Abstract
Aims
Mesenchymal stem cells (MSCs) present in the heart cannot differentiate into cardiomyocytes, but may play a role in pathological conditions. Therefore, the aim of this study was to scrutinise the role and mechanism of MSC differentiation in vivo during heart failure.
Methods and Results
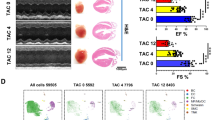
We performed single-cell RNA sequencing of total non-cardiomyocytes from murine and adult human hearts. By analysing the transcriptomes of single cells, we illustrated the dynamics of the cell landscape during the progression of heart hypertrophy, including those of stem cell antigen-1 (Sca1)+ stem/progenitor cells and fibroblasts. By combining genetic lineage tracing and bone marrow transplantation models, we demonstrated that non-bone marrow-derived Sca1+ cells give rise to fibroblasts. Interestingly, partial depletion of Sca1+ cells alleviated the severity of myocardial fibrosis and led to a significant improvement in cardiac function in Sca1-CreERT2;Rosa26-eGFP-DTA mice. Similar non-cardiomyocyte cell composition and heterogeneity were observed in human patients with heart failure. Mechanistically, our study revealed that Sca1+ cells can transform into fibroblasts and affect the severity of fibrosis through the Wnt4-Pdgfra pathway.
Conclusions
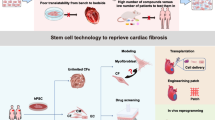
Our study describes the cellular landscape of hypertrophic hearts and reveals that fibroblasts derived from Sca1+ cells with a non-bone marrow source largely account for cardiac fibrosis. These findings provide novel insights into the pathogenesis of cardiac fibrosis and have potential therapeutic implications for heart failure.
Graphical abstract
Non-bone marrow-derived Sca1+ cells differentiate into fibroblasts involved in cardiac fibrosis via Wnt4-PDGFRα pathway.








Similar content being viewed by others
Data availability
The single-cell RNA sequencing data have been deposited in the Gene Expression Omnibus under accession number GSE198833 and GSE222144. The data underlying this article will be shared on reasonable request to the corresponding author.
References
Emmons-Bell S, Johnson C, Roth G (2022) Prevalence, incidence and survival of heart failure: a systematic review. Heart 108(17):1351–1360. https://doi.org/10.1136/heartjnl-2021-320131
Hall C, Gehmlich K, Denning C, Pavlovic D (2021) Complex relationship between cardiac fibroblasts and cardiomyocytes in health and disease. J Am Heart Assoc. 10(5):e019338. https://doi.org/10.1161/JAHA.120.019338. Epub 2021 Feb 15.
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ Res 118(6):1021–1040. https://doi.org/10.1161/CIRCRESAHA.115.306565
Frangogiannis NG (2021) Cardiac fibrosis. Cardiovasc Res 117(6):1450–1488. https://doi.org/10.1093/cvr/cvaa324
Frieler RA, Mortensen RM (2015) Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131(11):1019–1030. https://doi.org/10.1161/CIRCULATIONAHA.114.008788
Tallquist MD (2020) Cardiac fibroblast diversity. Annu Rev Physiol 10(82):63–78. https://doi.org/10.1146/annurev-physiol-021119-034527
Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM (2014) Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 124(7):2921–2934. https://doi.org/10.1172/JCI74783. (Epub 2014 Jun 17)
Czubryt MP, Hale TM. Cardiac fibrosis: Pathobiology and therapeutic targets. Cell Signal. 2021 Sep;85:110066. https://doi.org/10.1016/j.cellsig.2021.110066. Epub 2021 Jun 17.
Moore-Morris T, Guimarães-Camboa N, Yutzey KE, Pucéat M, Evans SM (2015) Cardiac fibroblasts: from development to heart failure. J Mol Med (Berl) 93(8):823–830. https://doi.org/10.1007/s00109-015-1314-y. (Epub 2015 Jul 14)
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13(8):952–961. https://doi.org/10.1038/nm1613. (Epub 2007 Jul 29)
Zhang L, Issa Bhaloo S, Chen T, Zhou B, Xu Q (2018) Role of resident stem cells in vessel formation and arteriosclerosis. Circ Res 122(11):1608–1624. https://doi.org/10.1161/CIRCRESAHA.118.313058
Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109(5):656–663. https://doi.org/10.1161/01.CIR.0000114522.38265.61. (Epub 2004 Jan 20)
Liu Q, Huang X, Zhang H, Tian X, He L, Yang R, Yan Y, Wang QD, Gillich A, Zhou B (2015) c-kit(+) cells adopt vascular endothelial but not epithelial cell fates during lung maintenance and repair. Nat Med 21(8):866–868. https://doi.org/10.1038/nm.3888. (Epub 2015 Jul 13)
van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD (2014) c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509(7500):337–341. https://doi.org/10.1038/nature13309. (Epub 2014 May 7)
Gong H, Wang T, Xu Q (2021) Resident stem cells in the heart. Med Rev 1(1):10–13. https://doi.org/10.1515/mr-2021-0003
Holmes C, Stanford WL (2007) Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25(6):1339–1347. https://doi.org/10.1634/stemcells.2006-0644
Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K, Braun T (2013) Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep 1(5):397–410. https://doi.org/10.1016/j.stemcr.2013.09.004
Tang J, Li Y, Huang X, He L, Zhang L, Wang H, Yu W, Pu W, Tian X, Nie Y, Hu S, Wang QD, Lui KO, Zhou B (2018) Fate mapping of Sca1+ cardiac progenitor cells in the adult mouse heart. Circulation 138(25):2967–2969. https://doi.org/10.1161/CIRCULATIONAHA.118.036210
Jolly AJ, Lu S, Strand KA, Dubner AM, Mutryn MF, Nemenoff RA, Majesky MW, Moulton KS, Weiser-Evans MCM (2022) Heterogeneous subpopulations of adventitial progenitor cells regulate vascular homeostasis and pathological vascular remodelling. Cardiovasc Res 118(6):1452–1465. https://doi.org/10.1093/cvr/cvab174
Vagnozzi RJ, Sargent MA, Lin SJ, Palpant NJ, Murry CE, Molkentin JD (2018) Genetic lineage tracing of Sca-1+ cells reveals endothelial but not myogenic contribution to the murine heart. Circulation 138(25):2931–2939. https://doi.org/10.1161/CIRCULATIONAHA.118.035210.Erratum.In:Circulation.2018Oct9;138(15):e424
Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, Kaffe E, Frommelt LS, Qu R, Knapp MS, Henriques A, Chalkidi N, Koliaraki V, Jiao J, Brewer JR, Bacher M, Blackburn HN, Zhao X, Breyer RM, Aidinis V, Jain D, Su B, Herschman HR, Kluger Y, Kollias G, Flavell RA (2020) Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 580(7804):524–529. https://doi.org/10.1038/s41586-020-2166-3. (Epub 2020 Apr 1)
Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE (2003) Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol 285(3):H1261-1269. https://doi.org/10.1152/ajpheart.00108.2003. (Epub 2003 May 8)
Du L, Sun X, Gong H, Wang T, Jiang L, Huang C, Xu X, Li Z, Xu H, Ma L, Li W, Chen T, Xu Q (2023) Single cell and lineage tracing studies reveal the impact of CD34+ cells on myocardial fibrosis during heart failure. Stem Cell Res Ther 14(1):33. https://doi.org/10.1186/s13287-023-03256-0
Haghverdi L, Lun ATL, Morgan MD, Marioni JC (2018) Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat Biotechnol 36(5):421–427. https://doi.org/10.1038/nbt.4091. (Epub 2018 Apr 2)
McGinnis CS, Murrow LM, Gartner ZJ (2019) DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst 8(4):329-337.e4. https://doi.org/10.1016/j.cels.2019.03.003. (Epub 2019 Apr 3)
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R (2019) Comprehensive integration of single-cell data. Cell 177(7):1888-1902.e21. https://doi.org/10.1016/j.cell.2019.05.031. (Epub 2019 Jun 6)
Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien R, Roose-Girma M, Modrusan Z, Arron JR, Bourgon R, Müller S, Turley SJ (2021) Cross-tissue organization of the fibroblast lineage. Nature 593(7860):575–579. https://doi.org/10.1038/s41586-021-03549-5. (Epub 2021 May 12)
Pu X, Zhu P, Zhou X, He Y, Wu H, Du L, Gong H, Sun X, Chen T, Zhu J, Xu Q, Zhang H (2022) CD34+ cell atlas of main organs implicates its impact on fibrosis. Cell Mol Life Sci 79(11):576. https://doi.org/10.1007/s00018-022-04606-6
Qiu X, Hill A, Packer J, Lin D, Ma YA, Trapnell C (2017) Single-cell mRNA quantification and differential analysis with Census. Nat Methods 14(3):309–315. https://doi.org/10.1038/nmeth.4150. (Epub 2017 Jan 23)
Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL (2014) The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32(4):381–386. https://doi.org/10.1038/nbt.2859. (Epub 2014 Mar 23)
Plummer NW, Ungewitter EK, Smith KG, Yao HH, Jensen P. A new mouse line for cell ablation by diphtheria toxin subunit A controlled by a Cre-dependent FLEx switch. Genesis. 2017 Oct;55(10):https://doi.org/10.1002/dvg.23067. doi: https://doi.org/10.1002/dvg.23067. Epub 2017 Sep 19.
Lee CH, Shah B, Moioli EK, Mao JJ (2010) CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 120(9):3340–3349. https://doi.org/10.1172/JCI43230. Epub 2010 Aug 2. Erratum in: J Clin Invest. 2015 Oct 1;125(10):3992.
Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, Driskell RR, Rosenthal N, Biernaskie J, Horsley V (2021) Fibroblasts: Origins, definitions, and functions in health and disease. Cell 184(15):3852–3872. https://doi.org/10.1016/j.cell.2021.06.024
Soliman H, Theret M, Scott W, Hill L, Underhill TM, Hinz B, Rossi FMV (2021) Multipotent stromal cells: one name, multiple identities. Cell Stem Cell 28(10):1690–1707. https://doi.org/10.1016/j.stem.2021.09.001
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA (2019) Targeting cardiac fibrosis with engineered T cells. Nature 573(7774):430–433. https://doi.org/10.1038/s41586-019-1546-z. (Epub 2019 Sep 11)
van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC (2008) Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol 214(3):377–386. https://doi.org/10.1002/path.2281
Tallquist MD, Molkentin JD (2017) Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol 14(8):484–491. https://doi.org/10.1038/nrcardio.2017.57. (Epub 2017 Apr 24)
Torsney E, Xu Q (2011) Resident vascular progenitor cells. J Mol Cell Cardiol 50(2):304–311. https://doi.org/10.1016/j.yjmcc.2010.09.006
Oh H, Chi X, Bradfute SB, Mishina Y, Pocius J, Michael LH, Behringer RR, Schwartz RJ, Entman ML, Schneider MD (2004) Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y Acad Sci 1015:182–189. https://doi.org/10.1196/annals.1302.015
Ni Z, Lyu L, Gong H, Du L, Wen Z, Jiang H, Yang H, Hu Y, Zhang B, Xu Q, Guo X, Chen T (2023) Multilineage commitment of Sca-1+ cells in reshaping vein grafts. Theranostics 13(7):2154–2175. https://doi.org/10.7150/thno.77735
Tang J, Wang H, Huang X, Li F, Zhu H, Li Y, He L, Zhang H, Pu W, Liu K, Zhao H, Bentzon JF, Yu Y, Ji Y, Nie Y, Tian X, Zhang L, Gao D, Zhou B (2020) Arterial Sca1+ vascular stem cells generate de novo smooth muscle for artery repair and regeneration. Cell Stem Cell 26(1):81-96.e4. https://doi.org/10.1016/j.stem.2019.11.010. (Epub 2019 Dec 27)
Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL (2003) Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101(2):517–523. https://doi.org/10.1182/blood-2002-06-1918. (Epub 2002 Aug 29)
Morcos MNF, Schoedel KB, Hoppe A, Behrendt R, Basak O, Clevers HC, Roers A, Gerbaulet A (2017) SCA-1 expression level identifies quiescent hematopoietic stem and progenitor cells. Stem Cell Reports 8(6):1472–1478. https://doi.org/10.1016/j.stemcr.2017.04.012. (Epub 2017 May 11)
assone NM, Li B, Patel MS, Devine MY, Firmiss PR, Gould AD, Kochan KS, Stubbee RA, Bowen DK, Dettman RW, Gong EM (2019) Stem cell antigen/Ly6a protects against bladder fibrosis in mice. Am J Physiol Renal Physiol. 317(6):F1503-F1512. https://doi.org/10.1152/ajprenal.00160.2019. Epub 2019 Sep 18.
Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S (2005) Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res 97(11):1142–1151. https://doi.org/10.1161/01.RES.0000193596.94936.2c. (Epub 2005 Oct 27)
Zhou H, Bian ZY, Zong J, Deng W, Yan L, Shen DF, Guo H, Dai J, Yuan Y, Zhang R, Lin YF, Hu X, Li H, Tang QZ (2012) Stem cell antigen 1 protects against cardiac hypertrophy and fibrosis after pressure overload. Hypertension 60(3):802–809. https://doi.org/10.1161/HYPERTENSIONAHA.112.198895. (Epub 2012 Jul 30)
Deng J, Ni Z, Gu W, Chen Q, Nowak WN, Chen T, Issa Bhaloo S, Zhang Z, Hu Y, Zhou B, Zhang L, Xu Q (2020) Single-cell gene profiling and lineage tracing analyses revealed novel mechanisms of endothelial repair by progenitors. Cell Mol Life Sci 77(24):5299–5320. https://doi.org/10.1007/s00018-020-03480-4. (Epub 2020 Mar 13)
Dong W, Zhao Y, Wen D, Lin Y, Zeng C, Gu J, Liao F, Li R, Zhang X, Wang D, Cai W, Duan J (2022) Wnt4 is crucial for cardiac repair by regulating mesenchymal-endothelial transition via the phospho-JNK/JNK. Theranostics 12(9):4110–4126. https://doi.org/10.7150/thno.71392
Haybar H, Khodadi E, Shahrabi S (2019) Wnt/β-catenin in ischemic myocardium: interactions and signaling pathways as a therapeutic target. Heart Fail Rev 24(3):411–419. https://doi.org/10.1007/s10741-018-9759-z
Brade T, Männer J, Kühl M (2006) The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc Res 72(2):198–209. https://doi.org/10.1016/j.cardiores.2006.06.025. (Epub 2006 Jun 29)
van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL (1989) Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A 86(12):4634–4638. https://doi.org/10.1073/pnas.86.12.4634
Acknowledgements
We are grateful to Ms. Chao Bi and Ms. Xiaoli Hong at the Core Facilities, Zhejiang University School of Medicine for technical assistance in confocal microscopy.
Funding
This work is supported by National Natural Science Foundation of China (82030008, 82270409, 31830039), Natural Science Foundation of Zhejiang Province (LQ23H020006), Science and technology Project of Zhejiang Province (2023C03087).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by TT, YG, XW. Data collection was performed by PT, HZ, LM. Analysis was performed by LD. The first draft of the manuscript was written by TT, LD, YH, QX and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (approval no. IIT20210018B-R2).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1L and Supplementary Fig. 3a–b.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, T., Du, L., Teng, P. et al. Stem cell antigen-1+cell-derived fibroblasts are crucial for cardiac fibrosis during heart failure. Cell. Mol. Life Sci. 80, 300 (2023). https://doi.org/10.1007/s00018-023-04957-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04957-8