Abstract
Background and aims
Hepatitis B virus (HBV)-associated liver cirrhosis (LC), a common condition with high incidence and mortality rates, is often associated with diabetes mellitus (DM). However, the molecular mechanisms underlying impaired glucose regulation during HBV-associated LC remain unclear.
Methods
Data from 63 patients with LC and 62 patients with LC-associated DM were analysed. Co-culture of NK cells and islet β cell lines were used to study the glucose regulation mechanism. A mouse model of LC was used to verify the effect of S100A8/A9 on the glucose regulation.
Results
Higher levels of interferon (IFN)-γ derived from natural killer (NK) cells and lower levels of insulin emerged in the peripheral blood of patients with both LC and DM compared with those from patients with LC only. IFN-γ derived from NK cells facilitated β cell necroptosis and impaired insulin production. Furthermore, S100A8/A9 elevation in patients with both LC and DM was found to upregulate IFN-γ production in NK cells. Consistently, in the mouse model for LC, mice treated with carbon tetrachloride (CCL4) and S100A8/A9 exhibited increased blood glucose, impaired insulin production, increased IFN-γ, and increased β cells necroptosis compared with those treated with CCL4. Mechanistically, S100A8/A9 activated the p38 MAPK pathway to increase IFN-γ production in NK cells. These effects were diminished after blocking RAGE.
Conclusion
Together, the data indicate that IFN-γ produced by NK cells induces β cell necroptosis via the S100A8/A9–RAGE–p38 MAPK axis in patients with LC and DM. Reduced levels of S100A8/A9, NK cells, and IFN-γ could be valuable for the treatment of LC with DM. Accumulation of S100A8/A9 in patients with LC may indicate the emergence of DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 250 million people suffer from hepatitis B virus (HBV) infection, and China comprises one-third of the global population [1, 2]. Sustained liver inflammation and activation of fibrogenic processes during chronic HBV infection can lead to cirrhosis, which may ultimately lead to hepatocellular carcinoma (HCC) in 25–40% of HBV carriers [3]. HBV-related cirrhosis is a primary cause of death worldwide [4]. Patients with HBV cirrhosis usually experience hospitalization, reduced quality of life, and high mortality.
Nearly 30% of patients with cirrhosis have diabetes mellitus (DM) owing to the critical role that the liver plays in glucose homeostasis [5, 6]. Patients with HBV infection who subsequently develop diabetes or metabolic syndrome have a greater chance of cirrhosis and decompensation [7]. DM is associated with an increased risk of developing HCC [8,9,10,11]. As a result of cirrhosis diagnosis and poorly controlled diabetes, patients with HBV cirrhosis have markedly higher mortality and complications of cirrhosis [12]. Liver diseases caused by HBV and diabetes are interconnected [13]. Understanding the relationship between cirrhosis and DM will help improve diabetes control in the management of HBV-associated cirrhosis.
Impaired glucose tolerance is associated with the loss of β-cells [14]. The role of β cell apoptosis in the pathogenesis of DM is well established [15]. However, the effect of β cell necroptosis on DM remains unclear owing to a paucity of associated studies. Necroptosis is morphologically similar to necrosis, but differs substantially in terms of the regulation of specific gene expression. Necroptosis exerts important effects on the development of many diseases, such as cardiovascular disease, neurodegeneration, cancer, and viral infection. Various stimuli can induce necroptosis without caspase activity. The death form of NIT-1 or INS-1 β cells caused by TNF-α is caspase-independent [16]. Moreover, hyperglycaemia and islet area loss emerged in ageing β-cell specific caspase 8 knockout mice and humans with caspase 8 mutations. Necroptosis is regulated by the protein kinase receptor-interacting serine/threonine protein kinases 1 and 3 (RIPK1 and RIPK3, respectively) [17]. Necroptosis may contribute to β cell loss since STZ-treated Ripk3−/− mice are resistant to hyperglycaemia [16]. Thus, β cell necroptosis may regulate glucose homeostasis.
Patients with liver cirrhosis (LC) have been extensively studied. There is a positive relationship between LC and diabetes risk in CHB patients with a long history [13]. This study aimed to gain a better understanding of the underlying mechanism of DM emergence. Here, we addressed the role of natural killer (NK) cell-derived interferon (IFN)-γ on β cell necroptosis.
Materials and methods
Human subjects
Healthy individuals were recruited through regular physical examination at The First Affiliated Hospital, College of Medicine, Zhejiang University. The clinical characteristics of LC and LC when accompanied with diabetes are presented in Table 1. All samples excluded HCV infection, Metabolic-dysfunction-associated fatty liver disease (MAFLD), and Metabolic dysfunction-associated steatohepatitis (MASH). The use of human blood was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University (IIT2020023A).
Animals model
C57 BL/6 male mice, 6 weeks, purchased from Hangzhou Ziyuan Experimental Animal Technical Company. Mice were intraperitoneally injected with Carbon tetrachloride (CCl4: corn oil = 1: 3, two injections per week, 160 μL/mouse). S100A8/A9 (1 μg/mouse) was injected intravenously at the same time of CCl4 injection. All experiments were performed in accordance with the institutional guidelines from the Animal Care and Use Committee of the Medical College of Zhejiang University (reference number 2021‐007). The mice were killed 24 h after CCl4 injections. The serum, liver, and pancreas were collected for further use.
Cell culture and treatment
The 1.1B4 islet β cell line (Kunming cell bank of Chinese Academy of Sciences, Kunming, China) was cultured in a RPMI 1640 medium (Sigma-Aldrich, Shanghai, China) containing 10% foetal bovine serum (FBS, Gibco, Newcastle, Australia) and 1% penicillin–streptomycin solution (Biological Industries, Shanghai, China). The βTC-6 mouse islet β cell line (Kunming cell bank of Chinese Academy of Sciences, Kunming, China) was cultured in a Dulbecco’s modified Eagle’s medium (Gibco, Newcastle, Australia) containing 10% FBS and 1% penicillin–streptomycin solution. All cells cultured at 37 °C in a 5% CO2 cell incubator.
To evaluate the death form of β cells, pan-caspase inhibitor zVAD-FMK (zVAD, MedChemExpress, Shanghai, China) and MLKL inhibitor Necrosulfonamide (NSA, MedChemExpress, Shanghai, China) were added in the culture medium. Cells were pretreated with zVAD (20 μM), NSA (1 μM), FPS-ZM1 (1 μM), or SB203580 (10 μM) for 30 min before culturing in medium containing 20% plasma from patients with LC, patients with LC and DM, or healthy people.
First, 1 \(\times {10}^{5}\) 1.1B4 islet β cells were first seeded in a 12-well plate for indirect co-culturing. The 1 \(\times {10}^{6}\) NK-92 MI cells were seeded in the upper chamber (0.4 μm aperture) 12 h later. The 5 \(\times {10}^{4}\) 1.1B4 islet β cells were first seeded in the 24-well plate for direct co-culturing. The 5 \(\times {10}^{3}\) NK-92 MI cells were seeded directly in the wells 12 h later.
Flow cytometry
Immunophenotypic analysis of whole blood or peripheral blood mononuclear cells (PBMCs) was conducted using monoclonal antibodies (mAbs) for the following: PE-Cy7-anti-Human CD56 (Biolegend, California, USA), PE-Cy7-anti-Human CD11b (Biolegend, California, USA), Percp-anti-Human CD45 (BD, NJ, USA), APC-Cy7-anti-Human CD14 (BD, NJ, USA), PE-anti-Human CD1c (Biolegend, California, USA), FITC-anti- Human CD3 (BD, NJ, USA), APC-Cy7-anti-Human HLA-DR (Biolegend, California, USA), PE-anti-Human RAGE (Abcam, Cambridge, UK) PE-anti-Human CD107a (Biolegend, California, USA), and APC-anti-Human NCR1 (Biolegend, California, USA). Intracellular proteins used were APC-anti-Human IFN-γ (BD, NJ, USA). Concentrations of plasma IFN-γ, TNF-α, IL-2, IL-4, IL-10, IL-6, IL-17, and IL-1β were detected using a human Th1/Th2/Th17 subsets detection kit (flow cytometry fluorescence luminescence method) (Jiangxi Cellgene Biotech Co. Ltd, Jiangxi, China). These values were determined based on the manufacturer’s instructions. The fluorochrome-conjugated single cells or cytokines were detected using BD FACS Canto II. The acquired data were analysed using FlowJo 10 or CBA-FCAP Array V3 software.
Propidium iodide (PI) staining
Single cells were stained with antibodies, followed by PI staining (Lianke Biotech, AP101-100-kit, Hangzhou, China) according to the manufacturer’s guidelines. Subsequently, flow cytometry (BD FACS Canto II, USA) was used to detect single cell suspensions. Data acquired were analysed using the FlowJo software.
Enzyme-linked immunosorbent assay
The concentrations of IFN-γ (BioLegend, USA), S100A8/A9 (BioLegend, USA), and insulin (Reddot Biotech, Canada; Abcam, UK) in plasma or cell culture supernatant were examined by enzyme-linked immunosorbent assays (ELISA). As described in a previous study [17], these were detected according to the manufacturer’s instruction.
Quantitative real-time polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from 1.1B4 and βTC-6 cells using TRIzol reagent (Takara, Kyoto, Japan). SYBR Green PCR Master Mix (Vazyme, Nanjing, China) was used to perform quantitative real-time PCR (qPCR) assays of mRNA. The qPCR conditions were: 95 °C for 30 s, followed by 40 cycles at 95 °C for 10 s, 60 °C for 30 s. Lastly, one PCR cycle was conducted at 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. Expression levels of target genes were normalised to the expression level of GAPDH (internal control) and calculated based on the comparative cycle threshold (CT) method (2−ΔΔCT).
Western blot analysis
1.1B4 and βTC-6 cells (5 \(\times {10}^{6}\) cells) were treated with 100 μL RIPA Lysis Buffer (Beyotime Biotechnology, Shanghai, China) containing Halt protease (100 \(\times\), Selleck, HOU, USA) for 30 min at 4 °C, followed by centrifugation at 13,523\(\times\)g at 4 °C for 10 min. The supernatant was collected and loaded onto a 10% polyacrylamide gel for electrophoresis.
Data-independent acquisition mass spectrometry (DIA-MS)
Plasma from patients was used for DIA-MS measurements. The resulting tandem MS/MS data were processed using the DIA-NN (v 1.8) search engine. The fold change (FC) was calculated as the average of the comparisons between biological replicates to determine differentially expressed proteins. The expression of proteins with a fold change > 1.5 and p < 0.05 were considered significant.
Statistical analyses
Data analysis was performed using the Prism 9 software (GraphPad, San Diego, CA, USA). Comparisons between two groups were done using the Student’s t test. Comparisons between more than two groups were done using One-way ANOVA followed by Tukey’s multiple comparison post hoc test. Data are presented as the mean ± S.E.M. and are representative of at least three independent experiments. Statistical significance was set at p < 0.05. *p ≤ 0.05, **p < 0.01, ***p < 0.001, ****p ≤ 0.0001, nsp > 0.05.
Results
Plasma factors from the LC + DM group induced β cell necroptosis
A fraction of patient with HBV-associated cirrhosis also have diabetes [18]. The underlying mechanism was investigated by comparing the data from 63 patients with LC and 62 patients with both LC and DM (Table 1). The composition of age, body mass index (BMI), gender, disease stage, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and total bilirubin (TBil) levels were similar between the LC and LC + DM groups. The levels of fasting plasma glucose (FPG) and prothrombin time (PT) were markedly higher in the LC + DM group than those of the LC group.
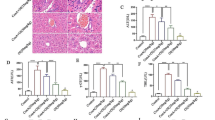
The function of β cells is influenced by the local environment in the islet, which is influenced by peripheral blood. Therefore, β cells were cultured in a medium containing plasma from the patients. The β cells cultured with LC + DM plasma displayed lower insulin levels and higher PI positive (PI+) percentages (Fig. 1a–b). Treatment with plasma from the LC + DM group resulted in β cell death, which was unaffected by zVAD and decreased by NSA (Fig. 1b, c). The expression of MLKL and p-MLKL significantly increased in the LC + DM group and decreased following NSA treatment (Fig. 1c, d). Analysis of the differentially expressed protein in plasma by MS/MS showed 399 upregulated and 39 downregulated proteins (Fig. 1e). Among the top 30 pathways changes, the necroptosis pathway was markedly activated in the LC + DM group according to analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (Fig. 1f). These data indicate that plasma from the LC + DM group induced β cell death via caspase-independent and MLKL-dependent necroptosis.
Plasma factors induce 1.1B4 pancreatic β cells necroptosis in patients with LC and DM. a–d After pretreating with NSA (1 μM), zVAD (20 μM), or PBS for 30 min, the 1.1B4 pancreatic β cells were cultured with medium containing plasma from HC, LC, or LC + DM for 24 h. a The pancreatic β cells were stained with DAPI (blue) and insulin (red). b The PI+ percentages and PI MFI of β cells were detected by flow cytometry. c The mRNA levels of MLKL were detected by qPCR. d The 1.1B4 pancreatic β cells were stained with DAPI (blue) and p-MLKL (red). e Heatmap of all differentially expressed proteins in plasma. f The proteins were divided into 4 groups according to fold changes. And the KEGG pathways were clustered according to every Q group. Data are presented as means ± S.E.M.; *p < 0.05, ***p < 0.001, nsp > 0.05
IFN-γ induced β cell necroptosis in the LC + DM group
Dysregulated cytokine production is considered as one of the main triggers of DM [19]. We compared the cytokine profiles in the LC + DM group with those in the LC group and healthy controls (HCs) to determine the key factors in LC + DM plasma responsible for this effect. IFN-γ and IL-17 levels were particularly increased in the LC + DM group (Fig. 2a). However, no significant differences were observed in the expression of TNF-α, IL-6, IL-4, IL-2, and IL-10 (Fig. 2a and S1a).
IFN-γ induce β cells necroptosis in patients with LC and DM. a The concentration of IFN-γ, IL-17, and TNF-α in the plasma from patients with LC and patients with LC and DM were detected by flow cytometry. n = 37–43. b Correlation analyses of inflammatory cytokines and glucose concentrations, as determined by Spearman’s rank test (***p < 0.001). p = 0.0008 for IFN-γ, p = 0.8065 for IL-17, p = 0.9098 for TNF-α, p = 9569 for IL-6, p = 0.2565 for IL-4, p = 0.7794 for IL-2, p = 0.8569 for IL-10. n = 34–37. c After stimulated by IFN-γ (100 ng/ml), IL-17 (100 ng/ml), and TNF-α (50 ng/ml) for 24 h, the 1.1B4 pancreatic β cells were stained with DAPI (blue) and insulin (red). d–g The 1.1B4 pancreatic β cells were stimulated with TNF-α, IFN-γ, or TNF-α and IFN-γ for 24 h. The culture medium was collected for detection of insulin (d), PI+ cells percentages (e), MLKL mRNA levels (f), MLKL and p-MLKL protein levels (g). h–k Mouse pancreatic βTC-6 cells were stimulated with TNF-α (50 ng/ml), IFN-γ (100 ng/ml), or TNF-α (50 ng/ml) and IFN-γ (100 ng/ml) for 24 h. The culture medium was collected for detection of insulin (h), PI+ cells percentages (i), MLKL mRNA levels (j), and MLKL and p-MLKL protein levels (k). Data are presented as means ± S.E.M.; *p < 0.05, **p < 0.01, ****p ≤ 0.0001, nsp > 0.05
Correlation analyses of inflammatory cytokines and glucose concentration revealed that IFN-γ was positively correlated with glucose, which contrasts with IL-17, IL-10, IL-6, TNF-α, IL-2, and IL-4 (Fig. 2b and S1b–h). Human pancreatic 1.1B4 β cells were stimulated with IFN-γ, IL-17, TNF-α, IL-6, and IL-4 to determine the effect on insulin production by β cells. IFN-γ reduced insulin levels by decreasing β cell numbers (Fig. 2c and S1i). To increase the cytotoxicity effects of IFN-γ, we combined TNF-α which can amplify the cytotoxicity of IFN-γ [20]; therefore, human pancreatic 1.1B4 β cells and mouse pancreatic βTC-6 cells were stimulated with TNF-α, IFN-γ, or TNF-α and IFN-γ (Fig. 2d–k). The culture medium was collected to measure the insulin levels. IFN-γ or TNF-α and IFN-γ reduced insulin production by β cells (Fig. 2d, h). The β cells (1.1B4 β cells and βTC-6 β cells) were vulnerable to IFN-γ-elicited necrosis, which was aggravated by TNF-α co-treatment according to the PI+ percentage determined by flow cytometry (Fig. 2e and 2). Furthermore, p-MLKL and MLKL were detected. These results indicated that IFN-γ contributed to β cell necroptosis, which is amplified by TNF-α co-treatment, in patients with LC + DM (Fig. 2f, g, j, and k).
Increased IFN-γ was mainly from CD56bright NK cells in LC patients with DM
IFN-γ is mainly secreted by T cells, NK cells, NKT cells, and dendritic cells (DCs), and monocytes [21, 22]. The percentages of T cells, NK cells, NKT cells, and DCs, and monocytes were analysed to determine the reason for the increased IFN-γ levels. A higher percentage of NK cells was detected in the PBMCs of the LC + DM group than in those of the LC group when IFN-γ+ subsets were analysed (Fig. 3a). A comparison of the LC + DM and LC groups showed an increase in NK cells percentages in the PBMCs of the LC + DM group (Fig. 3b). The percentages of CD56 bright NK cells in IFN-γ+ subsets and CD45+ subsets were higher in the LC + DM group than those in the LC group (Fig. 3c and d). The IFN-γ MFI in CD56 bright NK cells from the LC + DM group was higher than that in the LC group (Fig. 3d). However, no significant difference was observed in CD56dim NK cells between the two groups (Fig. S2a, b).
Increased IFN-γ was mainly from CD56bright NK cells in patients with LC and DM. a–d The percentages of IFN-γ+ cells, T cells, NK cells, NKT-like cells, DCs, and monocytes in PBMCs from LC patients and LC with DM patients were detected by flow cytometry, n = 22–63. e The enrichment Bar chart shows the 20 functions of the most significant enrichment. The vertical axis represents the description information of the corresponding KEGG pathway, and the horizontal axis represents the enrichment significance P value of—Log10 conversion. f Correlation analyses of NK percentages and glucose concentration, as determined by Spearman’s rank test. n = 45. g–j NK cells separated from patients PBMCs were cocultured with the pancreatic 1.1B4 β cells for 24 h. Effect cells: target cells = 1:10. The IFN-γ (g) and insulin (h) in supernatant were detected by ELISA. n = 3. The percentages of PI+ pancreatic 1.1B4 β cells were detected by flow cytometry (i). The protein levels of p-MLKL, MLKL, p-RIP3, RIP3, p-RIP1, and RIP1 in pancreatic 1.1B4 β cells were detected by western blot (j). Data are presented as means ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001, ****p ≤ 0.0001, nsp > 0.05
The enrichment bar chart showing the 20 functions of the most significant enrichment according to protein MS showed that necroptosis and NK cell-mediated cytotoxicity were significantly enriched in the LC + DM group (Fig. 3e). Moreover, the Pearson’s correlation test revealed a clear association between NK cells or their subsets and plasma glucose concentration (Fig. 3f, S2c, and d). NK cells from the LC + DM group produced more IFN-γ than did those from the LC group when cultured with 1.1B4 β cells (Fig. 3g). NK cells from the LC + DM group showed decreased insulin production and induced more β cells necroptosis than NK cells from the LC group (Fig. 3h–j). These results indicated that NK cells induced β cells necroptosis by producing IFN-γ.
NK cells induced β cell necroptosis via increasing IFN-γ levels
NK92-MI cells treated with medium containing plasma from the LC and LC + DM groups were collected and cocultured with 1.1B4 β cells directly or separately with transwells to further investigate the manner in which NK cells performed on β cells. The LC + DM group showed increased IFN-γ and decreased insulin expression compared with the LC group (Fig. 4a, b, e, and f). Increased cell necrosis percentages (PI+ %), p-RIP1, p-RIP3, and p-MLKL expression were observed in the LC + DM plasma-treated group (Fig. 4c, d, g, and h–j). IFN-γ induces β cell necroptosis. Collectively, these results showed that NK cells from the LC + DM group induced β cells necroptosis by increasing IFN-γ secretion.
NK cells induce β cells necroptosis via increasing IFN-γ level. NK92-MI cells were cultured in 1640 medium supplemented with 20% plasma (from healthy people, patients with LC, or patients with LC and DM) and 1% penicillin–streptomycin solution at 37 ℃ for 40 h. a–d, i The NK92-MI cells were cocultured with 1.1B4 β cells directly for 24 h (effect cells: target cells = 1:10). a, b The levels of IFN-γ (a) and insulin (b) in cell culture supernatant were detected by ELISA. n = 3–4. c The percentage of PI+ cells in 1.1B4 β cells were detected by flow cytometry. n = 3–4. d The mRNA levels of MLKL in 1.1B4 β cells were detected by qPCR. n = 3. i The protein levels of p-MLKL, MLKL, p-RIP3, RIP3, p-RIP1, and RIP1 in 1.1B4 β cells were detected by western blot. e–h, j The NK92-MI cells were cocultured with 1.1B4 β cells separated by transwells for 24 h (effect cells: target cells = 10:1). e, f The levels of IFN-γ (e) and insulin (f) in cell culture supernatant were detected by ELISA. n = 3. g The percentage of PI+ cells in 1.1B4 β cells were detected by flow cytometry. n = 3. h The mRNA levels of MLKL in 1.1B4 β cells were detected by qPCR. Data are presented as means ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001, ****p ≤ 0.0001, nsp > 0.05. j The protein levels of p-MLKL, MLKL, p-RIP3, RIP3, p-RIP1, and RIP1 in 1.1B4 β cells were detected by western blot
Plasma factors activated NK cells to secrete IFN-γ and increased CD107a and RAGE levels in patients with LC and DM
Plasma from the LC and LC + DM groups were used for NK-92 MI cell culture to determine the reason for NK cell activation. We found a significant increase in CD56bright NK cells and IFN-γ MFI in CD56bright NK cells in the LC + DM group (Fig. 3d). Furthermore, the LC + DM group exhibited elevated levels of RAGE and CD107a expression in NK cells (Fig. 5a). To further investigate the effect of plasma, a medium containing heaths or patient plasma was used for NK-92 MI culture. Plasma obtained from the LC + DM group showed enhanced the levels of IFN-γ, CD107a, NCR1, and RAGE in NK-92 MI cells, particularly within the CD56bright NK cell subsets, when compared with those in the LC group. (Fig. 5b–k). These results indicated that plasma factors activate NK cells to secret IFN-γ and increase the levels of CD107a and RAGE in patients with LC and DM.
Plasma factors activate NK cells to secret IFN-γ and increase the levels of RAGE and CD107a in patients with LC and DM. a The expression of RAGE, CD107a, and NCR1 on NK cells from LC patients and LC with DM patients were detected by flow cytometry. An unpaired two-tailed Student’s t-test was conducted. n = 25–45. b–k NK92-MI cells were cultured with 1640 medium supplemented with 20% plasma (from healthy people, patients with LC, or patients with LC and DM) and 1% penicillin–streptomycin solution at 37 ºC for 40 h. The percentages of IFN-γ+ NK cells (b), RAGE+ NK cells, CD107a+ NK cells, and NCR1+ NK cells (d) were detected by flow cytometry. n = 3–4. The IFN-γ MFI in CD56bright (c) and CD56dim (h) NK cells were detected by flow cytometry. n = 4.The percentages of RAGE+ CD56bright (e) and CD56dim (i) NK cells were detected by flow cytometry. n = 3–4. The percentages of CD107a+ CD56bright (f) and CD56dim (j) NK cells were detected by flow cytometry. n = 4. The percentages of NCR1+ CD56bright (g) and CD56dim (k) NK cells were detected by flow cytometry. n = 3–4. Data are presented as means ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001, ****p ≤ 0.0001, nsp > 0.05
S100A8/A9-RAGE combination triggered NK activation via the P38 MAPK signalling pathway
Activation of MAPK pathways, such as extracellular signal-regulated kinase (ERK) and p38 kinases, leads to the release of IFN-γ and various cytokines in NK cells [22, 23]. The NF-κB and MAPK signalling pathways in NK-92 MI cells were detected after treatment with plasma from healthy people, patients with LC, or patients with LC and DM. The expression of p-p38 and IFN-γ increased in NK-92 MI cells treated with LC + DM plasma compared with NK-92 MI cells treated with plasma from patients with LC (Fig. 6a, b). Our results showed that 1.1B4 β cells did not secrete IFN-γ, and pharmacological inhibitors of p38 MAPK, SB203580, alone did not affect IFN-γ secretion in 1.1B4 β cells. Additionally, these inhibitors did not affect the expression of p-MLKL, p-RIP3, and p-RIP1 in 1.1B4 β cells (Fig. S3a). Furthermore, pharmacological inhibitors of p38 MAPK inhibited IFN-γ production in NK-92 MI cells treated with plasma from patients with LC and DM (Fig. 6b). However, p-p65 was not inhibited by SB203580 (Fig. S3b). And, SB203580 decreased the expression of p-MLKL, p-RIP3, and p-RIP1 in 1.1B4 β cells treated with LC + DM plasma (Fig. 6c). These data indicated that plasma factors from patients with LC and DM activated NK cells to secrete IFN-γ via p38 MAPK but not the p65 signalling pathway. And this activation induces necroptosis in 1.1B4 β cells.
S100A8/A9 from neutrophils activate P38 MAPK signalling pathway via binding with RAGE. a–c After pretreated with SB203580 (10 μM) or not for 30 min, the NK-92 MI cells were cultured in medium containing plasma from HC, LC, or LC + DM patients. The NF-κB and P38 MAPK signalling pathways were detected by western blot (a, b). The concentration of IFN-γ in supernatant was detected by ELISA (b). n = 4. The protein levels of p-MLKL, MLKL, p-RIP3, RIP3, p-RIP1, and RIP1 in 1.1B4 β cells were detected by western blot (c). d Volcano plot of the total amount of differentially expressed proteins. The red dots indicate a significant increase, the blue dots indicate a significant decrease, and the gray dots indicate no significant difference. e The concentrations of S100A8/A9 in plasma from patients were examined by ELISA. n = 32–40. f The percentages of neutrophils and monocytes in peripheral blood were recorded from clinical laboratory. n = 62–63. g Correlation analyses of neutrophils percentages and S100A8/A9 concentration, as determined by Spearman’s rank test. n = 26. h Correlation analyses of IFN-γ concentration and S100A8/A9 concentration, as determined by Spearman’s rank test. n = 24. i–l After culturing with medium containing 20% plasma from LC patients for 40 h, the NK-92 MI cells were stimulated with S100A8/A9 (4 μg/ml) followed by PBS or FPS-ZM1 (1 μM) treatment. i The relative expression of p-P38 and P38 was detected by western blot. j, k The IFN-γ+ NK cells (j) and PI+ 1.1B4 β cells (k) were detected by flow cytometry. The expression of p-MLKL, MLKL, p-RIP3, RIP3, p-RIP1, and RIP1 in 1.1B4 β cells was detected by western blot (k). l The levels of insulin in cell culture supernatant were detected by ELISA. Data are presented as means ± S.E.M.; *p < 0.05, ***p < 0.001, ****p ≤ 0.0001, nsp > 0.05
S100A8/A9 expression was increased in diabetic mice. The plasma expression of S100A8/A9 in the LC + DM group was markedly increased compared to that in the LC group according to MS/MS and enzyme-linked immunosorbent assay (ELISA) (Fig. 6d and e). Furthermore, the heatmaps of functional cluster analysis-based protein domain enrichment depicted that the S-100/ICaBP type calcium binding domain significantly increased in the LC + DM group compared with that in the LC group (Fig. S3c). S100A8/A9 is mainly produced by myeloid cells, such as monocytes and neutrophils [24]. We found increased percentages of neutrophils, but not monocytes (Fig. 6f). Neutrophils and S100A8/A9 were positively correlated (Fig. 6g). S100A8/A9 did not directly affect the viability of 1.1B4 β cells (Fig. S3d), and did not affect the expression of TNF-α and RAGE in NK cells (Fig. S3e, f). In addition, there was a positive correlation between S100A8/A9 and IFN-γ levels (Fig. 6h). These data indicated that S100A8/A9 derived from neutrophils may increase IFN-γ production by activating the p38 signalling pathway in NK cells.
The 1.1B4 β cells were cocultured with NK-92 MI cells which treated with plasma from patients with LC, plasma from patients with LC and S100A8/A9, or plasma from patients with LC, S100A8/A9, and FPS-ZM1 (Fig. 6i–l). The levels of p-p38 and IFN-γ in NK-92 MI cells were increased by S100A8/A9 and blocked by FPS-ZM1 (Fig. 6i, j). Meanwhile, the PI+ %, the protein levels of p-MLKL, p-RIP3, and p-RIP1 in 1.1B4 β cells increased in the LC + S100A8/A9 group and decreased in LC + S100A8/A9 + FPS-ZM1 group (Fig. 6k). As expected, the insulin levels in the supernatant decreased in the LC + S100A8/A9 group and increased in the LC + S100A8/A9 + FPS-ZM1 group (Fig. 6l). Collectively, these results showed that S100A8/A9 from neutrophils activate the p38 MAPK signalling pathway by binding with RAGE.
S100A8/A9 impaired glucose hemostasis in CCl4-induced liver fibrosis model by increasing NK-derived IFN-γ
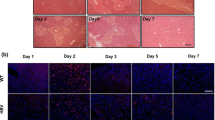
To mimic the condition of cirrhosis, mice were intraperitoneally injected with CCl4. S100A8/A9 was injected intravenously. The H&E and Masson’s trichrome staining of the livers indicated that there was no noteworthy difference in the degree and distribution of liver fibrosis (blue) between the CCl4 and the CCl4 + S100A8/A9 administration groups (Fig. 7a). Serum ALT and AST levels were similar in both groups (Fig. 7b). H&E staining of pancreas tissue showed that the islets in the CCl4 + S100A8/A9 group were destroyed and became less abundant than those in the CCl4 group (Fig. 7a). Insulin levels were also detected by ELISA and immunofluorescence (IF), which showed that the insulin levels were decreased in the S100A8/A9 treated group (Fig. 7c and j). Serum glucose levels increased in the S100A8/A9 treated group (Fig. 7d). Furthermore, the levels of p-MLKL were increased in the S100A8/A9 treated group (Fig. 7j). The concentration of IFN-γ and the percentages of IFN-γ+ NK cells from the liver and pancreas in the CCl4 + S100A8/A9 group were higher than those in the CCl4 group (Fig. 7e–i). These results were in accordance with those from human blood samples. However, the percentages of CD107a+ NK cells in the liver and pancreas were similar between these two groups (Fig. S4a, b). These results showed that S100A8/A9-activated IFNγ+ NK cells drive β-cell necroptosis in a CCl4-induced liver fibrosis model.
S100A8/A9 impaired glucose hemostasis in CCl4-induced liver fibrosis model by increasing NK-derived IFN-γ. The mice were injected with CCl4 intraperitoneally and S100A8/A9 intravenously for 3 weeks. The serum, livers and pancreas were collected. n = 5. a The H&E (left) and Masson’s trichrome staining (right) of liver. The H&E staining of pancreas. b The levels of ALT and AST in serum were examined by dry biochemical instrument. c–e The levels of insulin (c), Glucose (d) and IFN-γ (e) in serum were detected by ELISA. f–i The levels of IFN-γ in NK cells from livers (f, h) and pancreas (g, i) were detected by flowcytometry. n = 4–5. Data are presented as means ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001, nsp > 0.05. j The expression levels of insulin (green) and p-MLKL (red) in pancreas were detected by IF
Discussion
Herein, we collected samples from patients with HBV-related cirrhosis, excluding those related to HCV, MAFLD, and MASH. In the HBV background, we demonstrated that plasma from patients with LC and DM increased β cell necroptosis, but not apoptosis. S100A8/A9 increase in patients with LC and DM induced NK cell secretion of IFN-γ by activating the P38 MAPK signalling pathway.
β cells synthesise, store, and secrete insulin, which is a key regulator of glucose homeostasis [25]. Diabetes is characterized by chronic hyperglycaemia and closely related to inflammatory stress-induced β cell loss [26], which causes decreased insulin and impaired glucose tolerance. We postulated that the plasma from patients with LC with DM promotes β cell death. Molecular mechanisms that modulate pancreatic β cells survival include necroptosis, autophagy, and pyroptosis [25]. This study found that plasma from patients with LC accompanied by DM increased β cell necroptosis.
Apoptosis can be induced by many stimuli that induce necroptosis under conditions of caspase inhibition. The secretion of proinflammatory cytokines, such as IL-1β, TNF-α, and IFN-γ, by infiltrating lymphocytes is important. Aberrant IFN-γ expression is associated with several autoinflammatory and autoimmune diseases [27, 28]. We found that the plasma levels of IFN-γ and glucose were positively correlated with each other. IFN-γ has also been implicated in insulin-dependent diabetes. Moreover, co-treatment with TNF-α and IFN-γ strongly elicits primary β-cells necroptosis [16, 29, 30]. Consistently, our results showed that IFN-γ increases the β cell necroptosis.
Human NK cells can be divided into two subsets based on their CD56 surface density–CD56bright and CD56dim–each with different phenotypic characteristics. Engagement of CD56 can induce NK cell activation resulting in degranulation, IFN-γ secretion, and morphological changes. This makes CD56 a potential co-activating receptor in NK cells [31]. Moreover, CD56 has been shown to regulate human NK cell cytotoxicity [32]. The CD56dim NK cell subset is more naturally cytotoxic, while the CD56bright subset can produce a large number of cytokines [33]. Our data indicated that the increased IFN-γ derived from NK cells was mainly from the CD56bright NK cell subset.
We verified the effect of NK cells from the peripheral blood of different patients or NK-92 MI cells treated with plasma from different patients on β cells by coculture. The PI+% of the direct coculture group and the transwell coculture group was similar. Therefore, the IFN-γ secreted by NK cells may be a major cause of β cells necroptosis.
Cytotoxicity occurs after engaging activating receptors, including Nkp46 (NCR1 in mice). Activation of NCR1 is followed by direct cytotoxicity mediated by perforin and proinflammatory cytokines [34]. Additionally, RAGE involves in the inflammatory process of DM [35,36,37]. Therefore, the expression of NCR1, CD107a, and RAGE in NK cells was detected. Increased RAGE expression was observed in NK cells from patients with LC and DM compared with those from patients with LC only.
RAGE expression is high in human DM and in animal models of DM, and it mediates deleterious effects on various organs [38, 39]. Activation of RAGE induces the expression of different NF-κB-regulated genes that encode proinflammatory cytokines [40]. It is thought that RAGE may be triggered by the immunoglobulin superfamily and a variety of inflammatory molecules, including calgranulins (S100) and high-mobility group protein 1 (HMBG1) [38]. The levels of AGE and HMBG1 showed no significant differences in the LC and LC + DM groups according to MS/MS, while S100A8/A9 was elevated in patients with LC and DM according to our data. The upregulation of S100A9 was involved in the development of MAFLD with DM [41]. S100A8/A9 has been used as a as a diagnostic and therapeutic target of diabetes mellitus [42]. Additionally, S100/calgranulin proteins are present at sites of inflammation at concentrations that activate RAGE in vivo [43]. S100A8/A9 can activate NK cells via interaction with RAGE in tumours [44]. Therefore, the impact of S100A8/A9 on IFN-γ release by NK cells and the associated signalling pathways were studied. The results showed that S100A8/A9 could activate the P38 MAPK signalling pathway and induce IFN-γ secretion by binding to RAGE.
This is the first report showing the expression of RAGE and its ligand S100A8/A9 in patients with LC and DM and providing further analysis of the underlying mechanism. Moreover, the effects of RAGE and S100A8/A9 on the biological function of NK cells were also tested. A preliminary mechanism was also confirmed for RAGE and S100A8/A9 in NK cell biology. This study provides a new target for the treatment of LC with DM and provides an important basis for the clinical monitoring indicators of patients with LC. However, the study limitations include a small sample size for research and a lack of further verification using animal experiments. These problems require further investigation in future research. Moreover, animal experiments lacking HBV infection background cannot replicate the conditions of HBV-associated LC in humans.
Although this article only discusses the mechanism of diabetes occurrence in HBV-related liver cirrhosis, reports indicate a close association between HCV infection and diabetes. Compared to non-infected individuals, a higher proportion of HCV-infected individuals are reported to have diabetes [45, 46]. The association between HCV infection and diabetes has been documented by several epidemiological studies in different ethnic groups, including a variety of endocrine abnormalities, cytokine production, insulin receptor insensitivity, liver injury, and autoimmune mediated mechanisms [47]. However, the mechanisms involving NK cells in causing diabetes during HCV infection are still poorly understood. Therefore, further research in the future could explore whether the mechanisms discussed in this article are applicable to HCV infection. In addition, the prevalence of diabetes in patients with MAFLD and MASH has also significantly increased. Patients with MAFLD have a greater than two-fold increased risk of developing DM [48]. However, unlike the mechanism of diabetes onset caused by HBV-related liver cirrhosis, MAFLD and MASH share many common risk factors with diabetes, such as obesity (especially abdominal obesity), insulin resistance, hypertension, and lipid abnormalities, which interact to increase the risk of diabetes [49].
Overall, our study revealed that IFN-γ secreted by NK cells leads to β cell necroptosis, which is caspase-independent and MLKL-dependent. Furthermore, S100A8/A9 activates the P38 MAPK signalling pathway in NK cells to produce IFN-γ by binding with RAGE. This finding suggests that modulation of S100A8/A9, NK cells, and IFN-γ could be of great value for the treatment of LC with DM. Moreover, our findings indicate that abnormal levels of S100A8/A9, NK cells, and IFN-γ in patients with LC may indicate the emergence of DM.
Data availability
The data used for this study, though not available in a public repository, will be made available to other researchers upon reasonable request.
References
Horng JH, Lin WH, Wu CR, Lin YY, Wu LL, Chen DS, Chen PJ (2020) HBV X protein-based therapeutic vaccine accelerates viral antigen clearance by mobilizing monocyte infiltration into the liver in HBV carrier mice. J Biomed Sci 27:70
Su S, Wong WC, Zou Z, Cheng DD, Ong JJ, Chan P, Ji F et al (2022) Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health 10:e278–e287
Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG et al (2018) Hepatitis B virus infection. Nat Rev Dis Primers 4:18035
Gines P, Krag A, Abraldes JG, Sola E, Fabrellas N, Kamath PS (2021) Liver cirrhosis. Lancet 398:1359–1376
Lee WG, Wells CI, McCall JL, Murphy R, Plank LD (2019) Prevalence of diabetes in liver cirrhosis: a systematic review and meta-analysis. Diabetes Metab Res Rev 35:e3157
Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR et al (2014) Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 60:2008–2016
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G et al (2012) Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 130:1639–1648
Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, Yamashita T et al (2007) Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol 102:1939–1946
Sun X, Chi X, Zhao Y, Liu S, Xing H (2022) Characteristics and clinical significance of intestinal microbiota in patients with chronic Hepatitis B cirrhosis and type 2 diabetes mellitus. J Diabetes Res 2022:1826181
Li X, Xu H, Gao P (2018) Diabetes mellitus is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis b virus infection in China. Med Sci Monit 24:6729–6734
Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N et al (2008) Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol 23:1739–1746
Hsiang JC, Gane EJ, Bai WW, Gerred SJ (2015) Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol 30:591–599
Zhao Y, Xing H, Wang X, Ou W, Zhao H, Li B, Li Y et al (2019) Management of diabetes mellitus in patients with chronic liver diseases. J Diabetes Res 2019:6430486
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110
Rhodes CJ (2005) Type 2 diabetes-a matter of beta-cell life and death? Science 307:380–384
Contreras CJ, Mukherjee N, Branco RCS, Lin L, Hogan MF, Cai EP, Oberst AA et al (2022) RIPK1 and RIPK3 regulate TNFα-induced beta-cell death in concert with caspase activity. Mol Metab 65:101582
Li X, Zhang Y, Wang J, Li Y, Wang Y, Shi F, Hong L et al (2022) zVAD alleviates experimental autoimmune hepatitis in mice by increasing the sensitivity of macrophage to TNFR1-dependent necroptosis. J Autoimmun 133:102904
Yip TC, Lee HW, Chan WK, Wong GL, Wong VW (2022) Asian perspective on NAFLD-associated HCC. J Hepatol 76:726–734
Wibowo H, Harbuwono DS, Tahapary DL, Kartika R, Pradipta S, Larasati RA (2021) Impact of sodium butyrate treatment in LPS-stimulated peripheral blood mononuclear cells of poorly controlled type 2 DM. Front Endocrinol (Lausanne) 12:652942
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M et al (2021) Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184(149–168):e117
Frey T, Swade K, Zwecker L, Llewellyn T, Vogt E, Monteferante K, English H (2019) Monocyte production of IFN-γ is interleukin-12 dependent in a model of mevalonate kinase deficiency. J Interferon Cytokine Res 39:364–374
Schoenborn JR, Wilson CB (2007) Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 96:41–101
Mavropoulos A, Sully G, Cope AP, Clark AR (2005) Stabilization of IFN-γ mRNA by MAPK p38 in IL-12- and IL-18-stimulated human NK cells. Blood 105:282–288
Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA et al (2020) Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 141:1080–1094
Rojas J, Bermudez V, Palmar J, Martinez MS, Olivar LC, Nava M, Tomey D et al (2018) Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res 2018:9601801
Hu S, Kuwabara R, Beukema M, Ferrari M, de Haan BJ, Walvoort MTC, de Vos P et al (2020) Low methyl-esterified pectin protects pancreatic beta-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr Polym 249:116863
Barrat FJ, Crow MK, Ivashkiv LB (2019) Interferon target-gene expression and epigenomic signatures in health and disease. Nat Immunol 20:1574–1583
Stanbery AG, Newman ZR, Barton GM (2020) Dysregulation of TLR9 in neonates leads to fatal inflammatory disease driven by IFN-gamma. Proc Natl Acad Sci USA 117:3074–3082
Stephens LA, Thomas HE, Ming L, Grell M, Darwiche R, Volodin L, Kay TW (1999) Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology 140:3219–3227
Irawaty W, Kay TW, Thomas HE (2002) Transmembrane TNF and IFN-α induce caspase-independent death of primary mouse pancreatic beta cells. Autoimmunity 35:369–375
Picard LK, Claus M, Fasbender F, Watzl C (2022) Human NK cells responses are enhanced by CD56 engagement. Eur J Immunol 52:1441–1451
Gunesch JT, Dixon AL, Ebrahim TA, Berrien-Elliott MM, Tatineni S, Kumar T, Hegewisch-Solloa E et al (2020) CD56 regulates human NK cell cytotoxicity through Pyk2. Elife. https://doi.org/10.7554/eLife.57346
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, Scope A et al (2018) NKp46 receptor-mediated interferon-gamma production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity 48:396–398
Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM, Hsieh SC, Yu CL (2020) The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) Signaling Inhibitors As Novel Therapeutic Strategies For Patients With AGE-related diseases. Molecules 25(23):5591
Wang X, Li Q, Han X, Gong M, Yu Z, Xu B (2021) Electroacupuncture alleviates diabetic peripheral neuropathy by regulating glycolipid-related GLO/AGEs/RAGE axis. Front Endocrinol (Lausanne) 12:655591
Wu TT, Chen YY, Chang HY, Kung YH, Tseng CJ, Cheng PW (2020) AKR1B1-induced epithelial-mesenchymal transition mediated by RAGE-oxidative stress in diabetic cataract lens. Antioxidants (Basel) 9(4):273
Machado-Lima A, Lopez-Diez R, Iborra RT, Pinto RS, Daffu G, Shen X, Nakandakare ER et al (2020) RAGE mediates cholesterol efflux impairment in macrophages caused by human advanced glycated albumin. Int J Mol Sci 21(19):7265
Zheng DL, Wu QR, Zeng P, Li SM, Cai YJ, Chen SZ, Luo XS et al (2022) Advanced glycation end products induce senescence of atrial myocytes and increase susceptibility of atrial fibrillation in diabetic mice. Aging Cell 21:e13734
Petriv N, Neubert L, Vatashchuk M, Timrott K, Suo H, Hochnadel I, Huber R et al (2021) Increase of alpha-dicarbonyls in liver and receptor for advanced glycation end products on immune cells are linked to nonalcoholic fatty liver disease and liver cancer. Oncoimmunology 10:1874159
Cai Q, Zhu J, Cui X, Xia Y, Gao H, Wang X, Cheng M (2022) S100A9 promotes inflammatory response in diabetic nonalcoholic fatty liver disease. Biochem Biophys Res Commun 618:127–132
Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J (2018) S100A8/A9 in inflammation. Front Immunol 9:1298
Yao D, Brownlee M (2010) Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59:249–255
Narumi K, Miyakawa R, Ueda R, Hashimoto H, Yamamoto Y, Yoshida T, Aoki K (2015) Proinflammatory proteins S100A8/S100A9 activate NK cells via interaction with RAGE. J Immunol 194:5539–5548
Elhawary EI, Mahmoud GF, El-Daly MA, Mekky FA, Esmat GG, Abdel-Hamid M (2011) Association of HCV with diabetes mellitus: an Egyptian case-control study. Virol J 8:367
Su PY, Chen YY, Yen HH, Huang SP, Liu IL, Zeng YH, Hsu YC et al (2021) Strategy for the micro-elimination of hepatitis C among Patients with diabetes mellitus—a hospital-based experience. J Clin Med 10(11):2509
Negro F, Alaei M (2009) Hepatitis C virus and type 2 diabetes. World J Gastroenterol 15:1537–1547
Ferguson D, Finck BN (2021) Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol 17:484–495
Lee WLWP, Yang ST, Liu CH, Chang WH, Lee FK (2022) To do one and to get more: Part II. Diabetes and metabolic dysfunction-associated fatty liver diseases. J Chin Med Assoc 85:1109–1119
Funding
This work was supported by the National Key Research and Development Program of China (2021YFA1301100), the Fundamental Research Funds for the Central Universities (2022ZFJH003), Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022012B).
Author information
Authors and Affiliations
Contributions
Xuehui Li, Zhongwen Wu, Lanjuan Li and Hongyan Diao contributed to the conception of the study; Xuehui Li, Liang Hong, Minghui Ru, Rui Cai, and Baohua Wang performed the experiment; Xuehui Li and Hongyan Diao contributed significantly to analysis and manuscript preparation; Xuehui Li, Baohua Wang, Zhongwen Wu, Lanjuan Li, and Hongyan Diao helped perform the analysis with constructive discussions; All authors contributed to writing of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no actual or potential conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Hong, L., Ru, M. et al. S100A8/A9-activated IFNγ+ NK cells trigger β-cell necroptosis in hepatitis B virus-associated liver cirrhosis. Cell. Mol. Life Sci. 81, 345 (2024). https://doi.org/10.1007/s00018-024-05365-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05365-2