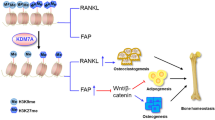
Abstract
Iron deficiency is a prevalent nutritional deficit associated with organ damage and dysfunction. Recent research increasingly associates iron deficiency with bone metabolism dysfunction, although the precise underlying mechanisms remain unclear. Some studies have proposed that iron-dependent methylation-erasing enzyme activity regulates cell proliferation and differentiation under physiological or pathological conditions. However, it remains uncertain whether iron deficiency inhibits the activation of quiescent mesenchymal stem cells (MSCs) by affecting histone demethylase activity. In our study, we identified KDM4D as a key player in the activation of quiescent MSCs. Under conditions of iron deficiency, the H3K9me3 demethylase activity of KDM4D significantly decreased. This alteration resulted in increased heterochromatin with H3K9me3 near the PIK3R3 promoter, suppressing PIK3R3 expression and subsequently inhibiting the activation of quiescent MSCs via the PI3K-Akt-Foxo1 pathway. Iron-deficient mice displayed significantly impaired bone marrow MSCs activation and decreased bone mass compared to normal mice. Modulating the PI3K-Akt-Foxo1 pathway could reverse iron deficiency-induced bone loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is a vital trace element within the human body and is predominantly stored in iron storage proteins such as hemoglobin in red blood cells, myoglobin in muscle cells, and ferritin [1,2,3,4,5]. Additionally, a small quantity of iron in its free form is involved in crucial metabolic enzyme activities [6, 7]. Due to its potent redox properties, excessive free iron can trigger the generation of reactive oxygen species (ROS) through the Fenton reaction, causing harm to the body [4, 7,8,9]. Conversely, iron deficiency can compromise oxygen transport and essential metabolic functions [10,11,12,13]. Consequently, a precise iron homeostatic mechanism governs the equilibrium between stored and free iron [14,15,16]. However, under pathological conditions, this mechanism may malfunction, leading to either iron overload or iron deficiency [1, 7]. While extensive research has examined the link between iron overload and bone metabolism [17,18,19,20], investigations into the impact of iron deficiency on bone metabolism are insufficient [21, 22].
Mesenchymal stem cells (MSCs) are multipotent, nonhematopoietic stem cells originating from the bone marrow [23, 24]. These cells can differentiate into osteoblasts, adipocytes, and chondrocytes [25, 26]. Under typical physiological conditions, MSCs remain in a quiescent state and exhibit low metabolic activity to preserve their stemness [27,28,29]. However, when exposed to factors such as injury or inflammatory stimuli, MSCs become activated and undergo asymmetric division, with some progeny committed to bone remodeling, while others return to a quiescent state [27, 29]. This mechanism ensures a reservoir of MSCs within the bone that can be mobilized when necessary. Despite the integral role of iron in numerous metabolic enzyme activities, whether iron deficiency hampers the mobilization of quiescent MSCs, potentially leading to imbalances in bone remodeling and the onset of osteoporosis, remains unclear.
Recent studies have illuminated the role of epigenetics in regulating cell fate [30, 31]. Specifically, epigenetic control is exerted through the conformation of specific chromatin, which, in turn, dictates the expression of key genes [32]. The precise epigenetic mechanisms governing MSCs fate, especially regarding quiescence and mobilization, remain largely uncharted. Among the histone lysine demethylases that catalyze the removal of methyl groups from histone tails, lysine demethylases (KDMs) have emerged as crucial players. These enzymes rely on Fe2+ and alpha-ketoglutarate (2-OG) as cofactors in the demethylation process [33, 34]. Notably, KDM4D is a histone demethylase responsible for eradicating methyl groups from lysine 9 on histone 3 (H3K9) [35, 36].
The phosphatidylinositol 3-kinase (PI3K)/serine and threonine kinase (Akt, also known as protein kinase B, PKB) signaling pathway is involved in many physiological processes and is an important signaling pathway in many diseases [37]. It can regulate cell survival, migration and metabolism and plays a role in angiogenesis and inflammatory factor recruitment. PI3K phosphorylates PIP2 to generate PIP3, which then recruits downstream signaling proteins, including Akt [38]. Activated Akt can phosphorylate many substrates, and Foxo1 is one of the most common downstream effectors of Akt [39]. In this process, PI3K/Akt signaling suppresses the expression of Foxo1 targets, which play crucial roles in various cellular processes, such as apoptosis induction, cell cycle arrest, catabolism, growth inhibition, and tissue-specific metabolic regulation. PIK3R3, a PI3K regulatory subunit, plays an important role in many cellular processes, including cell proliferation, differentiation and survival [40].
Our study demonstrated that KDM4D activity is dependent on iron and plays a critical role in regulating MSCs quiescence and mobilization. Mechanistically, under iron deficiency conditions, H3K9me3 modification at the PIK3R3 promoter increased, resulting in the downregulation of PIK3R3 expression. This change, in turn, impeded the mobilization of quiescent MSCs through the PI3K/Akt/Foxo1 pathway. This study further elucidates the mechanism of MSCs activation disorders caused by iron deficiency and provides new insight and targets for improving iron deficiency-induced bone loss.
Results
Iron deficiency inhibits KDM4D demethylase activity and hinders quiescent MSC mobilization
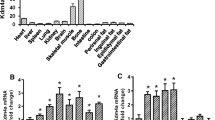
MSCs were exposed to varying DFO concentrations, and cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay. No significant differences in cell viability were observed when MSCs were treated with DFO at concentrations ranging from 0 to 30 µM (Fig. 1A). We found that total iron levels in MSCs gradually decreased with increasing DFO concentrations (Fig. 1B). In addition, Western blot analysis revealed that with increasing DFO concentration, the abundance of both the ferritin H (FTH) and ferritin L (FTL) subunits, which are essential iron storage proteins in mammals, decreased markedly, while the expression of TfR1, a molecule related to cellular iron absorption, gradually increased (Fig. 1C). These results are sufficient to demonstrate that MSCs were iron-deficient after DFO treatment. Consistent with existing research, serum starvation maintained MSCs in a quiescent state [41], while the addition of nutrients such as FBS could activate quiescent MSCs (Fig. 1D). Higher DFO concentrations resulted in more MSCs remaining in the G0 quiescent state (Fig. 1D). Concurrently, the number of EdU-positive MSCs decreased with increasing DFO concentrations (Fig. 1E). Due to the critical role of KDM enzymes in the quiescence-activation balance of MSCs and the iron-dependent nature of their demethylase activity, we detected KDM4A, KDM4D, and FTO expression levels and found that they were significantly elevated during quiescent MSCs activation (Fig. 1F). Western blotting confirmed that KDM4D was strongly induced upon serum supplementation (Fig. 1G). KDM4D primarily functions as a histone demethylase that targets H3K9me3 [35, 36]. To verify that iron deficiency inhibits KDM4D demethylase activity, we conducted in vitro demethylase assays. Recombinant KDM4D protein was exposed to calf thymus histones in vitro. The results revealed that DFO treatment significantly inhibited the H3K9me3 demethylase activity of KDM4D (Fig. 1H). We assessed the impact of iron deficiency on H3K9me3 expression during MSC activation through Western blotting. Compared with quiescent MSCs, activated MSCs displayed notably higher levels of KDM4D expression but significantly lower levels of H3K9me3 expression (Fig. 1I). KDM4D expression remained relatively stable after DFO treatment, but the downregulation of H3K9me3 expression was significantly reversed (Fig. 1I). These results demonstrate that iron deficiency inhibits KDM4D enzymatic activity and hinders quiescent MSC activation.
Iron deficiency directly inhibits KDM4D demethylase activity, hindering quiescent MSC mobilization. (A)Relative viability of MSCs treated with 0, 10, 20 and 30 µM DFO (n = 9). (B) Total iron content in MSCs treated with 0, 10, 20 and 30 µM DFO (n = 9). (C) Western blot analysis of the expression of TfR1, FTH, and FTL in MSCs treated with 0, 10, 20 and 30 µM DFO (n = 9). (D) Compared to that of the FBS group, the percentage of G0 MSCs increased after serum starvation (SS). Additionally, the percentage of G0 cells gradually increased with DFO concentrations of 0, 10, 20, and 30 µM in FBS (n = 9). (E) The number of EdU+ MSCs decreased in comparison to that of the FBS group after SS. Moreover, the number of EdU+ MSCs gradually decreased with increasing DFO concentrations (0, 10, 20, and 30 µM) in FBS. (F) The mRNA expression of histone demethylases was assessed by real-time RT‒PCR. (G) Protein expression of KDM4A, KDM4D, and FTO in MSCs treated with SS or FBS. (H) In vitro demethylation assays showing that KDM4D is catalytically inactive during DFO treatment. The band intensities of H3K9me3 were normalized relative to the intensities of their H3 bands. (I) Western blot analysis of KDM4D and H3K9me3 expression in MSCs treated as indicated (n = 9)
KDM4D plays an important role in the mobilization of quiescent MSCs
To investigate the impact of KDM4D on quiescent MSC activation, we used siRNA to knock down endogenous KDM4D. qPCR and Western blotting revealed that upon KDM4D knockdown, H3K9me3 levels increased significantly (Fig. 2A, B). Additionally, knocking down KDM4D led to a greater proportion of cells in the G0 phase (Fig. 2C) and a notable reduction in EdU-positive activated cells (Fig. 2D). Notably, the inhibitory effect on quiescent MSC activation was equivalent when either KDM4D was knocked down or DFO was administered alone (Fig. 2E). However, introducing DFO stimulation after KDM4D knockdown did not further hinder MSCs activation (Fig. 2E). EdU staining corroborated these findings (Fig. 2F), collectively demonstrating that KDM4D positively regulates quiescent MSCs activation.
KDM4D plays an important role in the mobilization process of quiescent MSCs. (A) The mRNA expression of KDM4D was measured by qRT–PCR after knockdown of KDM4D by siRNA (n = 9). (B) Western blot analysis of KDM4D and H3K9me3 protein expression in the KDM4D knockdown or control group (n = 9). (C) The G0% of the MSCs transfected with siKDM4D was greater than that of the control group (n = 9). (D) The number of EdU+ cells in the MSCs transfected with siKDM4D was lower than that in the control group (n = 9). (E) Flow cytometric detection of G0-phase MSCs treated as indicated (n = 9). (F) EdU staining for detecting activated MSCs treated as indicated (n = 9). (G) KDM4D mRNA expression in KDM4D-overexpressing MSCs treated with or without DFO (n = 9). (H) Western blot analysis of KDM4D and H3K9me3 expression in KDM4D-overexpressing MSCs treated with or without DFO (n = 9). (I) Flow cytometry detection of G0-phase MSCs treated as indicated (n = 9). (J) EdU staining for detecting activated MSCs treated as indicated (n = 9)
Furthermore, we constructed a lentivirus for KDM4D overexpression. qPCR and Western blotting indicated that KDM4D was overexpressed significantly (Fig. 2G, H). Moreover, KDM4D overexpression downregulated H3K9me3 expression under iron deficiency conditions (Fig. 3H). Flow cytometry revealed that KDM4D overexpression markedly activated MSCs subjected to DFO-induced quiescence (Fig. 3I), resulting in more EdU-positive cells than in the DFO-treated group (Fig. 3J). These results demonstrate that KDM4D plays a crucial role in the activation of quiescent MSCs.
Iron deficiency suppresses PIK3R3 expression via KDM4D-mediated H3K9me3 demethylation. (A) RNA-seq heatmap of control MSCs and DFO-treated MSCs (n = 3). (B) RNA-seq heatmap of siNC-transfected MSCs and siKDM4D-transfected MSCs (n = 3). (C, D) The pathways enriched in the DEGs according to KEGG analysis. (E) Venn diagram of DFO-treated MSCs or siKDM4D-transfected MSCs. (F) mRNA expression of PIK3R3 was measured by qRT–PCR in DFO-treated MSCs or siKDM4D-transfected MSCs. (G) Western blotting analysis of PIK3R3 and p-Akt expression in DFO-treated MSCs or siKDM4D-transfected MSCs. (H) CUT&Tag-seq average binding profiles and heatmaps depicting occupancy of KDM4D and H3K9me3 in the MSCs treated with DFO. (I) KEGG analyses of the CUT&Tag-seq data showing a decrease in the occupancy of KDM4D in the MSCs treated with DFO. (J) KEGG analyses of the CUT&Tag-seq data showing increased occupancy of H3K9me3 in the MSCs treated with DFO. (K) Venn diagram of CUT&Tag-seq data showing decreased occupancy of KDM4D and increased occupancy of H3K9me3 and DEGs according to RNA-seq in MSCs treated with DFO. (L) Signal traces of CUT&Tag-seq data showing KDM4D and H3K9me3 occupancy on PIK3R3 in MSCs treated with DFO
Iron deficiency suppresses PIK3R3 expression via KDM4D-mediated H3K9me3 demethylation
To investigate the impact of iron-dependent KDM4D activity on quiescent MSC mobilization, we conducted transcriptome sequencing to analyze gene expression changes following DFO treatment and KDM4D knockdown (Fig. 3A, B). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that DFO treatment enriched differentially expressed genes in cell growth-related pathways, such as the PI3K-Akt signaling pathway and the Foxo signaling pathway. Similarly, KEGG analysis following KDM4D knockdown also highlighted the PI3K-Akt signaling pathway (Fig. 3C, D). The common differentially expressed genes between the DFO treatment group and the KDM4D knockdown group were considered significant, and a Venn diagram analysis identified 10 such genes. Among these genes, PIK3R3 was downregulated following both DFO treatment and KDM4D knockdown (Fig. 3E). The literature indicates that PIK3R3 is a crucial regulatory subunit of PI3K that is responsible for regulating the activation of the PI3K-Akt signaling pathway [40, 42]. To verify the changes in PIK3R3 expression, we performed qPCR and Western blotting assays, which confirmed that both DFO treatment and KDM4D knockdown significantly reduced PIK3R3 expression, concomitant with a notable decrease in Akt phosphorylation (Fig. 3F, G).
To determine whether iron deficiency diminishes PIK3R3 expression via KDM4D-mediated H3K9me3 demethylation, we used cleavage under targets and tagmentation sequencing (CUT&Tag-seq) with anti-KDM4D and anti-H3K9me3 antibodies on MSCs subjected to DFO treatment or left untreated. Heatmap results from the total CUT&Tag-seq signal data revealed a significant reduction in KDM4D gene body occupancy after DFO treatment, while H3K9me3 gene body occupancy notably increased (Fig. 3H). KEGG analysis of genes with reduced KDM4D signaling post-DFO treatment indicated enrichment in pathways related to cell division, including DNA replication (Fig. 3I). Similarly, KEGG analysis of genes exhibiting enhanced H3K9me3 signaling following DFO treatment revealed significant enrichment in the PI3K-Akt signaling pathway, Foxo signaling pathway, and cell cycle signaling pathway (Fig. 3J).
To correlate chromatin binding with direct gene expression changes, we integrated DFO-treated RNA-seq data with CUT&Tag-seq data. Venn diagram analysis revealed five genes, including PIK3R3, that exhibited differential expression in post-DFO treatment RNA-seq data, along with decreased KDM4D signals and increased H3K9me3 signals in CUT&Tag-seq data (Fig. 3K). A representative view of the CUT&Tag-seq results indicates a significant reduction in KDM4D binding near the PIK3R3 promoter in MSCs post-DFO treatment, coupled with a notable increase in the H3K9me3 peak signal (Fig. 3L). These results suggest that PIK3R3 may be a key downstream target and that iron deficiency inhibits PIK3R3 expression through KDM4D-regulated H3K9me3 demethylation.
Iron deficiency modulates MSC activation via the PI3K-Akt pathway
To investigate the influence of iron-dependent KDM4D demethylase activity on MSCs activation through the PI3K-Akt pathway, we conducted Western blotting to assess PIK3R3 expression and Akt phosphorylation. Our findings revealed that KDM4D overexpression under normal conditions had no significant impact on PIK3R3 expression or Akt phosphorylation. However, under conditions of iron deficiency, KDM4D overexpression notably counteracted the inhibitory effects of iron deficiency on PIK3R3 expression and Akt phosphorylation (Fig. 4A). Subsequently, we used the PI3K agonist 740 Y-P and the Akt agonist SC79 to activate the PI3K-Akt pathway [43,44,45]. Western blotting results demonstrated that 740 Y-P and SC79 substantially increased Akt phosphorylation levels under iron-deficient conditions (Fig. 4B). Flow cytometry assays indicated that 740 Y-P and SC79 effectively reversed the inhibitory effects of iron deficiency on quiescent MSC activation (Fig. 4C). Similarly, activating the PI3K-Akt pathway with 740 Y-P and SC79 under iron-deficient conditions led to a greater proportion of MSCs in an EdU-positive activation state (Fig. 4D). Flow cytometry and EdU staining showed that in the absence of DFO, 740 Y-P and SC79 had no significant effect on the activation of MSCs (Supplementary Fig. 1A, B).
Iron deficiency modulates MSC activation via the PI3K-Akt pathway. (A) Western blot analysis of KDM4D, H3K9me3, PIK3R3 and p-Akt expression in MSCs treated as indicated (n = 9). (B) Western blot analysis of p-Akt expression in MSCs treated as indicated (n = 9). (C) Flow cytometric detection of G0-phase MSCs treated as indicated (n = 9). (D) EdU staining for detecting activated MSCs treated as indicated (n = 9). (E) Western blot analysis of KDM4D, H3K9me3 and p-Akt expression in MSCs treated as indicated (n = 9). (F) Flow cytometric detection of G0-phase MSCs treated as indicated (n = 9). (G) EdU staining for detecting activated MSCs treated as indicated (n = 9)
We further investigated whether KDM4D knockdown had effects similar to those of DFO treatment. Consistent with the effects of DFO treatment, Western blotting results demonstrated that KDM4D knockdown significantly reduced Akt phosphorylation levels (Fig. 4E). Stimulation with 740 Y-P and SC79 following KDM4D knockdown effectively elevated Akt phosphorylation levels (Fig. 4E). Flow cytometric analyses revealed a greater percentage of quiescent MSCs after KDM4D knockdown (Fig. 4F). Importantly, 740 Y-P and SC79 treatment successfully mitigated the inhibitory impact of KDM4D knockdown on quiescent MSC activation (Fig. 4F). EdU staining further confirmed that compared to KDM4D knockout, treatment with 740 Y-P and SC79 resulted in more cells in the EdU-positive activation state (Fig. 4G). These results demonstrate that iron deficiency blocks the PI3K-Akt pathway, leading to impaired MSCs activation. Activating the PI3K-AKT pathway with 740 Y-P and SC79 can reverse MSCs activation disorders caused by iron deficiency.
Restoration of quiescent MSC activation by the Foxo1 inhibitor AS1842856
We assessed the downstream signal of the PI3K-Akt pathway through Western blotting and observed a significant decrease in Foxo1 phosphorylation levels after DFO treatment or KDM4D knockdown, while the phosphorylation levels of Foxo3a, mTOR, GSK3, and other molecules remained largely unaffected (Fig. 5A). Notably, Foxo1 is known to localize in the nucleus and directly interact with DNA to regulate the expression of genes [46,47,48,49,50]. Upon phosphorylation by p-Akt, Foxo1 translocates to the cytoplasm, which inhibits its ability to regulate gene transcription [51]. Immunofluorescence staining revealed reduced cytoplasmic localization of Foxo1 following DFO treatment and KDM4D knockdown compared to that of the control conditions (Fig. 5B).
Restoration of quiescent MSC activation by the Foxo1 inhibitor AS1842856 (AS). (A) Western blot analysis of p-Foxo1, p-Foxo3a, p-GSK3 and p-mTOR expression in MSCs treated as indicated (n = 9). (B) Immunofluorescence staining for Foxo1 in MSCs treated as indicated (n = 9). (C) Western blot analysis of p-Foxo1 expression in MSCs treated as indicated (n = 9). (D) Flow cytometric detection of G0-phase MSCs treated as indicated (n = 9). (E) EdU staining for detecting activated MSCs treated as indicated (n = 9). (F) Western blot analysis of p-Foxo1 expression in MSCs treated as indicated (n = 9). (G) Flow cytometry detection of G0-phase MSCs treated as indicated (n = 9). (H) EdU staining for detecting activated MSCs treated as indicated (n = 9)
AS1842856, an inhibitor of Foxo1, can bind to Foxo1 and suppress its transcriptional activity [43, 51]. Western blotting results demonstrated that DFO treatment significantly decreased Foxo1 phosphorylation levels, whereas AS1842856 treatment did not alter Foxo1 phosphorylation levels following DFO treatment (Fig. 5C). Flow cytometry showed that DFO treatment inhibited quiescent MSC mobilization, while AS1842856 treatment effectively reversed this inhibitory effect (Fig. 5D). EdU staining corroborated these findings, revealing a greater number of EdU-positive activated cells in the AS1842856 group than in the DFO-treated group (Fig. 5E). Flow cytometry and EdU staining showed that in the absence of DFO, AS1842856 had no significant effect on the activation of MSCs (Supplementary Fig. 1A, B).
We further investigated whether inhibiting Foxo1 transcriptional activity could counteract the inhibitory effects of KDM4D knockdown on MSC activation. Western blotting indicated that Foxo1 phosphorylation levels significantly decreased after KDM4D knockdown, whereas AS1842856 treatment did not alter Foxo1 phosphorylation levels (Fig. 5F). Notably, after KDM4D knockout, more cells were in the G0 phase, and AS1842856 treatment led to the activation of more quiescent MSCs (Fig. 5G). EdU staining confirmed that there were more EdU-positive activated cells after AS1842856 treatment than after KDM4D knockout alone (Fig. 5H). The apoptosis and osteogenic differentiation of MSCs play important roles in maintaining bone mass. We used H2O2 to induce MSC apoptosis and stained the cells with PI and annexin V for flow cytometry. Annexin V-positive cells were considered apoptotic cells. We used siRNAs to reduce KDM4D expression in advance or 20 µM DFO while inducing apoptosis. Neither DFO treatment nor a reduction in KDM4D expression had a significant effect on MSCs apoptosis (Supplementary Fig. 2A). Furthermore, we evaluated the effect of iron deficiency on MSCs osteogenic differentiation. The results showed that DFO treatment inhibited the in vitro osteogenic differentiation of MSCs (Supplementary Fig. 3A). These results demonstrate that Foxo1 is a downstream target of the P13K-Akt pathway and that inhibiting Foxo1 transcriptional activity through AS1842856 can reverse MSCs activation disorders caused by iron deficiency. In addition, iron deficiency can inhibit the osteogenic differentiation of MSCs but has no significant effect on apoptosis.
Reversal of the iron deficiency-induced bone mass decline in vivo
Dysregulated bone remodeling contributes significantly to bone mass decline [52]. To investigate the impact of iron deficiency on femoral bone mass in vivo, we established an iron deficiency mouse model. C57 mice aged 3–5 weeks were fed an iron-deficient diet for two months, and the hemoglobin, hematocrit, serum iron and transferrin saturation levels indicated that the experimental mice exhibited iron deficiency, in contrast to the control mice (Fig. 6A, B). Micro-CT and HE staining revealed a reduced bone volume in the iron-deficient group (Fig. 6C-E). However, when the iron-deficient mice were treated with 740 Y-P, SC79, or AS1842856, a notable increase in bone volume in the mouse femur was observed (Fig. 6C-E). For further analysis, we collected femoral bone marrow from various groups of mice and conducted flow cytometric assessments. CD45−, Sca-1+, and Pdgfr-α+ cells were identified as MSCs, while Ki67+ cells represented activated cells within the bone marrow (Fig. 6F). The iron-deficient group exhibited fewer Ki67-positive MSCs, and treatment with 740 Y-P, SC79, or AS1842856 led to a significant increase in Ki67-positive MSCs (Fig. 6G). Furthermore, we isolated MSCs from the bone marrow of mice in each group. Consistent with the in vitro results, the phosphorylation levels of Akt and Foxo1 in the bone marrow MSCs of iron-deficient mice were significantly reduced. Moreover, when we treated iron-deficient mice with 740 Y-P or SC79, the phosphorylation of Akt and Foxo1 in bone marrow MSCs was significantly reversed. AS1842856 did not alter the phosphorylation levels of Akt or Foxo1 (Supplementary Fig. 4A). We also examined activated MSCs levels in the femurs of different groups of mice using immunofluorescence staining of CD105 in femoral MSCs. In the iron-deficient group, fewer Ki67-positive MSCs were observed, but treatment with 740 Y-P, SC79, or AS1842856 significantly increased the population of Ki67-positive MSCs (Fig. 6H). These in vivo results confirmed that activation of the PI3K/Akt/Foxo1 pathway can reverse the diminished mobilization capacity of quiescent MSCs induced by iron deficiency and restore bone mass.
Reversal of iron deficiency-induced bone mass decline in vivo. (A) Hemoglobin and hematocrit levels in the different groups of mice (n = 9). (B) Serum iron concentrations and transferrin saturation levels of different groups of mice (n = 9). (C) HE staining of the different groups of mice (n = 9). (D) Representative micro-CT images of different groups of mice (n = 9). (E) Quantitative µCT analyses of the distal ends of femurs (n = 9). (F) The gating strategy for the identification of Ki67-positive MSCs in the bone marrow of femurs. (G) Flow cytometric analysis of activated MSCs in the bone marrow of different groups of mice (n = 9). (H) Immunofluorescence staining for CD105 and Ki67 in the femurs of different groups of mice (n = 9)
Discussion
This study investigated the impact of iron deficiency on the equilibrium between quiescence and activation in MSCs. The results highlight the pivotal role of iron-dependent KDM4D demethylase activity as the primary mechanism responsible for impairing the activation of quiescent MSCs. Iron deficiency significantly dampened KDM4D demethylase activity, resulting in heightened enrichment of H3K9me3 in the promoter region of the PIK3R3 gene and subsequently reducing gene expression. Consequently, the activation of quiescent MSCs was hindered by the PI3K-Akt-Foxo1 pathway. KDM4D overexpression effectively counteracted the inhibitory effects of iron deficiency on the PI3K-Akt-Foxo1 pathway, thereby mitigating the suppression of quiescent MSC activation. Furthermore, treatment with inhibitors targeting the PI3K-Akt-Foxo1 pathway in iron-deficient mice led to a significant reversal of both the iron deficiency-induced reduction in femoral bone mass and impaired bone marrow MSC activation.
Iron, a vital trace element, plays a multifaceted role in the body [2, 4, 5, 7, 10]. Its role extends beyond its function as an oxygen carrier due to its transition metal properties. Iron is crucial for the activity of numerous respiratory and metabolic enzymes, and both iron excess and iron deficiency can result in adverse health effects. Iron overload can lead to an overabundance of ROS through the Fenton reaction, causing oxidative damage [8, 9]. In contrast, iron deficiency can weaken overall metabolic activity, precipitating related diseases [10, 22]. The intricate relationship between iron metabolism and bone health remains underexplored, and the impact of iron deficiency on bone metabolism is even less well understood. MSCs, derived from bone marrow and serving as primary osteoblasts, are multipotent stem cells capable of quiescence [24, 27]. Our study demonstrated that iron deficiency affects KDM4D enzyme activity, leading to decreased PIK3R3 expression through epigenetic regulation and inhibition of quiescent MSC activation via the PI3K-Akt-Foxo1 pathway. This effect may be a significant contributor to the bone remodeling decline triggered by iron deficiency. Hence, our study underscores the potential efficacy of treatments targeting the PI3K-Akt-Foxo1 pathway using inhibitors or agonists to address bone mass reduction resulting from iron deficiency across various diseases.
KDM4D plays a crucial role in DNA replication by reducing H3K9me3 levels [34, 36]. Our study revealed that KDM4D expression significantly increases during the activation of quiescent MSCs and that KDM4D knockdown markedly inhibits quiescent MSC activation. These results indicate that iron deficiency inhibits quiescent MSC activation by influencing KDM4D activity. In vitro enzyme activity assays further showed that DFO treatment significantly inhibits KDM4D activity. Elevating KDM4D expression under iron deficiency conditions effectively counteracted the inhibitory effects of iron deficiency on quiescent MSC activation. Our findings suggest that iron-dependent KDM4D demethylase activity plays a crucial role in attenuating quiescent MSC activation under iron deficiency conditions. Further exploration of downstream pathways revealed that iron-dependent KDM4D demethylase activity impacts the PI3K-Akt-Foxo1 pathway, thereby regulating the equilibrium between quiescence and activation in MSCs.
We subsequently demonstrated in vivo that the inhibitory effects of iron deficiency on bone remodeling could be counteracted by influencing the PI3K-Akt-Foxo1 pathway. Our results revealed diminished bone mass and fewer activated MSCs in iron-deficient mice than in control mice. Treatment with PI3K or Akt agonists or a Foxo1 inhibitor significantly increased femoral bone mass in iron-deficient mice, nearly restoring it to normal levels. Furthermore, the inhibitor treatments led to a substantial increase in the number of Ki67-positive MSCs. These findings collectively illustrate that iron deficiency hinders the activation of quiescent MSCs via the PI3K-Akt-Foxo1 pathway, consequently resulting in reduced bone mass in mice. Our results offer new therapeutic strategies and targets for treating osteoporosis caused by iron deficiency.
Bone is a dynamic tissue that rebuilds itself through the remodeling process to maintain its mechanical properties and calcium homeostasis [53]. Osteoclasts and osteoblasts play crucial roles in bone resorption and bone formation, respectively. Osteoblasts originate not only from the osteogenic layer of the periosteum but also from the stromal cells in the bone marrow. The differentiation of MSCs into osteoblast progenitors in the bone marrow and the mobilization of osteoblast progenitors to the bone surface are key steps in osteoblast maturation and mineralization [54,55,56]. Our study revealed that iron deficiency caused a decrease in bone mass in mice, possibly due to the difficulty in activating bone marrow MSCs under iron deficiency, resulting in fewer osteoblasts being mobilized to the bone surface and leading to impaired bone formation ability. On the other hand, iron deficiency may directly inhibit the osteogenic differentiation of osteoblasts, leading to a decrease in bone mass. Many studies have reported that iron overload inhibits MSCs osteogenic differentiation [57, 58], but studies on the direct effect of iron deficiency on MSCs osteogenic differentiation are lacking.
In conclusion, our study underscores the important role of iron deficiency in bone loss. The iron-dependent demethylase activity of KDM4D regulates quiescent MSC activation, and dysfunctional quiescent MSCs activation contributes to bone remodeling disorders. Research on the relationship between iron deficiency and bone metabolism is limited, and our study provides experimental evidence for iron supplementation in individuals with iron deficiency-associated osteoporosis. Additionally, we elucidated a potential novel mechanism: under conditions of iron deficiency, H3K9me3 levels near the PIK3R3 promoter increase, resulting in decreased expression of PIK3R3, which influences quiescent MSCs activation via the PI3K-Akt-Foxo1 pathway. These insights may offer novel treatment strategies and targets for addressing osteoporosis resulting from iron deficiency.
Materials and methods
Chemicals and reagents
The following commercially available reagents were used for this study: DFO (HY-B0988, MCE), 740 Y-P (B5246, APExBIO), SC79 (HY-18749, MCE), and AS1842856 (B8219, APExBIO).
Animals
C57BL/6 male mice were housed in the pathogen-free barrier facility of Sun Yat-sen University in accordance with institutional guidelines and approved by the regional board. Mice were maintained in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle and free access to distilled water. The animals were kept in stainless steel mesh wire bottom cages with no bedding material and no exposure to feces. Mice were randomly assigned to one of the following five groups, with nine mice in each group: normal control (NC), iron deficiency (ID), ID + 740 Y-P (10 mg/kg per day by intraperitoneal injection for one month), ID + SC79 (20 mg/kg every 5 days by intraperitoneal injection for one month) and ID + AS1842856 (50 mg/kg per day by gavage administration for one month). All mice were approximately 21 days of age on day 0 of treatment. The mice were fed a control diet (Dyets, 110700, 37 ppm Fe) or an ID diet (Dyets, D115109, approximately 1 ppm Fe) for 8 weeks and were weighed 2–3 times per week from the first day on the diets up to and including the day of sacrifice.
Cell isolation and culture
The isolation and expansion of human MSCs followed established procedures. Bone marrow was collected from healthy donors, and MSCs were isolated through density gradient centrifugation. Subsequently, these cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 atmosphere. The culture medium was refreshed every 3 days. Upon reaching 90% confluence in the flasks, the MSCs were trypsinized using 0.25% trypsin containing 0.53 mM ethylenediaminetetraacetic acid (EDTA) and then reseeded in new flasks for expansion. Notably, all MSCs used in the experiments were utilized before they reached passage 2.
Quiescence and activation induction
For induction of cell quiescence in vitro, MSCs were seeded at a density of 5 × 104 per well in 6-well cell culture plates. Subsequently, the culture medium was replaced with DMEM without FBS. After three days of serum starvation culture, the MSCs were then stimulated with DMEM supplemented with 10% FBS with or without DFO. Unless otherwise specified, 20 µM DFO was used to stimulate MSCs for 24 h. The MSCs were collected and utilized in subsequent experiments.
CCK-8 assay
A total of 1 × 104 MSCs per well were seeded for use in a cytotoxicity assay. At the indicated time points, fresh culture medium supplemented with CCK-8 reagent was added (Dojindo, Japan). After 2 h of culture, the absorbance of the wells was measured at 450 nm. Medium without cells was used as a negative control.
Western blotting analysis
Total protein was extracted from the cells with RIPA buffer containing protease and phosphatase inhibitors. Twenty micrograms of total protein from each sample was resolved by 10% or 15% sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE), transferred to a polyvinylidene difluoride membrane (Millipore), blocked in 5% nonfat milk and immunoblotted with various primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Anti-KDM4A (ab191433, 1:5000), anti-KDM4D (ab93694, 1:200) and anti-FTO (ab280081, 1:1000) antibodies were purchased from Abcam. Anti-Akt (4691, 1:1000), anti-phospho-Akt (4060, 1:2000), anti-Foxo1 (2880, 1:1000), anti-phospho-FoxO1 (9461, 1:1000), anti-PIK3R3 (9461, 1:1000) and anti-GAPDH (5174, 1:1000) antibodies were purchased from Cell Signaling Technology.
Quantitative real-time PCR
Quantitative real-time PCR (qRT–PCR) was performed as previously described [59]. Briefly, total RNA was isolated from MSCs using an RNA Quick Purification kit (ESscience) according to the manufacturer’s instructions and was transcribed into cDNA using a PrimeScript™ RT reagent kit (TaKaRa). qRT–PCR was then performed on a Light-Cycler® 480 PCR System (Roche) using SYBR® Premix Ex Taq™ (TaKaRa). Gene expression was calculated by the ΔΔCT method using GAPDH as the reference gene.
In vitro histone demethylation assay
The in vitro histone demethylation assay was performed as previously described [60]. In brief, 5 µg of calf thymus histones (Sigma; H9250) was incubated with 2–4 µg of KDM4D protein in demethylation buffer (comprised of 20 mM Tris-HCl, pH 7.3, 150 mM NaCl, 1 mM α-ketoglutarate, 50 µM FeSO4, and 2 mM ascorbic acid) at 37 °C overnight. Subsequently, the reaction mixtures were resolved on a gel and analyzed by Western blotting utilizing H3K9me3- and H3-specific antibodies.
RNA interference and lentiviral construction
To silence KDM4D expression in MSCs, three KDM4D siRNAs, along with a negative control siRNA, were designed and obtained from Guangzhou IGE Biotechnology, Ltd. Transfection was performed using Thermo Fisher’s Lipofectamine RNAi MAX reagent following the manufacturer’s guidelines. We verified the effectiveness of these siRNAs at both the RNA and protein levels and selected the most efficient siRNAs for subsequent experiments.
For KDM4D overexpression, lentiviruses were constructed by OBiO Technology, along with their corresponding vector controls. MSCs were exposed to lentiviruses at a concentration of 109 TU/mL with 5 µg/mL polybrene during transfection. The transfection process lasted for 24 h, maintaining a multiplicity of infection of 50. Subsequent experiments were conducted on the fourth day following transfection.
RNA sequencing and data analysis
RNA was extracted and used to construct a cDNA library. Subsequently, we conducted RNA sequencing on the BGISEQ-500 platform. The sequencing data were filtered with SOAPnuke (v1.5.2), and the resulting clean reads were mapped to the reference genome GCF_000001405.39_GRCh38.p13. The alignment of the clean reads to the reference coding gene set was carried out using Bowtie2 (v2.2.5), followed by the calculation of the expression level for each gene using RSEM (v1.2.12). For sequencing data analysis, tasks such as heatmap clustering, Venn diagram generation, and KEGG analysis were conducted utilizing BGI Dr. Tom 2.0.
CUT&Tag assay
The CUT&Tag assay was conducted using the NovoNGS CUT&Tag 3.0 High-Sensitivity Kit (catalog no. N259-YH01; NovoProtein). MSCs (1.0 × 105) were subjected to two washes with 0.5 mL of wash buffer. Subsequently, the cells were mixed with ConA beads and incubated with an anti-KDM4D antibody or an anti-H3K9me3 antibody overnight at 4 °C. Next, a goat anti-rabbit IgG antibody (NovoProtein) was diluted at a ratio of 1:500 and added to the sample. The sample was then incubated for 1 h at room temperature (RT). The cells were further washed and subsequently incubated with pAG-Tn5 for 1 h at RT. Afterward, MgCl2 was introduced to activate tagmentation for 1 h at 37 °C. The isolated DNA was processed using NovoNGS DNA Extract Beads and dissolved in TE buffer. DNA was amplified with N5 and N7 primers, which was enriched by PCR to create sequencing-ready libraries. Following PCR, the libraries were purified using NovoNGS DNA Clean Beads, and the library quality was assessed using an Agilent Bioanalyzer 2100 system.
CUT&Tag-seq data analysis
CUT&Tag-seq data analysis was conducted as previously described with slight modifications [61]. In summary, we filtered and trimmed the raw reads to eliminate adaptor sequences and low-quality reads using TrimGalore (version 0.6.6) with the following parameters: -q 20 –phred33 –stringency 3. Clean reads were then mapped to the human genome version hg38 using Bowtie2 (version 2.5.1) with the parameters -p 6 -q, retaining only the uniquely mapped reads. Peak calling was carried out using MACS2 with the following command: ‘macs2 -q 0.05–call-summits –nomodel –shift 100 –extsize 200 –keep-dup all’. To simulate the peaks called per input read, we utilized aligned and deduplicated BAM files without additional filtering. To normalize the CUT&Tag-seq data (in reads per kilobase per million [RPKM]), we used the bamCoverage command and generated heatmaps with the computeMatrix and plotHeatmap commands in deepTools (version 2.3.6.0). The normalized CUT&Tag-seq data were then visualized using the Integrative Genomics Viewer (IGV).
EdU incorporation analysis
To assess DNA synthesis, we used the BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 555 (Beyotime, China) in accordance with the manufacturer’s protocol [62]. The working solution containing EdU was added to the cell culture medium and coincubated with MSCs for 2 h. After fixation and permeabilization, the MSCs were treated with the Click reaction solution for 30 min at room temperature in the absence of light. The cells were then stained with DAPI (Thermo Fisher, USA) for 10 min. All the resulting images were captured using an LSM 5 Exciter confocal imaging system (Carl Zeiss).
Immunofluorescence staining assay
In the cell immunofluorescence assay, MSCs were fixed with 4% paraformaldehyde and permeabilized with 1% Triton X-100. After the cells were blocked with goat serum, they were incubated overnight at 4 °C with primary antibodies. Following washing, the cells were treated with a fluorescein-labeled secondary antibody (Alexa Fluor® 488; 1:1000) for 1 h and then stained with DAPI (Thermo Fisher) for 10 min.
For the tissue immunofluorescence assay, femurs were fixed in 4% paraformaldehyde and decalcified in a 15% EDTA solution. Subsequently, the samples were paraffin-embedded and sectioned at 5 μm. The sections were dewaxed and subjected to antigen retrieval by overnight heating at 60 °C. They were then permeabilized with 0.5% Triton X solution and blocked with 3% BSA. For immunostaining, the slides were incubated overnight at 4 °C with mouse anti-Ki67 antibody (CST) and rabbit anti-CD105 antibody (Abcam). Afterward, the sections were washed and incubated with Alexa Fluor 555-conjugated goat anti-rabbit IgG (Abcam) or Alexa Fluor 488-conjugated goat anti-mouse IgG (Abcam) for 1 h at room temperature. Subsequently, the sections were washed and further stained with DAPI (Beyotime) to label the nuclei. Finally, the samples were mounted and imaged using an LSM 5 Exciter confocal imaging system (Carl Zeiss).
Flow cytometry
To assess the quiescence-activation status, we collected MSCs using 0.25% trypsin and fixed them with precooled 75% ethanol. Subsequently the MSCs were permeabilized using the FIX & PERM™ Cell Permeabilization Kit (GAS004, Invitrogen, USA) according to the manufacturer’s instructions. The sections were then incubated with anti-Ki-67 eFluor® 450 for 1 h and propidium iodide (PI) for 10 min. Subsequently, we used a FACSCelesta Multicolor Flow Cytometer (BD, USA) for detection of the samples, and the analysis was conducted using FlowJo software version 10. For the evaluation of MSC activation in mice, femurs were collected from sacrificed mice. We trimmed both ends of the femurs with scissors and washed them with PBS using a syringe needle until they turned white. The collected cells were then fixed and permeabilized using FIX & PERM Cell Permeabilization Reagents (Thermo Fisher). Subsequent steps included staining with an eFluor450-conjugated anti-Ki-67 antibody (Thermo Fisher), a fluorescein isothiocyanate (FITC)-conjugated anti-CD45 antibody (BioLegend), phycoerythrin (PE)-conjugated anti-Sca-1 antibody (BioLegend), and allophycocyanin (APC)-conjugated anti-PDGFR-α antibody (BioLegend). Cells that were negative for CD45 and positive for both Sca-1 and PDGFR-α were identified as MSCs in the bone marrow. Ki67-positive cells were further measured to analyze MSC activation in mice.
Hematoxylin and eosin (H&E) staining
The femurs were initially fixed in 4% paraformaldehyde and then decalcified in a 15% EDTA solution. Next, the samples were paraffin-embedded and sectioned into 5 μm slices. The sections were subsequently dewaxed and stained with hematoxylin for 5 min. After a 10-minute PBS wash, the sections were subjected to eosin staining for 3 min. The sections were then dehydrated using ethanol and cleared with a dimethylbenzene solution. Finally, the slides were mounted and examined using a microscope.
Micro-CT analysis
To assess bone mass and microarchitecture across different groups, we utilized a micro-CT scanner, the Inveon MM system (Siemens). The image acquisition involved 360 rotational steps, with a pixel size of 8.82 μm, an 80 kV voltage, a 500 µA current, and an exposure time of 1,500 ms. Subsequently, we calculated key parameters, including BV/TV and Cb.Th. These calculations adhered to the guidelines provided by the American Society for Bone and Mineral Research.
Statistical analysis
Each experiment involved a minimum of three biological replicates. The data are presented as the mean ± standard deviation (SD). For comparisons between two groups, an unpaired Student’s t test was applied. In cases where more than two groups were compared, one-way ANOVA was utilized. GraphPad Prism 8.0 software was used for data analysis. Significance levels are denoted as *P < 0.05 and **P < 0.01.
Data availability
The authors agree with the publication of all the data involved in this article. No data from other entities were used in this study. The RNA-seq and CUT&Tag-seq data have been deposited in the NCBI Gene Expression Omnibus database under GEO accession number GSE245686.
Abbreviations
- KDM4D:
-
Histone Lysine Demethylase 4D
- MSCs:
-
Mesenchymal Stem Cells
- PI3K:
-
Phosphoinositide 3-Kinase
- AKT:
-
Protein Kinase B
- Foxo1:
-
Forkhead Box Protein O1
- H3K9me3:
-
Trimethylated Histone 3 Lysine 9
- PIK3R3:
-
Phosphoinositide-3-Kinase Regulatory Subunit 3
- 2-OG:
-
Alpha-Ketoglutarate
- ROS:
-
Reactive Oxygen Species
- DFO:
-
Deferoxamine
References
Gao H, Jin Z, Bandyopadhyay G et al (2022) Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab 34:1201–1213. https://doi.org/10.1016/j.cmet.2022.07.006
Dutt S, Hamza I, Bartnikas TB (2022) Molecular mechanisms of iron and heme metabolism. Annu Rev Nutr 42:311–335. https://doi.org/10.1146/annurev-nutr-062320-112625
Fang X, Ardehali H, Min J et al (2023) The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20:7–23. https://doi.org/10.1038/s41569-022-00735-4
Ward DM, Cloonan SM (2019) Mitochondrial iron in human health and disease. Annu Rev Physiol 81:453–482. https://doi.org/10.1146/annurev-physiol-020518-114742
Gorman MJ (2023) Iron homeostasis in insects. Annu Rev Entomol 68:51–67. https://doi.org/10.1146/annurev-ento-040622-092836
Camarena V, Huff TC, Wang G (2021) Epigenomic regulation by labile iron. Free Radic Biol Med 170:44–49. https://doi.org/10.1016/j.freeradbiomed.2021.01.026
Ward RJ, Zucca FA, Duyn JH et al (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060. https://doi.org/10.1016/S1474-4422(14)70117-6
Li L, Guo F, Liu H et al (2022) Iron overload in alcoholic liver disease: underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell Mol Life Sci 79. https://doi.org/10.1007/s00018-022-04239-9
Wang W, Jing X, Du T et al (2022) Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free Radic Biol Med 190:234–246. https://doi.org/10.1016/j.freeradbiomed.2022.08.018
Pasricha S, Tye-Din J, Muckenthaler MU et al (2021) Iron deficiency. Lancet 397:233–248. https://doi.org/10.1016/S0140-6736(20)32594-0
Hara Y, Yanatori I, Tanaka A et al (2020) Iron loss triggers mitophagy through induction of mitochondrial ferritin. Embo Rep 21:e50202. https://doi.org/10.15252/embr.202050202
Nurtjahja-Tjendraputra E, Fu D, Phang JM et al (2006) Iron chelation regulates cyclin d1 expression via the proteasome: a link to iron deficiency–mediated growth suppression. Blood 109:4045–4054. https://doi.org/10.1182/blood-2006-10-047753
Jiang Y, Li C, Wu Q et al (2019) Iron-dependent histone 3 lysine 9 demethylation controls b cell proliferation and humoral immune responses. Nat Commun 10:2935. https://doi.org/10.1038/s41467-019-11002-5
Zhou H, Chen J, Fan M et al (2023) Klf14 regulates the growth of hepatocellular carcinoma cells via its modulation of iron homeostasis through the repression of iron-responsive element-binding protein 2. J Exp Clin Cancer Res 42. https://doi.org/10.1186/s13046-022-02562-4
Santos M, Anderson CP, Neschen S et al (2020) Irp2 regulates insulin production through iron-mediated cdkal1-catalyzed trna modification. Nat Commun 11:296. https://doi.org/10.1038/s41467-019-14004-5
Wang H, Shi H, Rajan M et al (2020) Fbxl5 regulates irp2 stability in iron homeostasis via an oxygen-responsive [2fe2s] cluster. Mol Cell 78:31–41. https://doi.org/10.1016/j.molcel.2020.02.011
Jing X, Du T, Chen K et al (2019) Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol 234:10123–10137. https://doi.org/10.1002/jcp.27678
Tao Z, Li T, Wei S (2022) Silymarin prevents iron overload induced bone loss by inhibiting oxidative stress in an ovariectomized animal model. Chem Biol Interact 366:110168. https://doi.org/10.1016/j.cbi.2022.110168
Piriyakhuntorn P, Tantiworawit A, Phimphilai M et al (2020) Impact of iron overload on bone remodeling in Thalassemia. Arch Osteoporos 15. https://doi.org/10.1007/s11657-020-00819-z
He Q, Yang J, Pan Z et al (2023) Biochanin a protects against iron overload associated knee osteoarthritis via regulating iron levels and nrf2/system xc-/gpx4 axis. Biomed Pharmacother 157:113915. https://doi.org/10.1016/j.biopha.2022.113915
Balogh E, Paragh G, Jeney V (2018) Influence of iron on bone homeostasis. Pharmaceuticals (Basel) 11:107. https://doi.org/10.3390/ph11040107
Yang J, Li Q, Feng Y et al (2023) Iron deficiency and iron deficiency anemia: potential risk factors in bone loss. Int J Mol Sci 24:6891. https://doi.org/10.3390/ijms24086891
Naji A, Eitoku M, Favier B et al (2019) Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 76:3323–3348. https://doi.org/10.1007/s00018-019-03125-1
Liu J, Gao J, Liang Z et al (2022) Mesenchymal stem cells and their microenvironment. Stem Cell Res Ther 13. https://doi.org/10.1186/s13287-022-02985-y
Xie Z, Yu W, Zheng G et al (2021) Tnf-α-mediated m6a modification of elmo1 triggers directional migration of mesenchymal stem cell in ankylosing spondylitis. Nat Commun 12. https://doi.org/10.1038/s41467-021-25710-4
Li J, Wang P, Xie Z et al (2019) Traf4 positively regulates the osteogenic differentiation of mesenchymal stem cells by acting as an e3 ubiquitin ligase to degrade smurf2. Cell Death Differ 26:2652–2666. https://doi.org/10.1038/s41418-019-0328-3
Alekseenko LL, Shilina MA, Lyublinskaya OG et al (2018) Quiescent human mesenchymal stem cells are more resistant to heat stress than cycling cells. Stem Cells Int 2018:1–15. https://doi.org/10.1155/2018/3753547
Cheung TH, Rando TA (2013) Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14:329–340. https://doi.org/10.1038/nrm3591
Lin C, He Y, Feng Q et al (2021) Self-renewal or quiescence? Orchestrating the fate of mesenchymal stem cells by matrix viscoelasticity via pi3k/akt-cdk1 pathway. Biomaterials 279:121235. https://doi.org/10.1016/j.biomaterials.2021.121235
Liu Z, Leung D, Thrush K et al (2020) Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell 19. https://doi.org/10.1111/acel.13229
Ling C, Rönn T (2019) Epigenetics in human obesity and type 2 diabetes. Cell Metab 29:1028–1044. https://doi.org/10.1016/j.cmet.2019.03.009
Sun L, Zhang H, Gao P (2022) Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 13:877–919. https://doi.org/10.1007/s13238-021-00846-7
Arifuzzaman S, Khatun MR, Khatun R (2020) Emerging of lysine demethylases (kdms): from pathophysiological insights to novel therapeutic opportunities. Biomed Pharmacother 129:110392. https://doi.org/10.1016/j.biopha.2020.110392
Manni W, Jianxin X, Weiqi H et al (2022) Jmjd family proteins in cancer and inflammation. Signal Transduct Target Ther 7. https://doi.org/10.1038/s41392-022-01145-1
Chandwani R, Fang TC, Dewell S et al (2023) Control of enhancer and promoter activation in the type i interferon response by the histone demethylase kdm4d/jmjd2d. Front Immunol 14. https://doi.org/10.3389/fimmu.2023.1146699
Dong F, Jiang S, Li J et al (2019) The histone demethylase kdm4d promotes hepatic fibrogenesis by modulating toll-like receptor 4 signaling pathway. Ebiomedicine 39:472–483. https://doi.org/10.1016/j.ebiom.2018.11.055
Touil Y, Zuliani T, Wolowczuk I et al (2013) The pi3k/akt signaling pathway controls the quiescence of the low-rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells 31:641–651. https://doi.org/10.1002/stem.1333
Manning BD, Toker A (2017) Akt/pkb signaling: navigating the network. Cell 169:381–405. https://doi.org/10.1016/j.cell.2017.04.001
Eijkelenboom A, Burgering BMT (2013) Foxos: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 14:83–97. https://doi.org/10.1038/nrm3507
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619. https://doi.org/10.1038/nrg1879
Li B, Sun C, Sun J et al (2019) Autophagy mediates serum starvation-induced quiescence in nucleus pulposus stem cells by the regulation of p27. Stem Cell Res Ther 10. https://doi.org/10.1186/s13287-019-1219-8
Wang G, Cao X, Lai S et al (2013) Pi3k stimulates dna synthesis and cell-cycle progression via its p55pik regulatory subunit interaction with pcna. Mol Cancer Ther 12:2100–2109. https://doi.org/10.1158/1535-7163.MCT-12-0920
Savai R, Al-Tamari HM, Sedding D et al (2014) Pro-proliferative and inflammatory signaling converge on foxo1 transcription factor in pulmonary hypertension. Nat Med 20:1289–1300. https://doi.org/10.1038/nm.3695
Wen L, Wang K, Zhang F et al (2020) Akt activation by sc79 to transiently re-open pathological blood brain barrier for improved functionalized nanoparticles therapy of glioblastoma. Biomaterials 237:119793. https://doi.org/10.1016/j.biomaterials.2020.119793
Li D, Guo Y, Cen X et al (2022) Lupeol protects against cardiac hypertrophy via tlr4-pi3k-akt-nf-κb pathways. Acta Pharmacol Sin 43:1989–2002. https://doi.org/10.1038/s41401-021-00820-3
Pandey A, Kumar GS, Kadakol A et al (2016) Foxo1 inhibitors: the future medicine for metabolic disorders? Curr Diabetes Rev 12:223
Accili D, Arden KC (2004) Foxos at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426. https://doi.org/10.1016/S0092-8674(04)00452-0
Ro S, Liu D, Yeo H et al (2013) Foxos in neural stem cell fate decision. Arch Biochem Biophys 534:55–63. https://doi.org/10.1016/j.abb.2012.07.017
Arden KC (2007) Foxos in tumor suppression and stem cell maintenance. Cell 128:235–237. https://doi.org/10.1016/j.cell.2007.01.009
Wang D, Wang Y, Zou X et al (2020) Foxo1 inhibition prevents renal ischemia–reperfusion injury via camp-response element binding protein/ppar‐γ coactivator‐1α‐mediated mitochondrial biogenesis. Br J Pharmacol 177:432–448. https://doi.org/10.1111/bph.14878
Gopal K, Al Batran R, Altamimi TR et al (2021) Foxo1 inhibition alleviates type 2 diabetes-related diastolic dysfunction by increasing myocardial pyruvate dehydrogenase activity. Cell Rep 35:108935. https://doi.org/10.1016/j.celrep.2021.108935
Xu J, Yu L, Liu F et al (2023) The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: a review. Front Immunol 14:1222129. https://doi.org/10.3389/fimmu.2023.1222129
Salhotra A, Shah HN, Levi B et al (2020) Mechanisms of bone development and repair. Nat Rev Mol Cell Biol 21:696–711. https://doi.org/10.1038/s41580-020-00279-w
Guan M, Yao W, Liu R et al (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18:456–462. https://doi.org/10.1038/nm.2665
Shu HS, Liu YL, Tang XT et al (2021) Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell 28:2122–2136. https://doi.org/10.1016/j.stem.2021.08.010
Zhou BO, Yue R, Murphy MM et al (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168. https://doi.org/10.1016/j.stem.2014.06.008
Balogh E, Tolnai E, Nagy B et al (2016) Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Et Biophys Acta (Bba) - Mol Basis Disease 1862:1640–1649. https://doi.org/10.1016/j.bbadis.2016.06.003
Xiao J, Zhang G, Chen B et al (2023) Quercetin protects against iron overload-induced osteoporosis through activating the nrf2/ho-1 pathway. Life Sci 322:121326. https://doi.org/10.1016/j.lfs.2022.121326
Qian Z, Ye J, Li J et al (2022) Decrotonylation of akt1 promotes akt1 phosphorylation and activation during myogenic differentiation. J Adv Res. https://doi.org/10.1016/j.jare.2022.10.005
Zoabi M, Nadar-Ponniah PT, Khoury-Haddad H et al (2014) Rna-dependent chromatin localization of kdm4d lysine demethylase promotes h3k9me3 demethylation. Nucleic Acids Res 42:13026–13038. https://doi.org/10.1093/nar/gku1021
Jinteng L, Peitao X, Wenhui Y et al (2023) Bmal1-ttk-h2bub1 loop deficiency contributes to impaired bm-msc-mediated bone formation in senile osteoporosis. Mol Therapy - Nucleic Acids 31:568–585. https://doi.org/10.1016/j.omtn.2023.02.014
Li H, Shen X, Ma M et al (2021) Zip10 drives osteosarcoma proliferation and chemoresistance through itga10-mediated activation of the pi3k/akt pathway. J Exp Clin Cancer Res 40:340. https://doi.org/10.1186/s13046-021-02146-8
Acknowledgements
We would like to thank the animal facility at Sun Yat-sen University. The authors thank American Journal Experts for providing English language editing of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82172385, 82172349, and 82272448), the Guangdong Natural Science Foundation (2023A1515030026 and 2023A1515010531), the Futian Healthcare Research Project (FTWS2022016), the Guangdong Provincial Clinical Research Center for Orthopedic Diseases (2023B110001) and the Excellent Medical Innovation Talent Program of the Eighth Affiliated Hospital of Sun Yat-sen University (YXYXCXRC202104).
Author information
Authors and Affiliations
Contributions
Shen Huiyong, Wu Yanfeng and Xie Zhongyu initiated and supervised the project. Che Yunshu, Huang Guo, Li Jinteng, Su Zepeng, Lin Jiajie, Zheng Guan, Ye Guiwen and Yu Wenhui performed the experiments and collected the data; Che Yunshu, Li Jinteng and Huang Guo contributed to the animal models and animal analysis. Xie Zhongyu, Che Yunshu, Su Zepeng, Lin Jiajie and Yu Wenhui analyzed and discussed the data. Che Yunshu prepared the original draft manuscript, and Xie Zhongyu and Shen Huiyong edited and finalized the manuscript. All authors critically read and commented on the manuscript and approved the final version for submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The Eighth Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from all subjects included in the study in accordance with the Declaration of Helsinki. All animal experimental procedures were approved by the Animal Ethical and Welfare Committee of The Eighth Affiliated Hospital, Sun Yat-sen University.
Consent for publication
All the authors agree to publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, Z., Che, Y., Huang, G. et al. Iron-dependent KDM4D activity controls the quiescence-activity balance of MSCs via the PI3K-Akt-Foxo1 pathway. Cell. Mol. Life Sci. 81, 360 (2024). https://doi.org/10.1007/s00018-024-05376-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05376-z