Abstract
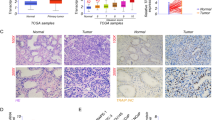
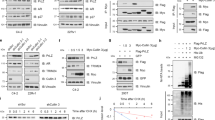
Phosphatase and tensin homolog (PTEN) loss tightly correlates with prostate cancer (PCa) progression and metastasis. Inactivation of PTEN leads to abnormal activation of PI3K/AKT pathway. However, results from clinical trials with AKT inhibitors in PCa have been largely disappointing. Identification of novel regulators of PTEN in PTEN-dysfunctional PCa is urgently needed. Here we demonstrated that the expression level of PTEN is inversely correlated with the signature score of unfolded protein response (UPR) in PCa. Importantly, PTEN suppresses the activity of ATF6α, via interacting to de-phosphorylate ATF6α and consequently inhibiting its nuclear translocation. Conversely, ATF6α promotes the ubiquitination and degradation of PTEN by inducing CHIP expression. Thus, ATF6α and PTEN forms a negative feedback loop during PCa progression. Combination of ATF6α inhibitor with AKT inhibitor suppresses tumor cell proliferation and xenograft growth. Importantly, this study highlighted ATF6α as a therapeutic vulnerability in PTEN dysfunctional PCa.







Similar content being viewed by others

Data availability
All data and material during the current study are available from the corresponding author on reasonable request.
References
Barbieri CE et al (2013) The mutational landscape of prostate cancer. Eur Urol 64(4):567–576. https://doi.org/10.1016/j.eururo.2013.05.029
Li J et al (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275(5308):1943–1947. https://doi.org/10.1126/science.275.5308.1943
Alimonti A et al (2010) Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42(5):454–458. https://doi.org/10.1038/ng.556
Lotan TL et al (2011) PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res 17(20):6563–6573. https://doi.org/10.1158/1078-0432.Ccr-11-1244
Han B et al (2009) Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol 22(8):1083–1093. https://doi.org/10.1038/modpathol.2009.69
Gray IC et al (1995) Loss of the chromosomal region 10q23-25 in prostate cancer. Cancer Res 55(21):4800–4803
Fruman DA, Rommel C (2014) PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 13(2):140–156. https://doi.org/10.1038/nrd4204
Crabb SJ et al (2021) Pan-AKT inhibitor Capivasertib With docetaxel and prednisolone in metastatic castration-resistant prostate cancer: a randomized, placebo-controlled phase II trial (ProCAID). J Clin Oncol 39(3):190–201. https://doi.org/10.1200/jco.20.01576
Cen B et al (2013) Elevation of receptor tyrosine kinases by small molecule AKT inhibitors in prostate cancer is mediated by Pim-1. Cancer Res 73(11):3402–3411. https://doi.org/10.1158/0008-5472.Can-12-4619
Chee KG et al (2007) The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer 5(7):433–437. https://doi.org/10.3816/CGC.2007.n.031
Sweeney C et al (2021) Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet 398(10295):131–142. https://doi.org/10.1016/s0140-6736(21)00580-8
Yuan L et al (2015) Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat Cell Biol 17(9):1169–1181. https://doi.org/10.1038/ncb3218
Bassi C et al (2013) Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341(6144):395–399. https://doi.org/10.1126/science.1236188
Goel A et al (2004) Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res 64(9):3014–3021. https://doi.org/10.1158/0008-5472.can-2401-2
Hetz C et al (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21(8):421–438. https://doi.org/10.1038/s41580-020-0250-z
Sheng X et al (2019) IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat Commun 10(1):323. https://doi.org/10.1038/s41467-018-08152-3
de la Calle CM et al (2022) The endoplasmic reticulum stress response in prostate cancer. Nat Rev Urol 19(12):708–726. https://doi.org/10.1038/s41585-022-00649-3
Chui MH et al (2019) Chromosomal instability and mTORC1 activation through PTEN loss contribute to Proteotoxic stress in Ovarian carcinoma. Cancer Res 79(21):5536–5549. https://doi.org/10.1158/0008-5472.Can-18-3029
Wang W et al (2018) KDM4B-regulated unfolded protein response as a therapeutic vulnerability in PTEN-deficient breast cancer. J Exp Med 215(11):2833–2849. https://doi.org/10.1084/jem.20180439
Haze K et al (2001) Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J 355(Pt 1):19–28. https://doi.org/10.1042/0264-6021:3550019
Forouhan M et al (2018) Paradoxical roles of ATF6α and ATF6β in modulating disease severity caused by mutations in collagen X. Matrix Biol. https://doi.org/10.1016/j.matbio.2018.03.004
Liu J et al (2017) Activation of UPR signaling pathway is associated with the malignant progression and poor prognosis in prostate cancer. Prostate 77(3):274–281. https://doi.org/10.1002/pros.23264
Wang L et al (2013) SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis 16(4):301–307. https://doi.org/10.1038/pcan.2013.25
Feng T et al (2016) Growth factor progranulin promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR signaling pathway. Oncotarget. https://doi.org/10.18632/oncotarget.11126
Plate L et al (2016) Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. eLife https://doi.org/10.7554/eLife.15550.
Myers MP et al (1998) The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A 95(23):13513–13518. https://doi.org/10.1073/pnas.95.23.13513
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273(22):13375–13378. https://doi.org/10.1074/jbc.273.22.13375
Lee YR et al (2018) The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 19(9):547–562. https://doi.org/10.1038/s41580-018-0015-0
Ahmed SF et al (2012) The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation. J Biol Chem 287(19):15996–16006. https://doi.org/10.1074/jbc.M111.321083
Maddika S et al (2011) WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol 13(6):728–733. https://doi.org/10.1038/ncb2240
Van Themsche C et al (2009) X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem 284(31):20462–20466. https://doi.org/10.1074/jbc.C109.009522
Wang X et al (2007) NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128(1):129–139. https://doi.org/10.1016/j.cell.2006.11.039
Misra J et al (2013) Transcriptional cross talk between orphan nuclear receptor ERRγ and transmembrane transcription factor ATF6α coordinates endoplasmic reticulum stress response. Nucleic Acids Res 41(14):6960–6974. https://doi.org/10.1093/nar/gkt429
Zhang W et al (2015) Feedback regulation on PTEN/AKT pathway by the ER stress kinase PERK mediated by interaction with the Vault complex. Cell Signal 27(3):436–442. https://doi.org/10.1016/j.cellsig.2014.12.010
Rebello RJ et al (2021) Prostate cancer. Nat Rev Dis Primers 7(1):9. https://doi.org/10.1038/s41572-020-00243-0
Ferraldeschi R et al (2015) PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol 67(4):795–802. https://doi.org/10.1016/j.eururo.2014.10.027
Toren P et al (2015) Combination AZD5363 with Enzalutamide significantly delays Enzalutamide-resistant prostate cancer in preclinical models. Eur Urol 67(6):986–990. https://doi.org/10.1016/j.eururo.2014.08.006
Kim MJ et al (2002) Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA 99(5):2884–2889. https://doi.org/10.1073/pnas.042688999
Di Cristofano A et al (2001) Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet 27(2):222–224. https://doi.org/10.1038/84879
Chen Z et al (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436(7051):725–730. https://doi.org/10.1038/nature03918
Yu Y, et al (2023) PTEN phosphatase inhibits metastasis by negatively regulating the Entpd5/IGF1R pathway through ATF6. iScience 26 (2): 106070 https://doi.org/10.1016/j.isci.2023.106070.
Jin Y, Saatcioglu F (2020) Targeting the unfolded protein response in hormone-regulated cancers. Trends Cancer 6(2):160–171. https://doi.org/10.1016/j.trecan.2019.12.001
Vandewynckel YP et al (2016) Next-generation proteasome inhibitor oprozomib synergizes with modulators of the unfolded protein response to suppress hepatocellular carcinoma. Oncotarget 7(23):34988–35000. https://doi.org/10.18632/oncotarget.9222
Benedetti R et al (2022) ATF6 prevents DNA damage and cell death in colon cancer cells undergoing ER stress. Cell Death Discov 8(1):295. https://doi.org/10.1038/s41420-022-01085-3
Spaan CN et al (2019) Expression of UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell proliferation and stemness by activating PERK signaling. Cell Death Dis 10(7):490. https://doi.org/10.1038/s41419-019-1729-4
Verfaillie T et al (2013) Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 332(2):249–264. https://doi.org/10.1016/j.canlet.2010.07.016
Wu J et al (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13(3):351–364. https://doi.org/10.1016/j.devcel.2007.07.005
Pachikov AN et al (2021) The non-canonical mechanism of ER stress-mediated progression of prostate cancer. J Exp Clin Cancer Res 40(1):289. https://doi.org/10.1186/s13046-021-02066-7
Salmena L et al (2008) Tenets of PTEN tumor suppression. Cell 133(3):403–414. https://doi.org/10.1016/j.cell.2008.04.013
Wu HH et al (2021) Hsp70 acts as a fine-switch that controls E3 ligase CHIP-mediated TAp63 and ΔNp63 ubiquitination and degradation. Nucleic Acids Res 49(5):2740–2758. https://doi.org/10.1093/nar/gkab081
Funding
This work was supported by the National Natural Science Foundation of China (Grants No. 82172818, 81972416), (Grant No. 81772760, 82072850), (Grant No. 81972436), Joint Research Fund of Natural Science of Shandong Province (ZR2019LZL014), Academic promotion program of Shandong First Medical University (LJ001), The Youth Innovation Technology Plan of Shandong University (Grant No. 2019KJK003), China Postdoctoral Science Foundation (2022M711900) and Natural Science Foundation of Shandong Province (ZR202111150013).
Author information
Authors and Affiliations
Contributions
BH, LW, TF, and RZ wrote the manuscript, performed, designed, and analyzed experiments; FS, HZ, and LG accomplished some of in vitro experiments; WC and MW analyzed experiments; TF, and YX accomplished some of in vivo studies; JH, MQ, JZ and LL analyzed IHC assay. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shandong University (Document No. ECSBMSSDU2021-1-61). All animal experimental protocols were performed following the Ethical Animal Care and Use Committee of Shandong University (Document No. ECSBMSSDU2021-2-126).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 2a.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, T., Zhao, R., Zhang, H. et al. Reciprocal negative feedback regulation of ATF6α and PTEN promotes prostate cancer progression. Cell. Mol. Life Sci. 80, 292 (2023). https://doi.org/10.1007/s00018-023-04940-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04940-3