Abstract
The pathogenesis of renal calcium-oxalate (CaOx) stones is complex and influenced by various metabolic factors. In parallel, palmitic acid (PA) has been identified as an upregulated lipid metabolite in the urine and serum of patients with renal CaOx stones via untargeted metabolomics. Thus, this study aimed to mechanistically assess whether PA is involved in stone formation. Lipidomics analysis of PA-treated renal tubular epithelial cells compared with the control samples revealed that α-linoleic acid and α-linolenic acid were desaturated and elongated, resulting in the formation of downstream polyunsaturated fatty acids (PUFAs). In correlation, the levels of fatty acid desaturase 1 and 2 (FADS1 and FADS2) and peroxisome proliferator-activated receptor α (PPARα) in these cells treated with PA were increased relative to the control levels, suggesting that PA-induced upregulation of PPARα, which in turn upregulated these two enzymes, forming the observed PUFAs. Lipid peroxidation occurred in these downstream PUFAs under oxidative stress and Fenton Reaction. Furthermore, transcriptomics analysis revealed significant changes in the expression levels of ferroptosis-related genes in PA-treated renal tubular epithelial cells, induced by PUFA peroxides. In addition, phosphatidyl ethanolamine binding protein 1 (PEBP1) formed a complex with 15-lipoxygenase (15-LO) to exacerbate PUFA peroxidation under protein kinase C ζ (PKC ζ) phosphorylation, and PKC ζ was activated by phosphatidic acid derived from PA. In conclusion, this study found that the formation of renal CaOx stones is promoted by ferroptosis of renal tubular epithelial cells resulting from PA-induced dysregulation of PUFA and phosphatidic acid metabolism, and PA can promote the renal adhesion and deposition of CaOx crystals by injuring renal tubular epithelial cells, consequently upregulating adhesion molecules. Accordingly, this study provides a new theoretical basis for understanding the correlation between fatty acid metabolism and the formation of renal CaOx stones, offering potential targets for clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is one of the most common urological diseases, and its incidence has exhibited rapid growth in recent years. Annual new incidence of kidney stones is approximately 100–400/105 in Europe, 1-19.1% in Asia, and 5.8% in China [1,2,3]. In addition, the substantial recurrence rates of urolithiasis ranging from 6 to 23% within the first year post-treatment and surging to 50% after a five-year interval, present a significant treatment challenge [4, 5]. Urolithiasis can be categorized into two principal types-calcium stones and non-calcium stones, with calcium oxalate (CaOx) stones constituting the majority and accounting for 65–80% of cases [5, 6].
The etiology and pathogenesis of renal CaOx stones are complex and unclear, and many factors are involved, including metabolic, genetic, environmental factors (such as the geographical region, climate, occupation, and diet), and local factors in the urinary tract (such as anatomical abnormalities, infection, obstruction, and foreign bodies). At present, effective and accurate preventive and therapeutic strategies for CaOx stones are still lacking.
Recent reports suggest that urolithiasis, as a systemic disorder, is closely related to metabolic syndrome (MS) [7, 8]. MS is associated with various metabolic disorders, including obesity (mainly abdominal obesity), fasting and postprandial hyperglycemia, hypertension, and dyslipidemia. In our previous report, the proportion of patients diagnosed with MS among urolithiasis patients was estimated at 29.9% (272/910) [9].
Studies have revealed that dyslipidemia is an independent risk factor for the formation of urinary calculi. In a retrospective study involving 52,184 patients, abnormal levels of blood lipids were found to correlate with an increased risk of kidney stones (hazard ratio, 2.2; 95% confidence interval,1.9–2.5; p < 0.001) [10]. Additionally, in our previous study, the proportion of patients accompanied by dyslipidemia among urolithiasis patients was estimated at 61.8% (562/910) [9]. This rate is much higher than the overall incidence of dyslipidemia in Chinese adults (≥ 35 years old), which stands at 34.7%. Although the correlation between dyslipidemia and the incidence of urolithiasis has become evident, the precise mechanism whereby dyslipidemia influences stone formation remains unclear.
Free fatty acids (FFAs), which encompass saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and ω 6- and ω 3-polyunsaturated fatty acids (PUFAs), are intermediate factors in lipid metabolism. Pathological upregulation of serum FFAs can stimulate oxidative stress, induce excessive cellular release of reactive oxygen species (ROS), and damage renal tubular cells [11, 12]. Mitochondrial flavoprotein long-chain acyl-CoA dehydrogenase can catalyze the mitochondrial FA oxidation and directly produce H2O2 in diseased kidneys [13]. Injury to renal tubular epithelial cells is considered an important cause of renal stone formation. Such injuries cause changes in the membrane structure and adhesion properties of these cells, thereby promoting the deposition of stone crystals [14].
Previously, to reveal the metabolic differences between patients with renal CaOx stones and healthy people, nontargeted metabolomics has been performed on urine samples from these individuals. Consequently, palmitic acid (PA), which is a major SFA, has been found significantly increased in the urine of these patients [15]. However, whether PA is involved in the formation of renal CaOx stones is unclear. Thus, this study aimed to mechanistically assess for such an involvement.
Methods and materials
Ethical statement
This study was pre-approved by the Ethics Committee of the Second Hospital of Tianjin Medical University (No. KY2022K060). All participants were informed of the study and signed the study consent form for the collection and analysis of their urine, serum, and stone samples.
Human urine and serum samples
Urine samples were collected from 137 patients with renal CaOx stones and 103 healthy controls. In our previous study, we conducted untargeted metabolomics on these urine samples and presented the discovery of PA as a notably differential metabolite in patients with renal CaOx stones [15]. Among these 137 patients, 82 and 55 patients were new-onset and relapse cases, respectively. The clinical characteristics of these patients and healthy controls are presented in Table S1.
Additionally, in this study, serum samples were obtained from 33 new-onset patients, 32 relapsed patients, and 20 healthy controls to assess whether serum PA level was also higher in patients with renal CaOx stones than in healthy individuals. These serum samples were collected after an overnight fast and stored at -80 ℃. The baseline features of each group are summarized in Table S2. Patients diagnosed with secondary causes of urolithiasis, monogenic-induced nephrolithiasis, renal dysfunction, urinary tract infection, liver disease, parathyroid dysfunction, anemia, cancer, congenital renal or urinary tract anomalies, or pregnancy were excluded from the study.
Animal experiments
Animal experiments were conducted following the approval and protocol of the Second Hospital of Tianjin Medical University and the Declaration of Helsinki. Wild-type male mice, aged 8 weeks and weighing 22–24 g, were obtained from SPF Biotechnology (SCXK 2019-0010), and then randomly assigned to the PA-treated and untreated group. To induce renal injury by using PA, mice were placed on a regular diet containing 7.5% PA (H8780, Solarbio) by weight for 20 weeks. The diet composition was formulated based on our preliminary experiments and previous reports [16,17,18]. The control group was fed a regular diet. After the 20-week period, all the mice were sacrificed, and blood samples (> 0.5 mL each) were collected through cardiac puncture and put into 1.5 ml tubes with heparin. Additionally, their kidneys were harvested.
Via renal histological analysis and continuous monitoring of renal function, the above-mentioned regimen was verified to induce injury to renal tubular epithelial cells of these mice fed with PA. Then, mice in each group were subjected to daily intraperitoneal injections of glyoxylic acid (Gly) (G10601, Sigma, 80 mg/kg dissolved in sterile saline) to establish the model of renal CaOx stones [19, 20].
Within the 20 weeks of feeding with PA, ferrostatin-1 (Fer-1) (HY 100,579, MCE, 2 mg/kg dissolved in 5% DMSO and then diluted in sterile saline), ζ-Stat (HY-123,979, MCE, 1 mg/kg dissolved in sterile saline) and GW6471 (HY-114,263, MCE, 10 mg/kg dissolved in 5% DMSO diluted in sterile saline) were administered daily via intraperitoneal injections starting from the 16th week until the establishment of the renal CaOx stone model. The corresponding control groups received an equivalent volume of sterile saline or DMSO (< 5%).
Cell experiments
HK-2 human renal tubular epithelial cells were purchased from the American Type Culture Collection and maintained in DMEM/F-12 containing 10% fetal bovine serum (Gibco) at 37 ℃ with 5% CO2. To induce cell injury, they were plated in 96-well plates and then treated with PA (P0500, Sigma) (IC50 estimated at approximately 400 µM) for 24 h. Afterward, their viability was assessed using the MTT assay (0.5 mg/ml) (M5655, Sigma) according to the instructions of the manufacturer. For cell experiments, such as the extraction of mRNA or proteins, HK-2 cells were seeded at a density of 2 × 105 cells/well in a 6-well plate.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections of 5 μm thickness were used. After deparaffinization, rehydration, and heat-mediated antigen retrieval by using 10 mM sodium citrate, endogenous peroxidase activity was blocked using 3% hydrogen peroxide. Then the sections were incubated overnight at 4 °C with appropriately diluted primary antibodies, followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (PV-6000, Zsbio). A DAB kit (ZLI-9018, Zsbio) was used for visualization, following the instructions of the manufacturer. The antibodies used are presented in Table S3.
Immunocytofluorescence
HK-2 cells were seeded into 12-well glass plates at a density of 30,000/well. After they were fixed at room temperature for 30 min by using 4% paraformaldehyde and then permeabilized using 0.1% Triton-100 for 15 min. Afterward, the cells were subjected to immunofluorescence staining. For dual renal immunofluorescence staining for phosphatidyl ethanolamine binding protein 1 (PEBP1) and 15-lipoxygenase (15-LO), kidney sections were subjected to antigen retrieval and then incubated overnight at 4 ℃ with primary antibodies against these proteins, followed by incubation with goat anti-mouse and anti-rabbit secondary antibodies (Table S3).
Hematoxylin and eosin (HE), periodic acid-Schiff (PAS), and Von Kossa staining
Kidney sections were subjected to HE staining to assess the injury to the renal tubular epithelial cells. To observe the structural changes in the epithelium and brush border of renal tubules, a PAS assay kit (G1280, Solarbio) was used according to the instructions of the manufacturer. For quantitative analysis of CaOx crystal deposition, a Von Kossa kit (150,687, Abcam) was used according to the instructions.
Monitoring of serum creatinine (scr) and blood urea nitrogen (BUN) levels of mice
To monitor the changes in the renal function of mice fed with PA, Scr and BUN assay kits (AS0174 and AS0172, SAB) were used according to the instructions of the manufacturers. For these assessments, blood was collected from mice at 5, 10, 15, and 20 weeks.
Enzyme-linked immunosorbent assay (ELISA)
ELISA kits were adopted to measure the levels of PA (69-26485, Mskbio), hyaluronic acid (HA) (69-22935, Mskbio), phosphatidic acid (69-99778, Mskbio), and arachidonic acid (AA) (69-10011, Mskbio) following the instructions.
RNA-sequencing
To examine the effect of PA on gene transcription, three paired and biologically duplicated HK-2 cell samples that were PA-treated or untreated were collected. The total RNA of each sample was extracted with TRIzol (15,596,026, Invitrogen), and then subjected to RNA high-throughput sequencing by Cloud-Seq Biotech. Briefly, RNA libraries were constructed and library sequencing was then performed on an Illumina Novaseq 6000 instrument by using 150 cycles. High-quality clean reads were aligned to the reference genome (UCSC HG19). Subsequently, guided by the Ensembl GTF-gene annotation file, the Cuffdiff software (a component of Cufflinks) was used to derive gene-level FPKM values, representing the mRNA levels. Differential mRNA levels were identified based on fold change (FC ≥ 2) and a significance threshold of p < 0.05. Accordingly, 797 and 469 genes were upregulated and downregulated in PA-treated HK-2 cells, respectively. The dataset was deposited in the Gene Expression Omnibus under accession number GSE240279, as per NCBI guidelines.
Quantitative reverse transcription–polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells by using the TRIzol reagent (15,596,026, Invitrogen), and then 5 µg of total RNA was reverse-transcribed in a 20 µL volume by using oligo dT primers and the Revertaid First-strand cDNA synthesis kit (K1622, ThermoFisher). The resulting cDNA was then subjected to PCR analysis by using the Applied Biosystems 7900 Real-Time PCR System (ThermoFisher) and the Faststart Universal SYBR green master mix (4,913,914,001, Roche). The relative expression levels of target genes were calculated using the 2-ΔΔCt method. GAPDH was used as an internal control. The sequences of the primers used are shown in Table S4.
Western blotting
HK-2 cells that were PA-treated or untreated were lysed using RIPA buffer (R0010, Solarbio) containing 1% cocktail proteinase inhibitors (78,441, ThermoFisher). Then, samples with equal amounts of protein (30 µg) were resolved using SDS-polyacrylamide gel electrophoresis (A1010, Solarbio) and transferred onto PVDF membranes (IPVH00010, Millipore). After blocking the membranes with 5% bovine serum albumin (BSA) (A8020, Solarbio), they were incubated with primary antibodies at 4 °C overnight. Afterward, the membranes were washed three times with Tris-buffered saline containing 0.05% Tween and then incubated with HRP-conjugated secondary antibodies (1:10,000 dilution) at room temperature for 1 h. Target bands were visualized using an ECL system (5200, Tanon). The expression levels of the target proteins were calculated using Image J based on the gray values of the corresponding bands. All the primary antibodies used are listed in Table S3.
Assessment of cellular ROS, Fe2+, lipid ROS, glutathione (GSH), and 4-hydroxynonenal (4-HNE) levels, and the activity of glutathione peroxidase 4 (GPX4)
Cellular ROS levels were assessed using the 2′,7′-dichlorofluorescin diacetate probe (D6470, Solarbio). Cellular Fe2+ levels were measured using an iron assay kit (ab83366, Abcam) by following the instructions of the manufacturer. In this assay, Fe2+ reacts with an iron probe, forming a stable colored complex with a maximum absorption peak at 593 nm. Cellular glutathione levels were assessed using a GSH/GSSG ratio detection assay kit (ab138881, Abcam). This assay employs a proprietary non-fluorescent dye, which undergoes significant fluorescence upon reacting with GSH, with the absorbance measurements performed at 490/520 nm.
Cellular 4-HNE levels were measured using a lipid-peroxidation (4-HNE) assay kit (ab238538, Abcam). This kit measures the 4-HNE adducts in cell lysates by comparing the absorbance of the samples to a known 4-HNE-BSA standard curve. The GPX4 activity was evaluated using an HT glutathione peroxidase assay kit GPX4 activity (7512-100-K, Trevigen). To evaluate intracellular and membrane lipid ROS accumulation, cells were stained with BODIPY (581/591) c11 (D3861, Invitrogen). All the measurements were performed by following the instructions.
Co-immunoprecipitation
Cells treated as indicated were collected and then lysed using 500 µL cell lysis buffer (88,804, ThermoFisher). Subsequently, 2–10 ug (per 500–1000 ug protein) of mouse anti-PEBP1 primary antibody (101,504, Santa Cruz) was added to the samples, followed by overnight incubation at 4 ℃. Next, 25 µL of pre-washed Protein A/G Magnetic Beads were introduced into the samples, which were then incubated at room temperature for 1 h. Afterward, the magnetic beads were isolated and washed. Then, 100 µL of elution buffer was added to the beads, and the mixture was shaken at room temperature for 10 min. Finally, the supernatants were collected to be analyzed via western blotting.
Transmission electron microscopy
HK-2 cells treated as indicated were fixed at 4 ℃ for 2 h by using 2.5% glutaraldehyde. After the dehydration, permeabilization, embedding, and sectioning, mounting steps, the samples were analyzed by using a transmission electron microscope (SU8100, Hitachi).
Lipidomics analysis
The medium- and long-chain FA (M/LCFA) profiles of PA-treated HK-2 cells were characterized via lipidomics analysis. To this end, 1 mL of a chloroform-methanol solution was added to the cell samples, followed by sonication for 30 min. Subsequently, 2 mL of a 1% sulfuric acid-methanol solution was introduced to the supernatant, which was then heated at 80 ℃ for 30 min. Afterward, 1 mL of n-hexane was added to extract the total lipid content of the sample. Then, 500 µL of the supernatant was combined with 25 µL of methyl n-nineteenth acid, serving as an internal standard. In the gas chromatography–mass spectrometry (GC-MS) analysis, an injection volume of 1 µL and a split ratio of 10:1 were used. The lipids were resolved using an Agilent DB-WAX capillary column (30 m×0.25 mm ID×0.25 μm) in a GC system. The initial temperature was set at 50 ℃ for 3 min, followed by an increase to 220 ℃ for 5 min at a rate of 10 ℃/min. Helium served as the carrier gas at a flow rate of 1 mL/min.
For quality control, each quality-control sample was randomly inserted to assess the stability of the mass-spectrometry separation and the reliability of the results. Mass spectrometry was performed using an Agilent 7890/5975c gas-mass spectrometer, with the sample-port, ion-source, and transmission-line temperatures set at 280, 230, and 250 °C, respectively, and the electron energy set as 70 eV. Peak areas and retention times were extracted using the MSD ChemStation software, and calibration curves were generated to calculate the concentrations of the M/LCFAs in the samples.
Statistical analysis
SPSS 21.0 and GraphPad Prism 8.0 were used for data analysis and graph generation. Comparative analyses between groups were executed using either t-tests or ANOVA. A p-value < 0.05, based on a two-sided test, was considered to indicate statistically significant. All the experiments were independently repeated three times, yielding consistent results.
Results
PA was significantly upregulated in patients with renal CaOx stones
In our previous study, via metabolomics profiling and statistical analysis involving 137 patients with renal CaOx stones compared with 103 healthy controls (Fig. 1A), we identified PA as an upregulated metabolite in these patients [15]. Notably, PA was the sole upregulated fatty acyl compound with statistical significance (p < 0.05) (Fig. 1B) (Table S5). The concentration of PA was elevated in the urine of these patients (Fig. 1C). Although there was no significant difference in urine PA level between the new-onset patients and the controls (Fig. 1D), the relapse group exhibited higher PA levels than both the control and new-onset groups (Fig. 1D).
PA was increased in the urine and serum of patients with renal CaOx stones. A Schema of untargeted metabolomics was performed using the urine of 137 patients and 103 healthy controls. B PA was the only upregulated fatty acid with statistical significance (p < 0.05). C The concentration of PA in the urine of patients and the controls. D The comparison of PA levels in the urine between the new-onset and recurrent patients, and the controls. E The concentration of PA in the serum of the renal CaOx stone patients and the controls. F The comparison of PA level in the serum between new-onset and recurrent patients, and the controls. *p < 0.05, **p < 0.01, ***p < 0.001; ns not statistically significant
Furthermore, serum PA levels were also elevated in the patients compared with the control levels (Fig. 1E), with the highest levels observed in the relapse group (Fig. 1F). Interestingly, unlike the urine PA levels, serum PA levels were higher in the new-onset patients than in the controls (Fig. 1F). The identification of PA as a marker associated with the incidence and recurrence of renal CaOx stones underscores the need for further in-depth investigation.
PA induced injury to renal tubular epithelial cells
Mice were fed a diet containing 7.5% PA for 20 weeks (Fig. 2A), during which no significant difference in mortality was observed among the two groups. However, kidneys from the PA group exhibited a paler and slightly edematous appearance (Fig. 2B). Additionally, the Scr and BUN levels in the PA group showed a gradual increase (Fig. 2C and D). Although there was no notable difference in body weight between the two groups (Fig. 2E), the kidney weights of the mice fed with PA were increased after 20 weeks (Fig. 2F). Notably, histological analysis revealed that the renal tubular cells of the PA-treated mice displayed severe injuries, characterized by cell swelling, marked vacuolation, cell exfoliation, and loss of the brush borders (Fig. 2G). Furthermore, kidney injury molecule 1 (KIM-1) protein, a specific biomarker for renal tubular injury, was significantly upregulated in the PA-treated mice, compared with the control levels (Fig. 2G).
Injury of renal tubular epithelial cells was induced by PA. A Timeline of the mice feeding with PA and monitoring of renal tubular damage. B The appearance of mice kidneys from the PA group. C-D Changes in Scr and BUN of mice were measured dynamically. E-F Differences in body weight and kidney weight of the two groups. G HE and PAS staining were performed to observe the structural damage to renal tubular epithelial cells of the PA-treated mice, and KIM-1 level was detected by IHC; DCFH-DA probe was used to measure the levels of ROS in HK-2 cells, original magnification, 100×, scale bar, 50 μm. H Changes of cellular activity with gradient concentrations of PA. I The KIM-1 in HK-2 cells was detected by western blotting. *p < 0.05, **p < 0.01, ****p < 0.0001, ns not statistically significant, compared with the control. Scale bar for IHC, 100 μm
The IC50 of PA in HK-2 cells was estimated at approximately 400 µM via cell activity inhibition assay (Fig. 2H). Oxidative stress and excessive production of ROS were detected in PA-treated HK-2 cells (Fig. 2G). Numerous studies have highlighted the contributory role of ROS in the progression of kidney stones [21, 22]. Similarly, the addition of PA to the cell culture significantly upregulated KIM-1 (Fig. 2I). Taken together, these findings suggest that PA injures renal tubular epithelial cells.
More CaOx crystals adhered to renal tubular epithelial cells upon the PA-induced injury
Following the confirmation of injury to renal tubular epithelial cells after the 20th week, Gly (80 mg/kg/d) was intraperitoneally injected to induce the formation of renal CaOx stone in the respective groups (Fig. 3A). As the damage progressed in HK-2 cells and renal tubules within the PA group, major adhesion molecules, including CD44, osteopontin (OPN), and hyaluronic acid (HA), were upregulated (Fig. 3B-D). Interestingly, several mice from the PA group unexpectedly died on the 5th day of the Gly administration. Consequently, the model-building timeframe was adjusted to 5 days, at which point the kidneys were harvested.
PA boosted adhesion molecule expression and promoted CaOx crystal deposition to renal tubular epithelial cells. A Timeline for inducing renal CaOx stone formation in the respective groups. B-C Western blotting and IHC were performed to measure the adhesion molecule levels of OPN and CD44 in HK-2 cells and renal tubules. D The level of hyaluronic acid (HA) in HK-2 cells was assayed. E and E1 The deposition of CaOx crystals (dark brown) in the whole kidney was analyzed by Von Kossa staining. F and F1 CaOx crystals-FITC (green) adhered to the HK-2 cell membranes were detected with fluorescence staining, original magnification, 100×, scale bar, 50 μm. **p < 0.01, ***p < 0.001, compared with the control. Scale bar for IHC, 100 μm
More CaOx crystals were found deposited in the renal tubules of the PA-treated mice (Fig. 3E and E1). Notably, in contrast to previous studies reporting that CaOx crystals were predominantly located at the junction between the renal cortex and medulla [23, 24], we detected widespread deposition of CaOx crystals throughout the entire kidneys in the PA group (Fig. 3E).
Similarly, in HK-2 cells, more FITC-labeled CaOx crystals adhered to the cell membranes following PA treatment compared with the control level (Fig. 3F and F1). These results indicate that PA can promote renal deposition of CaOx crystals by injuring renal tubular epithelial cells.
PA activated peroxisome proliferator-activated receptor α (PPARα), which in turn upregulated FA desaturase 1 and 2 (FADS1/2) to enhance PUFA synthesis
Acting as a central player in intracellular FA metabolism, PA undergoes several significant metabolic transformations, including glyceride synthesis, desaturation, and β-oxidation (Fig. 4A). Following the PA treatment, a comprehensive shift was observed in the levels of enzymes associated with PA metabolism in both HK-2 cells and mice kidneys. Specifically, long-chain acyl-CoA synthetase 1 (ACSL1), the initiating enzyme in β-oxidation, was activated, and diglycerol acyltransferase 1 (DGAT1) was upregulated. Conversely, the activity of stearoyl-coenzyme A desaturase 1 (SCD1), a principal Δ9 desaturase involved in MUFA desaturation, was suppressed (Fig. 4B, B1 and C). In contrast, Δ5 and Δ6 desaturases, namely FADS1 and FADS2, which are pivotal in the synthesis of ω-3 and ω-6 PUFAs, were upregulated (Fig. 4B, B1 and D). Additionally, two enzymes responsible for very long-chain FA elongation, ELOVL2 and ELOVL5, were also activated (Fig. 4B, B1 and C). These changes in PA metabolism predominantly correlated with increased β-oxidation, glyceride synthesis, and PUFA metabolism, as well as diminished MUFA desaturation.
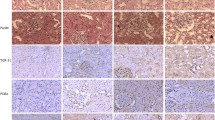
PPARα activated by PA enhanced FADS1/2 expression to upregulate PUFA synthesis. A Overview of the metabolic transformations of PA. B and B1 The levels of enzymes associated with PA metabolism were evaluated by western blotting in HK-2 cells. C The expression of enzymes associated with PA metabolism in renal tubules was detected by IHC. D The FADS1/2 levels were measured in renal tubules. E-F Heatmap and the levels of most M/LCFAs in the PA-treated HK-2 cells via lipidomics. G The mRNA expression of FADS1/2 in HK-2 cells was determined by qPCR. H The concentration of AA in HK-2 cells was detected by the Elisa kit. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns not statistically significant, compared with the control; #p < 0.05, ####p < 0.0001, compared with the PA group. Scale bar for IHC, 100 μm
Untargeted lipidomics analysis was employed to detect alterations in M/LCFA levels following PA treatment. The relative standard deviation (RSD) values of the quality-control samples were consistently < 30%, indicating the high precision of the measurements (Fig. S1 A and B). The levels of most M/LCFAs in the PA-treated HK-2 cells were disrupted (Fig. 4E), and the cellular PA (C16:0) concentration was increased by 2.36 folds (Fig. 4F).
Following the PA treatment, the levels of total SFAs in HK-2 cells increased by 1.85 folds (Fig. 4F). However, the major MUFAs oleic acid (C18:1N9) and palmitoleic acid (C16:1N7) were downregulated due to the suppression of SCD1 (Fig. 4F). Notably, major downstream PUFAs, including arachidonic acid (AA, C20:4N6), eicosapentaenoic acid (EPA, C20:5N3), were upregulated, whereas essential α-linoleic acid (LA, C18:2N6) and α-linolenic acid (ALA, C18:3N3) were downregulated (Fig. 4F).
It is well-established that PA cannot be directly converted into PUFAs in humans. PUFAs are synthesized through the desaturation and elongation of the essential PUFAs, catalyzed by enzymes such as FADS1 and FADS2, which utilize precursors such as LA and ALA. Intriguingly, the levels of LA and ALA decreased following the PA treatment (Fig. 4F). This observation led to the hypothesis that PA metabolism may influence the expression of FADS1 and FADS2, subsequently promoting the synthesis of the downstream PUFAs derived from LA or ALA.
Our results from protein-protein-interaction (PPI) analysis suggested that FADS1/2 is regulated by PPARα (Fig. S1C), which is a critical regulatory factor in FFA metabolism [25], abundantly expressed in proximal tubular cells. Dietary FFAs, including PA, serve as natural ligands that activate PPARα [26]. We found that the FADS1 and FADS2 levels in the PA-treated HK-2 cells and mice kidneys that were pre-treated with the selective PPARα antagonist GW6471 were lower than those not pre-treated with GW6471 (Fig. 4D and G). Consequently, the levels of AA, the downstream PUFA, also decreased (Fig. 4H).
PA enhanced PUFA peroxidation, which in turn induced ferroptosis of renal tubular epithelial cells
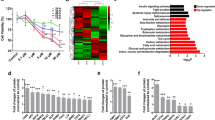
PUFAs are susceptible to oxidation, forming lipid peroxides via the Fenton Reaction when exposed to oxidative stress. This process leads to the increased production of a specific product, 4-HNE (Fig. 5A). It is well-established that lipid peroxidation of PUFAs is a prominent feature of ferroptosis. Transcriptomics analysis revealed notable alterations in the mRNA levels of many factors associated with the ferroptosis pathway (Fig. 5B). In corroboration, the mitochondria exhibited signs of atrophy and rounding, characterized by the disappearance of cristae and increased membrane density, as observed through transmission electron microscopy (Fig. 5C). Importantly, pretreatment with Fer-1 (2 µM) (Fig. S2A), a selective and efficient inhibitor of ferroptosis, mitigated these changes (Fig. 5A and C).
PA enhanced PUFA peroxidation to induce ferroptosis of renal tubular epithelial cells. A The level of lipid peroxide 4-HNE was upregulated in PA-treated HK-2 cells. B Heatmaps of the mRNA levels of genes related to the ferroptosis pathway were analyzed through transcriptomics. C changes in mitochondrial microstructure were observed through transmission electron microscopy, scale bar, 500 nm. D Western blotting was used to measure the protein levels of ACSL4, FTH1, TFRC, and GPX4 in HK-2 cells. E-G The concentration of Fe2+, GPX4 activity, and level of GSH, were detected in HK-2 cells. H The mRNA levels of SLC3A2 and SLC7A11 were detected in PA-treated HK-2 cells. I Cell activity was measured in PA-treated HK-2 cells. J The levels of 4-HNE, GPX4, TFRC, FTH1, and ACSL4 in renal tubules were evaluated by IHC. K Lipid peroxides in HK-2 cells were measured by BODIPY 581/591 C11 probe, original magnification, 250×, scale bar, 20 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the control; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the PA group. Scale bar for IHC, 100 μm
The expression levels of GPX4, transferrin receptor (TFRC), ferritin heavy chain 1 (FTH1), and acyl-CoA synthetase long-chain family member 4 (ACSL4), all of which are involved in the regulation of lipid or iron metabolism [27], were changed in the PA-treated renal tubules and HK-2 cells, and these changes could be ameliorated by Fer-1 pretreatment (Fig. 5D and J). Moreover, an overload of Fe2+ within the PA-treated HK-2 cells was observed (Fig. 5E), accompanied by a reduction in GPX4 activity (Fig. 5F) and GSH level (Fig. 5G), despite a significant upregulation of solute carrier family 3 member 2 (SLC3A2) and solute carrier family 7 member 11 (SLC7A11) (Fig. 5H) (Fig. S2D). These observations suggest that the system xc- is not constrained by PA. Fer-1 pre-treatment mitigated the reduction in GPX4 activity and GSH level (Fig. 5F and G), and the increase in Fe2+ level (Fig. 5E). In parallel, the cell viability was recovered (Fig. 5I). Notably, Fer-1 pre-treatment of HK-2 cells downregulated the PUFA peroxides in the membrane of PA-treated HK-2 cells, assessed using the Bodipy C11 fluorescence dye, lipid ROS generation was inhibited (Fig. 5K).
To evaluate the impact of ferroptosis on the expression of adhesion molecules, HK-2 cells were treated with 10 µM Erastin (Fig. S2B), a specific inducer of ferroptosis. The treated cells showed significant upregulation of OPN, CD44, and TFRC (Fig. S2C).
These findings underscore the involvement of the ferroptosis pathway in renal tubular injury and the expression of adhesion molecules.
Phosphatidic acid derived from PA activated protein kinase C (PKC) ζ, which in turn promoted the formation of the PEBP1/15-LO complex to accelerate PUFA peroxidation
In the process of ferroptosis, lipid peroxidation is not solely catalyzed by Fe2+ but is also influenced by lipoxygenases (LOs) [28]. The formation of the PEBP1/15-LO complex, which significantly promotes the generation of PUFA peroxides, was observed [29]. However, it is important to note that PEBP1 is bound to RAF1 under physiological conditions and dissociates from RAF1 upon phosphorylation [29]. Our results from PPI analysis suggested that PEBP1 undergoes phosphorylation through interaction with PKC ζ (Fig. S3A) [30].
As an atypical subtype of protein kinase C, PKC ζ can be specifically activated by a limited number of second messengers, such as phosphatidic acid [31]. Interestingly, C16:0-CoA and glycerol-3-phosphate can be converted into lysophosphatidic acid (LPA) by glycerol-3-phosphate acetyltransferase 1 (GPAT1) (Fig. 6A and A1) (Fig S3C), and LPA can subsequently generate phosphatidic acid in HK-2 cells (Fig. 6B) [32].
Phosphatidic acid increased by PA treatment activated PKC ζ to promote the formation of the PEBP1/15-LO complex, and accelerate PUFA peroxidation. A The GPAT1 level in PA-treated HK-2 cells was evaluated by western blotting. B The concentration of phosphatidic acid in PA-treated HK-2 cells. C Western blotting was performed to analyze the protein levels of PKC ζ, p-PKC ζ, PEBP1, and p-PEBP1. D PEBP1/15-LO complex in PA-treated HK-2 cells was detected in the CoIP experiment. E Immunofluorescence was used to confirm the formation of PEBP1 /15-LO complex in renal tubules, original magnification, 200×, scale bar, 25 μm. F The levels of PKC ζ, p-PKC ζ, PEBP1, and p-PEBP1 were measured by IHC in renal tubules. G The concentration of 4-HNE in PA-treated HK-2 cells after pre-treatment of Fer-1 or ζ-Stat. H Cell viability after Fer-1 or ζ-stat pretreatment in HK-2 cells was detected by MTT assay. I Hypothesis for the PUFA lipid peroxidation induced by PA promoting ferroptosis of renal tubular cells. J The levels of GPX4, TFRC, FTH1, and ACSL4 in HK-2 cells after pre-treatment of Fer-1 or ζ-Stat. **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the control; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the PA group. Scale bar for IHC, 100 μm
The increased phosphorylation of PKC ζ and PEBP1 correlated with elevated levels of phosphatidic acid in both HK-2 cells and renal tubules following PA treatment (Fig. 6C). Pre-treatment with 3 µM ζ-Stat (Fig S3B), a specific inhibitor of PKC ζ, reduced the PEBP1 phosphorylation by restraining the PKC ζ phosphorylation (Fig. 6C and F). Co-immunoprecipitation experiments showed that PEBP1 was disassociated from 15-LO upon ζ-Stat pre-treatment (Fig. 6D). Furthermore, PEBP1 and 15-LO proteins co-localized more in the renal tubules of PA-fed mice than in the controls, and this effect was suppressed by the ζ-Stat pretreatment (Fig. 6E). As the ζ-Stat pre-treatment inhibited the formation of the PEBP1/15-LO complex, PUFA peroxidation decreased, resulting in downregulation of 4-HNE (Fig. 6G). Therefore, it can be inferred that PKC ζ, activated by phosphatidic acid derived from PA, phosphorylates PEBP1 to promote PUFA peroxidation catalyzed by the PEBP1/15-LO complex (Fig. 6I). With the Fer-1 or ζ-Stat pre-treatment, the increase in the 4-HNE level was suppressed, cell viability was restored (Fig. 6H), and the levels of TFRC, FTH1, GPX4, and ACSL4 were recovered in both PA-treated HK-2 cells and the kidneys of PA-treated mice (Figs. 5J and 6J) (Fig S4).
In summary, these findings underscore that PA induces the formation of the PEBP1/15-LO complex, which in turn stimulates PUFA peroxidation and ferroptosis.
Inhibition of PUFA peroxidation reduced renal deposition of CaOx crystals
The above findings showed that PA contributes to the formation of renal CaOx stones. As the PA-induced lipid peroxidation was alleviated by the Fer-1 or ζ-Stat pre-treatments, the PA-induced injury to renal tubular epithelial cells was alleviated and the viability of PA-treated HK-2 cells was restored (Fig. 6H). Furthermore, these pre-treatments suppressed the upregulation of KIM-1, OPN, CD44 proteins, and HA in HK-2 cells and the kidneys of mice treated with PA (Fig. 7A-C) (Fig S5). Importantly, the renal deposition of CaOx crystals was reduced (Fig. 7D and D1). Consistent with the in vivo results, the adhesion of CaOx crystals to the cell membrane was significantly suppressed in PA-treated HK-2 cells pre-treated with Fer-1 or ζ-Stat, compared with those that were not pre-treated (Fig. 7E and E1).
Inhibition of PUFA peroxidation reduced renal CaOx crystal deposition. A-C KIM-1, OPN, CD44 proteins, and HA in HK-2 cells and the kidneys of mice treated with PA were measured after pre-treatment of Fer-1 or ζ-Stat. D and D1 Deposition of CaOx crystals (dark brown) in the whole kidneys was evaluated by Von Kossa staining. E and E1 Fluorescence staining was performed to observe CaOx crystals-FITC (green) adhered to the HK-2 cell membranes, original magnification, 100×, scale bar, 50 μm. **p < 0.01 compared with the control; #p < 0.05, ##p < 0.01, compared with the PA group. Scale bar for IHC, 100 μm
Discussion
Plasma FFAs constitute a vital energy reservoir and contribute to the intermediary factors in lipid metabolism. Notably, FFAs are recognized for their capacity to induce injury to renal tubular epithelial cells, primarily through their lipotoxic effects [11, 13, 33,34,35]. Via nontargeted metabonomics, PA has been found to be the only upregulated FA among all the FFAs between patients with renal CaOx stones and healthy controls.
Previous studies have reported that excessive PA can increase myocardial FFA uptake and lead to mitochondrial structural remodeling with a significant reduction in minimum diameter, accompanied by loss of the mitochondrial reticulum and increased mitochondrial fission [36]. PA has also been implicated in liver fibrosis and insulin resistance. It has been shown to interfere with nucleocytoplasmic transport and glucose-induced insulin secretion [37, 38]. However, the role of PA in the formation of renal CaOx stones remains unclear.
Injury to renal tubular epithelial cells is considered to be an important cause of the formation of renal stones [39]. Changes in cell-membrane structure and adhesion sites for stone crystals happened in damaged cells [40, 41]. The adhesion molecules, such as OPN, HA, sialoglycoprotein, fibronectin, and collagen, were increased in the injured cells [42]. In the study, the expression of adhesion molecules in renal tubular epithelial cells was increased after PA treatment. In addition, CaOx crystals were more susceptible to adhering to the membrane of damaged epithelial cells.
As the hub of intracellular FFA metabolism, PA metabolism encompasses three primary pathways, including β-oxidation, glyceride synthesis, and desaturation into MUFAs [43, 44]. Notably, the expression levels of genes implicated in these three pathways were significantly changed in PA-treated renal tubular epithelial cells. Consequently, there was an overall upregulation of SFAs, whereas the principal MUFAs, namely oleic acid and palmitoleic acid, were downregulated, as revealed by our non-targeted lipidomics analysis. Although the total PUFA levels did not exhibit significant changes after PA treatment, enzymes associated with PUFA metabolism were significantly upregulated. Additionally, downstream PUFAs, such as AA, EPA, and DHA, were all found to be upregulated.
As is well known, PA cannot be directly converted into PUFAs in humans. However, LC-PUFAs can derive from LA or ALA through a consecutive series of desaturation and chain-elongation reactions mediated by FADS1/2 and ELOVLs [45, 46]. We observed that the levels of the two essential PUFAs LA and ALA were reduced in PA-treated HK-2 cells, and this change was accompanied by an increase in downstream LC-PUFA levels. Therefore, in investigating the potential association between PA and the upregulation of FADS1/2, it was established that FADS1/2 can indeed be modulated by PPARα [47, 48], which serves as the native receptor for PA [49, 50]. In corroboration, we observed that the PA-induced FADS1/2 and AA upregulation in HK-2 cells was suppressed upon their pre-treatment with the PPARα antagonist GW6471. Although PPARα inhibition by GW6471 can decrease FADSs expression, it has not been considered a therapeutic target because of its multiple regulatory functions, including promoting fatty acid oxidation and mediating inflammatory responses [51].
PUFAs are considerably vulnerable to oxidation, leading to the formation of lipid peroxides. Supplying cells with PUFAs can improve their susceptibility to ferroptosis [52]. Upon activation of ACSL4, free PUFAs can be esterified and incorporated into membrane phospholipids, a process facilitated by LPCAT3 [53]. Among the phospholipids containing PUFAs, phosphatidylethanolamines (PEs) containing AA are the predominant substrates susceptible to peroxidation during the process of ferroptosis [54].
Our transcriptomics analysis in this study revealed changes in the expression levels of genes related to the ferroptosis pathway after PA addition. Moreover, mitochondria displayed characteristic manifestations commonly associated with ferroptosis. It was found that the expression and activity of GPX4 were decreased with GSH exhaustion in the PA-treated renal tubular epithelial cells. However system xc- was not inhibited, and SLC7A11 and SLC3A2 were always upregulated. Therefore, we inferred that the reduction in GPX4 activity is mainly caused by GSH depletion. It is noteworthy that the PA-induced injury to renal tubular epithelial cells was caused by dramatic peroxidation of PUFAs, as evidenced by the results from the Fer-1 pre-treatment.
PUFA peroxidation catalyzed by LOs is another form of ferroptosis in addition to the Fenton Reaction mediated by Fe2+ [55]. LOs are effective in the oxygenation of free PUFAs. 15-LO can catalyze the formation of pro-ferroptosis 15-OOH-eicosatetraenoic acid (HpETE), which includes AA. HpETE is subsequently esterified into PE, forming HpETE-PE. A previous study has shown that 15-LO can form a complex with PEBP1 to accelerate PUFA peroxidation [56]. However, the role of PA in the formation of the PEBP1/15-LO complex has remained unknown. PEBP1, also known as RAF1 kinase inhibitory protein (RKIP1), is bound to and inhibits the RAF1 kinase under physiological conditions. Phosphorylation of PEBP1 disrupts its association within this complex, allowing PEBP1 to interact with new partners, including 15-LO [57, 58]. Our PPI analysis revealed that PKC ζ was involved in PEBP1 phosphorylation [30]. We found that PKC ζ can induce PEBP1 phosphorylation, thereby facilitating the binding of PEBP1 to 15-LO. PKC ζ is an atypical PKC isoform and can be activated solely by specific second messengers, such as phosphatidic acid, as opposed to conventional second messengers, including diacylglycerol (DAG) and Ca2+ [30, 31]. Although phosphatidic acid is one of the intermediates in the synthesis of glycerides from PA, intracellular phosphatidic acid was upregulated in HK-2 cells upon PA treatment. This upregulation occurred through a consecutive series of catalytic reactions involving ACSL1 and GPAT1. Increased formation of the PEBP1/15-LO complex due to the activation of PKC ζ increased PUFA peroxidation, which in turn induced the ferroptosis of renal tubular epithelial cells.
Through the aforementioned experiments, we substantiated that PA, which is an SFA, upregulates PUFAs in renal tubular epithelial cells by activating PPARα. Additionally, PA promotes PUFA peroxidation by facilitating the interaction between PEBP1 and 15-LO via the activation of PKC ζ. Consequently, these processes induce ferroptosis in renal tubular cells and elevate the deposition of CaOx crystals on renal tubules due to the upregulation of adhesion molecules (Fig. 8).
In summary, our study shows that PA contributes to the formation of renal CaOx stones. Functioning as a pivotal instigator of ferroptosis by inducing downstream PUFA synthesis and peroxidation. PA causes injury to renal tubular epithelial cells and facilitates the formation of renal CaOx stones. These findings underscore the critical and intricate role of PA in nephrolithiasis.
Data availability
All data are available from the corresponding author upon reasonable request.
Abbreviations
- PA:
-
Palmitic acid
- CaOx:
-
Calcium oxalate
- MS:
-
Metabolic syndrome
- FFA:
-
Free fatty acid
- SFA:
-
Saturated fatty acid
- PAS:
-
Periodic acid-schiff
- Scr:
-
Serum creatinine
- AA:
-
Arachidonic acid
- M/LCFA:
-
Medium- and long-chain fatty acid
- Gly:
-
Glyoxylic acid
- LA:
-
α-linoleic acid
- ALA:
-
a-linolenic acid
- Fer-1:
-
Ferrostatin-1
References
Turk C, Petrik A, Sarica K et al (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69. https://doi.org/10.1016/j.eururo.2015.07.041. :475 – 82
Liu Y, Chen Y, Liao B et al (2018) Epidemiology of urolithiasis in Asia. Asian J Urol 5:205–214. https://doi.org/10.1016/j.ajur.2018.08.007
Zeng G, Mai Z, Xia S et al (2017) Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int 120:109–116. https://doi.org/10.1111/bju.13828
Khan A (2018) Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int Urol Nephrol 50:799–806. https://doi.org/10.1007/s11255-018-1849-2
Khan SR, Pearle MS, Robertson WG et al (2017) Kidney stones. Nat Rev Dis Primers 3:17001. https://doi.org/10.1038/nrdp.2017.1
Ye Z, Zeng G, Yang H et al (2020) The status and characteristics of urinary stone composition in China. BJU Int 125:801–809. https://doi.org/10.1111/bju.14765
Boyd C, Wood K, Whitaker D et al (2018) The influence of metabolic syndrome and its components on the development of nephrolithiasis. Asian J Urol 5:215–222. https://doi.org/10.1016/j.ajur.2018.06.002
Shojaei-Zarghani S, Safarpour AR, Askari H et al (2023) Metabolic syndrome and nephrolithiasis; a cross sectional population-based study on the baseline data of the PERSIAN kavar cohort study. Urology 173:61–67. https://doi.org/10.1016/j.urology.2022.11.004
Fu Q, Xie L, Diao C et al (2022) The impacts of metabolic syndrome on the risk of severe urolithiasis. Urolithiasis 50:423–430. https://doi.org/10.1007/s00240-022-01328-z
Masterson JH, Woo JR, Chang DC et al (2015) Dyslipidemia is associated with an increased risk of nephrolithiasis. Urolithiasis 43:49–53. https://doi.org/10.1007/s00240-014-0719-3
Noels H, Lehrke M, Vanholder R et al (2021) Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol 17:528–542. https://doi.org/10.1038/s41581-021-00423-5
Mori Y, Ajay AK, Chang JH et al (2021) KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab 33:1042–1061e7. https://doi.org/10.1016/j.cmet.2021.04.004
Zhang YX, Bharathi SS, Beck ME et al (2019) The fatty acid oxidation enzyme long-chain acyl-CoA dehydrogenase can be a source of mitochondrial hydrogen peroxide. Redox Biol 26:101253. https://doi.org/10.1016/j.redox.2019.101253
Asselman M, Verhulst A, De Broe ME et al (2003) Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 14:3155–3166. https://doi.org/10.1097/01.Asn.0000099380.18995.F7
Wang R, Xie LG, Zhang JD et al (2022) Characterization of the metabolites and construction of a novel diagnostic panel in calcium oxalate urolithiasis by electrospray ionization - mass spectrometry (ESI-MS). Metabolomics Anal Lett 55:1997–2010. https://doi.org/10.1080/00032719.2022.2040523
Zhang P, Yu YH, Qin YF et al (2019) Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem 65:54–65. https://doi.org/10.1016/j.jnutbio.2018.08.016
Anusornvongchai T, Nangaku M, Jao TM et al (2018) Palmitate deranges erythropoietin production via transcription factor ATF4 activation of unfolded protein response. Kidney Int 94:536–550. https://doi.org/10.1016/j.kint.2018.03.011
Lu HM, Hao LY, Li ST et al (2016) Elevated circulating stearic acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway. Diabetologia 59:1247–1257. https://doi.org/10.1007/s00125-016-3900-0
Unno R, Kawabata T, Taguchi K et al (2020) Deregulated MTOR (mechanistic target of rapamycin kinase) is responsible for autophagy defects exacerbating kidney stone development. Autophagy 16:709–723. https://doi.org/10.1080/15548627.2019.1635382
Liu HR, Ye T, Yang XQ et al (2019) H19 promote calcium oxalate nephrocalcinosis-induced renal tubular epithelial cell injury via a ceRNA pathway. Ebiomedicine 50:366–378. https://doi.org/10.1016/j.ebiom.2019.10.059
Li Y, Lu XL, Yu ZH et al (2023) Meta-data analysis of kidney stone disease highlights ATP1A1 involvement in renal crystal formation. Redox Biol 61:102648. https://doi.org/10.1016/j.redox.2023.102648
Wigner P, Grebowski R, Bijak M et al (2021) The molecular aspect of nephrolithiasis development. Cells 10:1926. https://doi.org/10.3390/cells10081926
Liu HR, Yang XQ, Tang K et al (2020) Sulforaphane elicts dual therapeutic effects on renal inflammatory injury and crystal deposition in calcium oxalate nephrocalcinosis. Theranostics 10:7319–7334. https://doi.org/10.7150/thno.44054
Ushimoto C, Sugiki S, Kunii K et al (2023) Dynamic change and preventive role of stress response via Keap1-Nrf2 during renal crystal formation. Free Radic Biol Med 207:120–132. https://doi.org/10.1016/j.freeradbiomed.2023.07.013
Matsuzaka T, Shimano H, Yahagi N et al (2002) Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J Lipid Res 43:107–114. https://doi.org/10.1016/S0022-2275(20)30193-0
Varga T, Czimmerer Z, Nagy L (2011) PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta Mol Basis Dis 1812:1007–1022. https://doi.org/10.1016/j.bbadis.2011.02.014
Stockwell BR (2022) Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185:2401–2421. https://doi.org/10.1016/j.cell.2022.06.003
Chen X, Li JB, Kang R et al (2021) Ferroptosis: machinery and regulation. Autophagy 17:2054–2081. https://doi.org/10.1080/15548627.2020.1810918
Wenzel SE, Tyurina YY, Zhao JM et al (2017) PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171:628–641e26. https://doi.org/10.1016/j.cell.2017.09.044
Corbit KC, Trakul N, Eves EM et al (2003) Activation of Raf-1 signaling by protein kinase C through a mechanism involving raf kinase inhibitory protein. J Biol Chem 278:13061–13068. https://doi.org/10.1074/jbc.M210015200
Limatola C, Schaap D, Moolenaar WH et al (1994) Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem J 304:1001–1008. https://doi.org/10.1042/bj3041001
Farese RV Jr, Walther TC (2023) Glycerolipid synthesis and lipid droplet formation in the endoplasmic reticulum. Cold Spring Harb Perspect Biol 15:a041246. https://doi.org/10.1101/cshperspect.a041246
Chen YT, Yan Q, Lv MY et al (2020) Involvement of FATP2-mediated tubular lipid metabolic reprogramming in renal fibrogenesis. Cell Death Dis 11:994. https://doi.org/10.1038/s41419-020-03199-x
Yamamoto T, Takabatake Y, Minami S et al (2021) Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy 17:1700–1713. https://doi.org/10.1080/15548627.2020.1782034
Kruger C, Nguyen TT, Breaux C et al (2019) Proximal tubular cell-specific ablation of carnitine acetyltransferase causes tubular disease and secondary glomerulosclerosis. Diabetes 68:819–831. https://doi.org/10.2337/db18-0090
Tsushima K, Bugger H, Wende AR et al (2018) Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circ Res 122:58–73. https://doi.org/10.1161/CIRCRESAHA.117.311307
Hou T, Tian Y, Cao Z et al (2022) Cytoplasmic SIRT6-mediated ACSL5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation. Mol Cell 82:4099–4115e9. https://doi.org/10.1016/j.molcel.2022.09.018
Zhang M, Yang C, Zhu M et al (2021) Saturated fatty acids entrap PDX1 in stress granules and impede islet beta cell function. Diabetologia 64:1144–1157. https://doi.org/10.1007/s00125-021-05389-4
Sun XY, Zhang H, Deng JW et al (2021) Regulatory effects of damaged renal epithelial cells after repair by porphyra yezoensis polysaccharides with different sulfation degree on the calcium oxalate crystal-cell interaction. Int J Nanomed 16:8087–8102. https://doi.org/10.2147/IJN.S320278
Lai Y, Liang X, Zhong F et al (2019) Allicin attenuates calcium oxalate crystal deposition in the rat kidney by regulating gap junction function. J Cell Physiol 234:9640–9651. https://doi.org/10.1002/jcp.27651
Thongboonkerd V (2019) Proteomics of Crystal-cell interactions: a model for kidney stone research. Cells 8:1076. https://doi.org/10.3390/cells8091076
Vinaiphat A, Thongboonkerd V (2018) Characterizations of PMCA2-interacting complex and its role as a calcium oxalate crystal-binding protein. Cell Mol Life Sci 75:1461–1482. https://doi.org/10.1007/s00018-017-2699-2
Carta G, Murru E, Banni S et al (2017) Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol 8:902. https://doi.org/10.3389/fphys.2017.00902
Piccolis M, Bond LM, Kampmann M et al (2019) Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Mol Cell 74:32–44e8. https://doi.org/10.1016/j.molcel.2019.01.036
Koletzko B, Reischl E, Tanjung C et al (2019) FADS1 and FADS2 polymorphisms modulate fatty acid metabolism and dietary impact on health. Annu Rev Nutr 39:21–44. https://doi.org/10.1146/annurev-nutr-082018-124250
Hammouda S, Ghzaiel I, Khamlaoui W et al (2020) Genetic variants in FADS1 and ELOVL2 increase level of arachidonic acid and the risk of Alzheimer’s disease in the Tunisian population. Prostaglandins Leukot Essent Fat Acids 160:102159. https://doi.org/10.1016/j.plefa.2020.102159
Athinarayanan S, Fan YY, Wang X et al (2021) Fatty acid desaturase 1 influences hepatic lipid homeostasis by modulating the PPARalpha-FGF21 Axis. Hepatol Commun 5:461–477. https://doi.org/10.1002/hep4.1629
Hsiao WT, Su HM, Su KP et al (2019) Deficiency or activation of peroxisome proliferator-activated receptor alpha reduces the tissue concentrations of endogenously synthesized docosahexaenoic acid in C57BL/6J mice. Nutr Res Pract 13:286–294. https://doi.org/10.4162/nrp.2019.13.4.286
Montaigne D, Butruille L, Staels B (2021) PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol 18:809–823. https://doi.org/10.1038/s41569-021-00569-6
Christofides A, Konstantinidou E, Jani C et al (2021) The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 114:154338. https://doi.org/10.1016/j.metabol.2020.154338
Bougarne N, Weyers B, Desmet SJ et al (2018) Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev 39:760–802. https://doi.org/10.1210/er.2018-00064
Yang WS, Kim KJ, Gaschler MM et al (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113:E4966–4975. https://doi.org/10.1073/pnas.1603244113
Yuan H, Li XM, Zhang XY et al (2016) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 478:1338–1343. https://doi.org/10.1016/j.bbrc.2016.08.124
Kagan VE, Mao GW, Qu F et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13:81–90. https://doi.org/10.1038/Nchembio.2238
Shah R, Shchepinov MS, Pratt DA (2018) Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. Acs Cent Sci 4:387–396. https://doi.org/10.1021/acscentsci.7b00589
Nagasaki T, Schuyler AJ, Zhao JM et al (2022) 15LO1 dictates glutathione redox changes in asthmatic airway epithelium to worsen type 2 inflammation. J Clin Invest 132:e151685. https://doi.org/10.1172/JCI151685
Yeung K, Seitz T, Li SF et al (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401:173–177. https://doi.org/10.1038/43686
Sun WY, Tyurin VA, Mikulska-Ruminska K et al (2021) Phospholipase iPLA(2)beta averts ferroptosis by eliminating a redox lipid death signal. Nat Chem Biol 17:465–476. https://doi.org/10.1038/s41589-020-00734-x
Acknowledgements
We thank the altruistic support of the patients.
Funding
This work was supported by the National Natural Science Foundation of China (82070725 to Author Shiyong Qi); The Science and Technology Project of Tianjin (21JCYBJC01300 to Chunyu Liu); Tianjin Health Research Project (TJWJ2022ZD004 to Chunyu Liu); The Fund of The Second Hospital of Tianjin Medical University (2023ZDSYS03 and 2022ydey04 to Rui Wang); The Science and Technology Project of Tianjin (22YDTPJC00190 to Haijie Xie); Scientific Research Program of Tianjin Education Commission (2021KJ228 to Haijie Xie).
Author information
Authors and Affiliations
Contributions
Rui Wang and Jingdong Zhang conducted experiments, obtained data, and prepared the original manuscript; Haotian Ren helped conduct animal experiments; Shiyong Qi edited the manuscript; Linguo Xie collected literature; Haijie Xie designed the figures; Zhiqun Shang and Chunyu Liu designed and supervised the study.
Corresponding authors
Ethics declarations
Ethical statement
Approval was granted by the Ethics Committee of the Second Hospital of Tianjin Medical University (Approval Number: KY2022K060). The study was performed following the Declaration of Helsinki. All participants were informed of the study and signed the study consent form for the collection and analysis of their samples.
Consent for publication
All human participants provided informed consent for the publication of the clinical characteristics and the analyzed data of their samples.
Conflicts of interest
The authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Zhang, J., Ren, H. et al. Dysregulated palmitic acid metabolism promotes the formation of renal calcium-oxalate stones through ferroptosis induced by polyunsaturated fatty acids/phosphatidic acid. Cell. Mol. Life Sci. 81, 85 (2024). https://doi.org/10.1007/s00018-024-05145-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05145-y