML [6, 7]. This protein exhibits a unique and highly cancer-specific behavior by partially translocating from the endoplasmic reticulum to the tumor cell surface [8, 9]. Overexpression of GRP78 is not only associated with drug resistance but also correlated with the level of malignancy in cancer cells [10,11,12]. Other investigators and we have developed CAR-T cells targeting csGRP78, which have shown promising results in eradicating AML, pancreatic cancer, and malignant brain tumors [6, 13,14,15,16]. Together, these characteristics make GRP78 a promising therapeutic target for AML-redirected T-cell therapy. However, the development of bispecific antibodies targeting csGRP78 has yet to be achieved. Therefore, we will utilize this target to design bispecific T-cell engagers to enhance the treatment of AML.
To develop bispecific T-cell engagers targeting csGRP78, we identified a cyclic peptide ligand, Pep42, that is shorter than the G4S linker of GRP78. Pep42 is formed by the disulphide bond between Cys1 and Cys13 to bind to csGRP78 stably. Guided by this natural design inspiration to target csGRP78-expressing cells and given that cyclic peptides exhibit enhanced protein hydrolysis resistance and structural stability [16, 17], we took advantage of this insight to develop a bispecific T-cell engager, GRP78-CD3/BiTE, which combines Pep42 with an anti-CD3 single-chain variable fragment (scFv). Here, we demonstrated that GRP78-CD3/BiTE specifically eradicates AML cells in vitro and in vivo by recruiting CD3+ T cells, laying the foundation for its application in targeted anti-AML research.
Materials and methods
Cell lines and reagents
KG-1a cells were cultured in IMDM supplemented with 10% fetal bovine serum (FBS) and 1x Penicillin/Streptomycin (Pen/Strep) at a temperature of 37 °C with 5% carbon dioxide (CO2). U937 and K562 cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1x Pen/Strep under the same temperature and CO2 conditions. AsPC-1, BxPC-3 and SMCC-7721 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1x Penicillin/Streptomycin (Pen/Strep) at a temperature of 37 °C with 5% carbon dioxide (CO2). The cell lines mentioned above were maintained in our lab and tested via short tandem repeat (STR) profiling. The cell culture media and supplements were purchased from Gibco (Thermo Fisher Scientific). The U937-eGFP-luciferase (U937-eGFP-Luci) cell line was established by infecting U937 cells with the pTomo-CMV-luciferase-eGFP-puro lentivirus (MOI = 10) and subsequently selected by puromycin (1 mg/ml) for 2 weeks to generate U937-eGFP-Luci cell lines. The KG-1a-luciferase (KG-1a-Luci) cell line was established by infecting KG-1a cells with the pTomo-CMV-luciferase-puro lentivirus (MOI = 10) and subsequently selected by puromycin (1 mg/ml) for 2 weeks to generate KG-1a-Luci cell lines. Chinese hamster ovary (CHO) cells were from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences and were cultured in F12K medium (Invitrogen) supplemented with 10% FBS and 1x Pen/Strep at a temperature of 37 °C with 5% CO2. The CellTrace CFSE (Carboxyfluorescein succinimidyl ester) was obtained from ThermoFisher Scientific.
Construction, expression and purification of GRP78-CD3/BiTE
GRP78-CD3/BiTE was constructed by recombinant DNA technology. The coding sequences for the variable heavy (VH) and variable light (VL) chain regions of the anti-human CD3 antibody, as well as the coding sequence of Pep42, were all derived from previously published sequences [18, 19]. We incorporated a flexible linker sequence of (G4S)3, (G4S)5 or a rigid peptide linker sequence of (EAAAK)3 (HE linker) or pyruvate dehydrogenase (PD linker) between the Pep42 and anti-CD3 scFv fragments. The BiTE molecules were tethered to an effector-less fragment crystallizable (Fc) domain conferring half-life extended (HLE) BiTEs. These fragments comprised a 6X His-tag at the carboxyl-terminus, were subcloned and inserted into the pCDH-CMV-MCS-EF1a-copGFP vector and purified from the supernatants from transfected CHO cells. The supernatant was collected, filtered through a 0.45-µm strainer and purified using the AKTA pure chromatography system (GE Healthcare, Pittsburgh, PA, USA). Purification of the monomeric protein was performed using immobilized nickel chelate chromatography, as described for other BiTE antibody constructs [20]. The quality of BiTE was assessed through Coomassie brilliant blue staining and western blot analysis. The monoclonal anti-6xHis-tag antibody (#12698, Cell Signaling Technology) and the secondary peroxidase-conjugated goat anti-rabbit IgG antibody (A6154, Sigma) were used for the western blot analysis. The linker sequences are listed in Supplemental Fig. 2a.
Isolation and culture of PBMC and T cells
Peripheral Blood Mononuclear Cells (PBMCs) were isolated from healthy donors and patients. Whole blood was diluted with phosphate-buffered saline (PBS) at a 1:1 ratio to isolate the PBMCs. The diluted blood was then gently layered onto a lymphoprepTM gradient (STEMCELL Technologies, Vancouver, Canada) in a 50 ml Falcon tube. The supernatant containing lysed erythrocytes was discarded and the cell pellet was washed twice with PBS. After washing, cells were resuspended in a culture medium (RPMI-1640 + 10%FBS + 1x Pen/Strep). Primary human T cells were isolated from the peripheral blood of healthy donors using the RosetteSep Human T-Cell Enrichment Cocktail (Stemcell Technologies) according to the manufacturer’s protocol. T cells were cultured in Advanced RPMI1640 (Gibco) supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin sulphate, and 200 U/ml IL2 (PeproTech) at 37 °C with 5% CO2. T cells were counted and stimulated by CD3/CD28 beads (Life Technologies) for 72 h. Blood samples were collected from patients diagnosed with AML or healthy individuals in the West China Hospital of Sichuan University (#20231797). Our protocol was in accordance with the principles of the Declaration of Helsinki.
Flow cytometry
The specified antigens were targeted using the following antibody clones: FITC-conjugated anti-CD34 (clone 581, BioLegend), FITC-conjugated anti-CD25 (clone BC96, BioLegend), FITC-conjugated anti-CD69 (clone FN50, BioLegend), and PE-conjugated anti-CD3 (clone SP34-2, BD Biosciences). For surface staining, cells were stained in 100 µL of wash buffer (PBS + 2%FBS) with antibodies specific for cell surface antigens for 1 h at 4 °C. After the incubation period, cells were washed twice with a wash buffer and acquired on the flow cytometer immediately. All flow cytometry studies were carried out on a BD Fortessa flow cytometer and the data were analyzed by FlowJo v10 analysis software.
Bispecific antibody binding assay
Binding studies for GRP78-CD3/BiTE and negative control CD19-CD3/BiTE to csGRP78-expressing cell lines and CD3+ T cells were conducted using flow cytometry. Briefly, cells were stained with bispecific T-cell engagers and then incubated with the anti-His tag antibody. Next, the samples were incubated with goat anti-rabbit IgG (H + L) secondary antibody conjugated with FITC (#A11008, Thermo Fisher Scientific, 1:1000) and the fluorescence of stained cells was measured using a flow cytometer.
T-cell activation and functional assays
Enriched T cells (Effectors (E)) were cocultured with CFSE-labeled cells (Targets (T)) in the presence of various concentrations of GRP78-CD3/BiTE or CD19-CD3/BiTE at an effector-to-target (E: T) ratio of 1:10 for 24 h. At the end of the incubation period, total cells were harvested and dead cells were labeled with PI following flow cytometry analysis. The primary human T cells were cocultured with target cells at an E: T ratio of 1:5 in triplicate wells with 100 ng/ml GRP78-CD3/BiTE or CD19-CD3/BiTE. After 48 h, activation of T cells was assessed by measuring the surface expression of CD69 and CD25 using flow cytometry analysis.
Enzyme-linked immunosorbent assay (ELISA) for cytokine secretion
To examine the cytokine production of T cells in response to BiTE treatment, the supernatant was collected. Then, the secretion of tumor necrosis factor α (TNF-α) (#550610, BD Biosciences), interferon-γ (IFN-γ) (#KHC4021, Invitrogen), and granzyme B (VAL142, R&D) was measured using ELISA kits specifically designed for these cytokines.
Immunofluorescence
A total of 1 × 10^6 PBMCs were collected and washed once with PBS, then incubated with anti-GRP78 (#PA1-014 A, ThermoFisher Scientific; 1:200 in 2% FBS PBS) for 60 min at 4 °C. Cells were centrifuged and washed once with PBS, followed by fixation with 4% paraformaldehyde for 20 min at 4 °C. Then, cells were washed once again with PBS and incubated in the blocking buffer (2.5% BSA/10% goat serum/0.1% Tween-20) for 1 h at RT. Incubation with goat anti-rabbit IgG (H + L) secondary antibody conjugated with Cyanine3 (#A10520, ThermoFisher Scientific, 1:1000) in blocking buffer for 1 h at RT. After washing once with PBS, cell nuclei were stained with DAPI in PBS for 10 min in the dark. The cells were centrifuged and washed three times with PBS, resuspended cells in 30 µl PBS and dropped the cells on the coverslip, mounted with Aqua-Polymount on the slide (#18606-20, Polysciences, Inc.).
Pharmacokinetics (PK)
Blood samples were obtained from the tail veins of NCG mice after injection of 0.5 mg/kg of GRP78-CD3/BiTE. The concentration of BiTE in the serum was measured using the His Tag ELISA Detection Kit (YX-080919 H, Yuan Xin). Pharmacokinetic parameters using a non-compartmental modeling approach were calculated with the PKSolver software.
The efficacy of the GRP78-CD3/BiTE in vivo
Six-week-old female NOD-Prkdcem26ll2rgem26Nju (NCG) mice were purchased from GemPharmatech Co., Ltd. (Jiangsu, China) and housed in a pathogen-free animal facility. Subsequently, NCG mice were intravenously administered with 1.0 × 10^6 U937-EGFP-Luci or KG1a-Luci cells via the tail vein one week after arriving at the local animal facility. After the randomization, mice received expanded T cells injection intravenously. Intravenous injections of various concentrations of BiTEs were repeated every other day via the tail vein. These were followed by serial bioluminescence imaging to quantify the progression of the tumor. The bioluminescence images were captured by the IVIS imaging system and quantified using Living Image software (aniview100). Mice were euthanized either when they exhibited signs of hind-limb paralysis or reached the predetermined endpoint of the experiment. Subsequently, the liver, heart, spleen, lung, and kidney were extracted and preserved in paraffin. These tissue samples were then subjected to staining with hematoxylin and eosin (HE) for further analysis.
Statistical analysis
Graphs were plotted and all the data were analyzed using the GraphPad Prism 8.0 statistical software. Unless otherwise stated, data was presented as the mean ± SD. Statistical differences between the two groups were analyzed using unpaired Student’s t-tests with Welch correction. Statistical differences among three or more groups were analyzed by one- or two-way ANOVA followed by Tukey’s multiple comparisons post hoc test. Statistical significance was defined as *P ≤ 0.05, ** P ≤ 0.01, and *** P ≤ 0.001.
Results
The construction of GRP78-CD3/BiTE
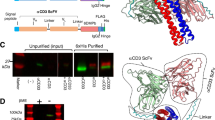
We have developed a bispecific T-cell engager that combines a ligand for csGRP78 with a CD3 antibody scFv fragment using a linker (GGGGS)3, with an Igκ signal peptide inserted at the 5’ end and a His tag added at the 3’ end. This fragment was cloned and inserted into the pCDH-CMV-MCS-EF1-copGFP plasmid (Fig. 1a). T cells were infected with the aforementioned lentiviral vectors carrying different BiTEs, thereby inducing the secretion of each BiTE by the respective batches of transduced T cells. Next, T cells were cocultured with target cells to observe their potential cytotoxic effects. Through this process, the Pep42 cyclic peptide ligand was identified and selected (Fig. S1). The different lentiviral plasmids containing the cyclic peptide ligand Pep42 with different linkers were subjected to HindIII enzyme digestion, resulting in the generation of five fragments (Fig. S2b), and the sequence fidelity was confirmed through sequencing analysis.
The expression and purification of GRP78-CD3/BiTE. (a) Plasmid construction of bispecific T-cell engagers. (b) Fluorescence efficiency of CHO cells infected with lentivirus. (c) Coomassie blue staining of SDS/PAGE gel of the GRP78-CD3/BiTE and CD19-CD3/BiTE protein. MW. Protein molecular weight markers
The purification of GRP78-CD3/BiTE
We then employed lentiviral vectors to establish a stable CHO cell line for the expression of GRP78-CD3/BiTE (Fig. 1b). Following the collection of the cell supernatant, and purification was performed using a nickel column. The expressions of GRP78-CD3/BiTE and GRP78-CD3/HLE BiTE were both confirmed by Coomassie brilliant blue staining and western blot analysis (Fig. 1c and Fig. S3a-b).
Binding of GRP78-CD3/BiTE to csGRP78-expressing tumor cells and CD3+ T cells
We selected two csGRP78-expressing acute myeloid leukemia cell lines (U937 and KG-1a) and the csGRP78-negative cell line (K562) to test the binding ability of GRP78-CD3/BiTE. The binding abilities of GRP78-CD3/BiTE to these three cell lines were 89.6%, 72.7%, and 1.84%, respectively (Fig. 2a). Of note, coculture with CD19-CD3/BiTE resulted in negligible binding to any of the three cell lines. T lymphocytes were isolated from the peripheral blood of healthy individuals and their binding capacities with GRP78-CD3/BiTE and CD19-CD3/BiTE were assessed. It was observed that both the novel engager and CD19-CD3/BiTE exhibited strong binding affinities with T lymphocytes (Fig. 2b and Fig. S3c-d).
GRP78-CD3/BiTE activates T cells in the presence of target cells and induces redirected T cell mediated cytotoxicity in vitro. (a) GRP78-CD3/BiTE was incubated with targeted cells, and binding was detected by flow cytometry. (b) GRP78-CD3/BiTE was incubated with T cells, and binding was detected by flow cytometry. (c) The percentage of tumor target lysis in GRP78-positive (U937 and KG-1a) and GRP78-negative (K562) cells was determined by flow cytometric analysis. (d) Cytokine levels were determined in the cell culture supernatant. (e) Activation markers CD25 and CD69 on CD3-positive T cells. The results are presented as the mean ± SD, data were analyzed by two-way ANOVA with multiple comparisons (c) or one-way ANOVA with multiple comparisons (d and e). * P < 0.05, ** P < 0.01, ***, P < 0.001, ns, not significant
GRP78-CD3/BiTE promotes T-cell activation and csGRP78-expressing tumor cell cytotoxicity
We compared the cytotoxic efficacies of BiTEs constructed with different linkers and different structures and found that this was similar across the constructs we generated (Fig. S2c and Fig. S3e). Therefore, we opted to proceed with further experiments using the conventional structure with the linker (G4S)3 and without an Fc fragment. In the next experiment, we cocultured T cells with acute myeloid leukemia cells with different concentrations of GRP78-CD3/BiTE. As shown, we found that this GRP78-CD3/BiTE mediated T-cell cytotoxicity against U937 and KG-1a cells expressing csGRP78 in a dose-dependent manner (Fig. 2c) but did not show killing ability against csGRP78-negative K562 cells. The coculture of T cells with acute myeloid leukemia cells resulted in the release of specific cytokines (TNF-α, IFN-γ, and GZMB), consistent with the observed cytotoxic effects (Fig. 2d). These results indicate that GRP78-CD3/BiTE is effective for activating and recruiting T cells to kill acute myeloid leukemia cells.
Next, we investigated the expression of CD69, an early marker of T-cell activation, and that of CD25, a late marker of T-cell activation. Since the majority of T cells in freshly isolated PBMCs are naive T cells, we extracted the primary T cells from PBMCs and cocultured primary T cells with target cells mediated by this engager, facilitating the observation of phenotypic changes in naive T cells. We observed the upregulation of T-cell activation markers CD69 and CD25 (Fig. 2e), further demonstrating that GRP78-CD3/BiTE could mediate and activate T cells to induce cytotoxicity.
GRP78-CD3/BiTE mediates T-cell cytotoxicity against csGRP78-expressing primary acute myeloid leukemia
We next wanted to determine whether this engager mediated the cytotoxicity of T cells against csGRP78-expressing primary AML cells. Under the concentration of 100 ng/ml of GRP78-CD3/BiTE, the cytotoxicity of tumor cells and T cells in primary AML blood samples was measured after coculturing for 24 h. The results demonstrated cytotoxicity of the engager towards primary AML samples that were positive for csGRP78 (Fig. 3b, e and h), while no cytotoxicity was detected in AML samples that were negative for GRP78 (Fig. S4b and e). In comparison to the control group, the treatment group exhibited higher levels of the cell cytokine TNF-α in the supernatant, indicating that GRP78-CD3/BiTE modulation of T-cell function within blood samples induced immune cytotoxicity only against csGRP78-expressing AML cells in the patients (Fig. 3c, f and i and Fig. S4c, f). In addition, immunofluorescence staining of primary AML samples confirmed that only patient AML samples with enriched csGRP78 on the plasma membrane showed cytotoxic effects (Fig. 3d, g, j and Fig. S4d, g).
GRP78-CD3/BiTE eradicates csGRP78-positive primary acute myeloid leukemia (AML) blasts. (a) csGRP78 positive AML patients’ information. (b, e, h) Cytotoxic assay measuring the specific lysis of target cells. AML patient PBMC were coincubated with T cells and BiTE for 24 h. (c, f, i) ELISA measurement of cytokine in the cell supernatant was determined following coculture. (d, g, j) Confocal image of representative immunofluorescence staining of cell surface GRP78 (red) and DAPI (blue) on indicated primary AML patients’ PBMC. The results are presented as the mean ± SD, data were analyzed by one-way ANOVA with multiple comparisons (* P < 0.05, ** P < 0.01, ***, P < 0.001)
GRP78-CD3/BiTE eliminates human acute myeloid leukemia cells in a xenograft mouse model
To facilitate the in vivo animal study of our novel-designed BiTE, we began by measuring the half-life of GRP78-CD3/BiTE and the negative control CD19-CD3/BiTE in NCG mice by administering a high dose of these engagers via tail vein injection. The half-lives of GRP78-CD3/BiTE and CD19-CD3/BiTE were found to be approximately 8.6 h and 3.25 h, respectively (Fig. 4), which enabled every other day (QAD) dosing with this BiTE [21, 22].
To gain further insight into the impact of GRP78-CD3/BiTE in eliminating AML in vivo, we validated the in vivo antileukemic cell toxicity of GRP78-CD3/BiTE using a human AML xenograft mouse model established by injecting U937-eGFP-Luci cells. As shown, mice treated with GRP78-CD3/BiTE (0.2 mg/kg or higher) exhibited conspicuous tumor clearance and prolonged survival, accompanied by an increase in the level of the cytokine TNF-α in the blood, compared with mice treated with either an injection of T cells or an injection of T cells with the negative control CD19-CD3/BiTE. In contrast, treatment with 0.04 mg/kg GRP78-CD3/BiTE mitigated tumor progression but did not completely eradicate the tumor (Fig. 5b-f). In the No Ab group and the negative control group, initial signs of the disease appeared within 15 days, such as decreased activity, lethargy and hair coats on the mice that were disordered and lacked luster, consistent with signs of tumor progression. An examination of several organs, including the heart, spleen, lung and kidney, revealed no structural changes in these organs in mice injected with BiTE compared to those in control mice, with the exception of the presence of tumor cell metastasis in the liver (Fig. 5g).
GRP78-CD3/BiTE suppresses tumorigenesis in an U937 xenograft model. (a) A schematic of the U937 leukemia cell xenograft model. NCG mice were injected via the tail vein with 1 × 10^6 U937-eGFP-Luci cells on day 0. CD19-CD3/BiTE (1 mg/kg) or GRP78-CD3/BiTE (three concentration gradients) were injected via the tail vein every other day from day 5. (b, c) Tumor burden was visualized by bioluminescence imaging following U937-eGFP-Luci cell transplantation. (d) Bioluminescent signal and weight for each treatment group over time. (e) Survival analysis of the mouse models. Statistical significance was determined using the Kaplan–Meier estimator with Mantel–Cox log rank to compare treated groups with the No Ab control group, ** P < 0.01. (f) Cytokines from serum were examined on day 7. (g) Representative pictures of the H&E staining of mouse tissue. The results are presented as the mean ± SD, data were analyzed by one-way ANOVA with multiple comparisons (d and f). * P < 0.05, ** P < 0.01, ***, P < 0.001, ns, not significant
In addition to the U937 cell line xenograft model, we also established an NCG xenograft model using KG-1a-Luci cells. By monitoring tumor growth, we observed a dramatic decrease in bioluminescence signals in mice following the injection of GRP78-CD3/BiTE, as indicated by an increase in the level of the cytokine TNF-α in the blood of the mice, indicating the clearance of the majority of the KG-1a leukemia cells in vivo and the efficient elimination of the tumor burden (Fig. 6b, c and e). The statistical analysis revealed that there were no obvious differences in the body weights of the mice and examination by HE staining indicated that the structural integrity of other organs was not markedly altered (Fig. 6d and f).
GRP78-CD3/BiTE suppresses tumorigenesis in a KG-1a xenograft model. (a) Schematic of the KG-1a-Luci leukemia cell xenograft model. GRP78-CD3/BiTE was injected via the tail vein every other day beginning on day 22. (b) Tumor burden was visualized by bioluminescence imaging every 8 days. (c) Bioluminescent signal for each treatment group over time. (d) The weight of each mouse. (e) Cytokines from serum were examined on day 24 and analyzed by unpaired two-tailed Student’s t-tests with Welch correction, * P < 0.05. (f) Representative images of H&E-stained of mouse tissue
Our results from the AML xenograft mouse experiments are consistent with the findings from the in vitro cytotoxicity assays. These data demonstrate that the engager is capable of exerting its biological activity in vivo and effectively eliminating acute myeloid leukemia cells.
Discussion
AML is a disease characterized by poor survival rates. Although immunotherapy based on CD3+ T cells redirection of CD19-expressing cells has become an effective treatment for B-cell-related malignancies, its efficacy in AML patients remains to be validated. Therefore, there is a clear and urgent need to identify suitable targets for developing more curative treatments for AML.
More recently, an increasing number of BiTEs have entered clinical trials and successfully gained approval for clinical applications. In 2022, three more T-cell engagers (TCEs) were approved. Namely tebentafusp, approved by the FDA for the treatment of gp100-expressing uveal melanoma, and mosunetuzumab and teclistamab, approved by the EMA for the treatment of CD20-expressing follicular lymphoma and BCMA-expressing multiple myeloma, respectively [23]. Indeed, the field of AML treatment has entered a new era, with FLT3×CD3, CD33×CD3, and CLEC12A×CD3 BiTEs also advancing to their clinical research stages [24,25,26]. In parallel, ongoing developments in the field of bispecific engagers, such as CD123×CD3, WT1×CD3, and CD34×CD3, are currently in the preclinical research stage [27,28,29]. Altogether, these BiTEs have demonstrated significant clinical activity in both hematologic malignancies and solid tumors.
The selection of appropriate targets for both hematologic and solid tumors holds significant importance. Above all, it is crucial to ensure that the chosen targets are highly expressed in tumor tissues, allowing drugs to recognize and exert their effects. Second, these targets must have low or even no expression in normal tissues, ensuring the safety of patients during drug administration and preventing harm to normal tissues caused by the drugs [3, 30]. Therefore, the selective cell membrane surface expression of GRP78 represents an ideal target, and this feature has attracted increasing attention from researchers [31]. GRP78 is a chaperonin belonging to the heat shock protein family, which plays a crucial role in maintaining cellular homeostasis. The expression of csGRP78 has high specificity on cell membrane surface across various tumor types, including but not limited to AML, ALL, and pancreatic adenocarcinoma (PAAD) [31,32,33]. Likewise, both CAR-T cells and BiTEs targeting the antigen CD19 have been approved for clinical use and have shown promising efficacy. Although CD19 CAR-T demonstrates a higher complete remission rate (CR) compared to BiTE, the high cost and potential side effects remain a major concern. Given that the BiTE known as blinatumomab has gained recognition in clinical settings for its cost-effectiveness and controllable side effects [34], we have chosen to develop a novel BiTE with the same target.
BiTEs have emerged as a promising alternative with unique advantages. The BiTE molecule utilizes a short peptide linker to connect the two scFv fragments, allowing for free rotation of the arms and enhancing the affinity for antigen. The small size of the scFv fragments reduces their immunogenicity, thereby increasing tissue penetration and ensuring proximity between T cells and target cells [21, 35, 36]. In addition, BiTEs not only have the ability to target the same therapeutic targets as CAR-T cells using T cells but also have the advantage of being easily produced and making simplified clinical applications. Furthermore, the side effects are more readily controlled through timely cessation of infusion, a feature that is favorable to patients, such as for patients treated with blinatumomab that opt to interrupt or discontinue infusions at short notice [37].
The scFv fragment and macromolecular protein are both commonly used for designing BiTEs targeting specific cells, while the use of peptides for BiTE design has not been extensively explored [38, 39]. Here, we have developed a novel bispecific cell engager targeting CD3 and csGRP78 using the cyclic peptide ligand Pep42, which consists of only 13 amino acids. Of note, the molecular weight of this conformation is approximately 30 kDa, which is comparable to BiTE molecules constructed using nanobodies [40, 41]. However, the molecular weight of blinatumomab, the smallest bispecific antibody currently available for the clinical treatment of AML, is approximately 55 kDa [42, 43]. Due to its low molecular weight, BiTE exhibits superior tumor penetration and can be applied not only in circulating blood but also in tissues and organs [44]. AML is a rapidly progressing malignant tumor that can readily infiltrate various tissues and organs [45]. In our studies using a xenograft mouse model, an increase in the dosage of GRP78-CD3/BiTE resulted in a reduction in the number of AML cells detected in the liver. The compact size of GRP78-CD3/BiTE likely enables it to translocate to regions that are difficult for large molecular antibodies or CAR-T to reach. This characteristic renders it highly valuable in both biological and clinical contexts [46].
On the other hand, it is worth noting that although the molecular weight is small, we estimated the pharmacokinetics of GRP78-CD3/BiTE and the negative control CD19-CD3/BiTE in mice and determined their half-lives to be approximately 8.6 h and 3.25 h, respectively. This negative control, which has a similar structure to blinatumomab, provides a basis for inferring that the half-life of the BiTE in this study is more stable than that of blinatumomab. Although there are limitations in using NCG mice, further validation in nonhuman primates is still needed. However, the current results could provide us with a reference. In comparison to similar BiTE structures, such as targeting CD33 with a half-life of 6.5–8.7 h in mice and targeting PSMA with a half-life of 6.3 h in mice, the stability of the BiTEs constructed using cyclic peptide structures in this study is comparable [21, 47]. A half-life extended (HLE) BiTE molecule, by fusing an immunoglobulin G (IgG) crystallizable fragment (Fc) domain to the core BiTE molecule structure, enables longer dosing intervals in patients, such as IBI379 with a half-life of 128 h in mice, AMG757 with a half-life of 9.8 days in NHP, and ERY974 with a half-life of 2.89 to 3.87 days in NHP [48,49,50]. Notwithstanding the addition of Fc fragments greatly extends the half-life, it also implies that the control of toxic side effects becomes more challenging. Therefore, the pursuit of an appropriate half-life is an important objective.
The safety of this engager targeting csGRP78 in nonexpressing tissue cells has been further demonstrated through the integration of previous CAR-T research utilizing the same ligand for csGRP78, as mouse and human GRP78 are highly conserved with a homology of up to 98.5% [6, 13,14,15,16]. And our study has shown that this engager does not cause obvious damage to organs in mice, which may indicate that csGRP78 has good safety. Nevertheless, due to the safety being observed only in mice, this study still has limitations that should be addressed in future research.
In summary, we have developed a bispecific cell engager targeting CD3+ T cells and csGRP78+ leukemia AML cells. Instead of using conventional scFv structures to target csGRP78, we utilized a small molecular weight cyclic peptide. In addition, we demonstrated its ability as a T-cell engager to eliminate csGRP78-expressing primary AML cells and to clear tumor cells in a xenograft mouse model. Our studies suggest that a proportion of AML patients could benefit from treatment with this engager therapy, following clinical trials to confirm its safety and efficacy profiles in the near future. GRP78-CD3/BiTE has the potential for clinical translation into an effective treatment.
Data availability
All data reported in this article are available upon request to the authors.
Abbreviations
- AML:
-
Acute myeloid leukemia
- OS:
-
Overall survival
- csGRP78:
-
Cell surface GRP78
- BiTE:
-
Bispecific T-cell engager
- TAA:
-
Tumor-associated antigen
- TCR:
-
T-cell receptor
- ALL:
-
Acute lymphoblastic leukemia
- PAAD:
-
Pancreatic adenocarcinoma
- TCEs:
-
T-cell engagers
- scFv:
-
Single-chain variable fragment
- CR:
-
Complete remission rate
- HLE:
-
Half-life extended
References
Gurnari C, Voso MT, Maciejewski JP, Visconte V (2020) From bench to Bedside and Beyond: therapeutic scenario in Acute myeloid leukemia. Cancers (Basel) 12(2). https://doi.org/10.3390/cancers12020357
Sasaki K, Ravandi F, Kadia T, DiNardo C, Borthakur G, Short N, Jain N et al (2022) Prediction of survival with intensive chemotherapy in acute myeloid leukemia. Am J Hematol 97(7):865–876. https://doi.org/10.1002/ajh.26557
Goebeler ME, Bargou RC (2020) T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol 17(7):418–434. https://doi.org/10.1038/s41571-020-0347-5
Allen C, Zeidan AM, Bewersdorf JP (2021) BiTEs, DARTS, BiKEs and TriKEs-Are antibody based therapies changing the Future treatment of AML? Life (Basel). 11(6). https://doi.org/10.3390/life11060465
Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, Bailey SR et al (2019) CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 37(9):1049–1058. https://doi.org/10.1038/s41587-019-0192-1
Hebbar N, Epperly R, Vaidya A, Thanekar U, Moore SE, Umeda M, Ma J et al (2022) CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat Commun 13(1):587. https://doi.org/10.1038/s41467-022-28243-6
Staquicini DI, D’Angelo S, Ferrara F, Karjalainen K, Sharma G, Smith TL, Tarleton CA et al (2018) Therapeutic targeting of membrane-associated GRP78 in leukemia and lymphoma: preclinical efficacy in vitro and formal toxicity study of BMTP-78 in rodents and primates. Pharmacogenomics J 18(3):436–443. https://doi.org/10.1038/tpj.2017.46
Ibrahim IM, Abdelmalek DH, Elfiky AA (2019) GRP78: a cell’s response to stress. Life Sci 226:156–163. https://doi.org/10.1016/j.lfs.2019.04.022
Gonzalez-Gronow M, Gopal U, Austin RC, Pizzo SV (2021) Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders. IUBMB Life 73(6):843–854. https://doi.org/10.1002/iub.2502
Huang H, Gao Y, Liu A, Yang X, Huang F, Xu L, Danfeng X et al (2019) EIF3D promotes sunitinib resistance of renal cell carcinoma by interacting with GRP78 and inhibiting its degradation. EBioMedicine 49:189–201. https://doi.org/10.1016/j.ebiom.2019.10.030
Rai R, Kennedy AL, Isingizwe ZR, Javadian P, Benbrook DM (2021) Similarities and Differences of Hsp70, hsc70, Grp78 and Mortalin as Cancer Biomarkers and Drug Targets. Cells 10(11). https://doi.org/10.3390/cells10112996
Zhang X, Wu R, Tian C, Wang W, Zhou L, Guo T, Yu J et al (2022) GRP78 blockade overcomes intrinsic resistance to UBA1 inhibitor TAK-243 in glioblastoma. Cell Death Discov 8(1):133. https://doi.org/10.1038/s41420-022-00950-5
Ibanez J, Hebbar N, Thanekar U, Yi Z, Houke H, Ward M, Nevitt C et al (2023) GRP78-CAR T cell effector function against solid and brain tumors is controlled by GRP78 expression on T cells. Cell Rep Med 4(11):101297. https://doi.org/10.1016/j.xcrm.2023.101297
Wang S, Wei W, Yuan Y, Sun B, Yang D, Liu N, Zhao X (2023) Chimeric antigen receptor T cells targeting cell surface GRP78 efficiently kill glioblastoma and cancer stem cells. J Transl Med 21(1):493. https://doi.org/10.1186/s12967-023-04330-0
Yuan Y, Fan J, Liang D, Wang S, Luo X, Zhu Y, Liu N et al (2024) Cell surface GRP78-directed CAR-T cells are effective at treating human pancreatic cancer in preclinical models. Transl Oncol 39:101803. https://doi.org/10.1016/j.tranon.2023.101803
Yu W, Zhang H, Yuan Y, Tang J, Chen X, Liu T, Zhao X (2022) Chimeric Antigen Receptor T Cells targeting cell surface GRP78 to Eradicate Acute myeloid leukemia. Front Cell Dev Biol 10:928140. https://doi.org/10.3389/fcell.2022.928140
Dougherty PG, Sahni A, Pei D (2019) Understanding cell penetration of cyclic peptides. Chem Rev 119(17):10241–10287. https://doi.org/10.1021/acs.chemrev.9b00008
Kim Y, Lillo AM, Steiniger SC, Liu Y, Ballatore C, Anichini A, Mortarini R et al (2006) Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry 45(31):9434–9444. https://doi.org/10.1021/bi060264j
Elfiky AA, Ibrahim IM (2021) Zika virus envelope - heat shock protein A5 (GRP78) binding site prediction. J Biomol Struct Dyn 39(14):5248–5260. https://doi.org/10.1080/07391102.2020.1784794
Jain NK, Barkowski-Clark S, Altman R, Johnson K, Sun F, Zmuda J, Liu CY et al (2017) A high density CHO-S transient transfection system: comparison of ExpiCHO and Expi293. Protein Expr Purif 134:38–46. https://doi.org/10.1016/j.pep.2017.03.018
Lee SC, Ma JSY, Kim MS, Laborda E, Choi SH, Hampton EN, Yun H et al (2021) A PSMA-targeted bispecific antibody for prostate cancer driven by a small-molecule targeting ligand. Sci Adv 7(33). https://doi.org/10.1126/sciadv.abi8193
Iizuka A, Nonomura C, Ashizawa T, Kondou R, Ohshima K, Sugino T, Mitsuya K et al (2019) A T-cell-engaging B7-H4/CD3-bispecific fab-scfv antibody targets human breast Cancer. Clin Cancer Res 25(9):2925–2934. https://doi.org/10.1158/1078-0432.CCR-17-3123
Arvedson T, Bailis JM, Urbig T, Stevens JL (2022) Considerations for design, manufacture, and delivery for effective and safe T-cell engager therapies. Curr Opin Biotechnol 78:102799. https://doi.org/10.1016/j.copbio.2022.102799
Brauchle B, Goldstein RL, Karbowski CM, Henn A, Li CM, Bucklein VL, Krupka C et al (2020) Characterization of a Novel FLT3 BiTE Molecule for the treatment of Acute myeloid leukemia. Mol Cancer Ther 19(9):1875–1888. https://doi.org/10.1158/1535-7163.MCT-19-1093
Laszlo GS, Gudgeon CJ, Harrington KH, Dell’Aringa J, Newhall KJ, Means GD, Sinclair AM et al (2014) Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood 123(4):554–561. https://doi.org/10.1182/blood-2013-09-527044
Cheng P, Chen X, Dalton R, Calescibetta A, So T, Gilvary D, Ward G et al (2022) Immunodepletion of MDSC by AMV564, a novel bivalent, bispecific CD33/CD3 T cell engager, ex vivo in MDS and melanoma. Mol Ther 30(6):2315–2326. https://doi.org/10.1016/j.ymthe.2022.02.005
Kroll KT, Mata MM, Homan KA, Micallef V, Carpy A, Hiratsuka K, Morizane R et al (2023) Immune-infiltrated kidney organoid-on-chip model for assessing T cell bispecific antibodies. Proc Natl Acad Sci U S A 120(35):e2305322120. https://doi.org/10.1073/pnas.2305322120
Arruda LCM, Stikvoort A, Lambert M, Jin L, Rivera LS, Alves RMP, De Moura TR et al (2022) A novel CD34-specific T-cell engager efficiently depletes acute myeloid leukemia and leukemic stem cells in vitro and in vivo. https://doi.org/10.3324/haematol.2021.279486. Haematologica
Bonnevaux H, Guerif S, Albrecht J, Jouannot E, De Gallier T, Beil C, Lange C et al (2021) Pre-clinical development of a novel CD3-CD123 bispecific T-cell engager using cross-over dual-variable domain (CODV) format for acute myeloid leukemia (AML) treatment. Oncoimmunology 10(1):1945803. https://doi.org/10.1080/2162402X.2021.1945803
Tabata R, Chi S, Yuda J, Minami Y (2021) Emerging immunotherapy for Acute myeloid leukemia. Int J Mol Sci 22(4). https://doi.org/10.3390/ijms22041944
Hernandez I, Cohen M (2022) Linking cell-surface GRP78 to cancer: from basic research to clinical value of GRP78 antibodies. Cancer Lett 524:1–14. https://doi.org/10.1016/j.canlet.2021.10.004
Angeles-Floriano T, Rivera-Torruco G, Garcia-Maldonado P, Juarez E, Gonzalez Y, Parra-Ortega I, Vilchis-Ordonez A et al (2022) Cell surface expression of GRP78 and CXCR4 is associated with childhood high-risk acute lymphoblastic leukemia at diagnostics. Sci Rep 12(1):2322. https://doi.org/10.1038/s41598-022-05857-w
Gopal U, Mowery Y, Young K, Pizzo SV (2019) Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling. J Biol Chem 294(38):13939–13952. https://doi.org/10.1074/jbc.RA119.009091
Subklewe M (2021) BiTEs better than CAR T cells. Blood adv. 5(2):607–612. https://doi.org/10.1182/bloodadvances.2020001792
Khalique H, Baugh R, Dyer A, Scott EM, Frost S, Larkin S, Lei-Rossmann J et al (2021) Oncolytic herpesvirus expressing PD-L1 BiTE for cancer therapy: exploiting tumor immune suppression as an opportunity for targeted immunotherapy. J Immunother Cancer 9(4). https://doi.org/10.1136/jitc-2020-001292
Wang Q, Chen Y, Park J, Liu X, Hu Y, Wang T, McFarland K et al (2019) Design and production of Bispecific Antibodies. Antibodies (Basel) 8(3). https://doi.org/10.3390/antib8030043
Guy DG, Uy GL (2018) Bispecific Antibodies for the treatment of Acute myeloid leukemia. Curr Hematol Malig Rep 13(6):417–425. https://doi.org/10.1007/s11899-018-0472-8
Godbersen C, Coupet TA, Huehls AM, Zhang T, Battles MB, Fisher JL, Ernstoff MS et al (2017) NKG2D ligand-targeted bispecific T-Cell Engagers lead to Robust Antitumor activity against Diverse Human tumors. Mol Cancer Ther 16(7):1335–1346. https://doi.org/10.1158/1535-7163.MCT-16-0846
Wu Y, Yi M, Zhu S, Wang H, Wu K (2021) Recent advances and challenges of bispecific antibodies in solid tumors. Exp Hematol Oncol 10(1):56. https://doi.org/10.1186/s40164-021-00250-1
de Bruin RCG, Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, Stam AG et al (2017) A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vgamma9Vdelta2-T cells. Oncoimmunology 7(1):e1375641. https://doi.org/10.1080/2162402X.2017.1375641
de Weerdt I, Lameris R, Ruben JM, de Boer R, Kloosterman J, King LA, Levin MD et al (2021) A bispecific single-domain antibody boosts autologous Vgamma9Vdelta2-T cell responses toward CD1d in chronic lymphocytic leukemia. Clin Cancer Res 27(6):1744–1755. https://doi.org/10.1158/1078-0432.CCR-20-4576
Robinson HR, Qi J, Cook EM, Nichols C, Dadashian EL, Underbayev C, Herman SEM et al (2018) A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood 132(5):521–532. https://doi.org/10.1182/blood-2018-02-830992
Zhao L, Li S, Wei X, Qi X, Liu D, Liu L, Wen F et al (2022) A novel CD19/CD22/CD3 trispecific antibody enhances therapeutic efficacy and overcomes immune escape against B-ALL. Blood 140(16):1790–1802. https://doi.org/10.1182/blood.2022016243
Paci A, Desnoyer A, Delahousse J, Blondel L, Maritaz C, Chaput N, Mir O et al (2020) Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: part 1, monoclonal antibodies, antibody-drug conjugates and bispecific T-cell engagers. Eur J Cancer 128:107–118. https://doi.org/10.1016/j.ejca.2020.01.005
Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Ogrinc Wagner A, Rataj F et al (2018) Bifunctional PD-1 x alphaCD3 x alphaCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood 132(23):2484–2494. https://doi.org/10.1182/blood-2018-05-849802
Bohme M, Kayser S (2021) Immune-based therapeutic strategies for Acute myeloid leukemia. Cancers (Basel) 14(1). https://doi.org/10.3390/cancers14010105
Jitschin R, Saul D, Braun M, Tohumeken S, Volkl S, Kischel R, Lutteropp M et al (2018) CD33/CD3-bispecific T-cell engaging (BiTE(R)) antibody construct targets monocytic AML myeloid-derived suppressor cells. J Immunother Cancer 6(1):116. https://doi.org/10.1186/s40425-018-0432-9
Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, Thomas M et al (2021) AMG 757, a half-life extended, DLL3-Targeted bispecific T-Cell engager, shows high potency and sensitivity in Preclinical models of Small-Cell Lung Cancer. Clin Cancer Res 27(5):1526–1537. https://doi.org/10.1158/1078-0432.CCR-20-2845
Ishiguro T, Sano Y, Komatsu SI, Kamata-Sakurai M, Kaneko A, Kinoshita Y, Shiraiwa H et al (2017) An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med 9(410). https://doi.org/10.1126/scitranslmed.aal4291
Fei K, Ni H, Zhu M, Kuang Z, Wu M, Wu Z, Wang F et al (2022) IBI379, a novel B cell maturation antigen/CD3 bispecific T-cell engager, displays high antitumor efficacy in preclinical models of multiple myeloma. Cancer Lett 536:215663. https://doi.org/10.1016/j.canlet.2022.215663
Acknowledgements
We would like to acknowledge the technical assistance of the Core Facility of West China Hospital (Li Chai, Yi Li and Xing Xu), and the Animal Experimental Center of West China Hospital. Manuscript editors Julian Heng and Xin Du (Remotely Consulting, Australia) provided professional English-language editing of this article (Manuscript Certificate No. 6Xh7Se0N).
Funding
This work was supported by the National Natural Science Foundation of China (82172701 to X Zhao), the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYYC20002 to X Zhao) and Sichuan Science and Technology Program (2024YFHZ0063 to X Zhao).
Author information
Authors and Affiliations
Contributions
X.Zhao and X.Zeng designed the study. X.Zeng performed most experiments, analyzed the data, and wrote the manuscript. HZ and TL collected the blood samples and provided the clinical data. JG and DY performed animal experiments, data collection and analysis. YZ, JT and NL provided administrative, technical, or material support. TL and X.Zhao reviewed and supervised the manuscript. All authors have read and approved to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All samples were approved by the ethics committee of the West China Hospital of Sichuan University with written informed consent (#20231797). Animal experiments were performed in strict accordance with rules approved by the Ethics Committee of the West China Hospital of Sichuan University West China Hospital (20220216013).
Consent to participate
All clinical samples were collected after written informed consent was included in the study.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interest exists.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, X., Zhang, H., Guo, J. et al. A novel bispecific T-cell engager using the ligand-target csGRP78 against acute myeloid leukemia. Cell. Mol. Life Sci. 81, 371 (2024). https://doi.org/10.1007/s00018-024-05410-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05410-0