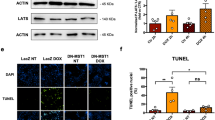
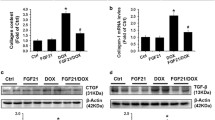
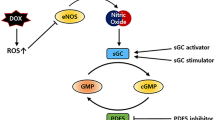
Abstract
Cardiotoxicity remains a major limitation in the clinical utility of anthracycline chemotherapeutics. Regulator of G-protein Signaling 7 (RGS7) and inflammatory markers are up-regulated in the hearts of patients with a history of chemotherapy particularly those with reduced left-ventricular function. RGS7 knockdown in either the murine myocardium or isolated murine ventricular cardiac myocytes (VCM) or cultured human VCM provided marked protection against doxorubicin-dependent oxidative stress, NF-κB activation, inflammatory cytokine production, and cell death. In exploring possible mechanisms causally linking RGS7 to pro-inflammatory signaling cascades, we found that RGS7 forms a complex with acetylase Tip60 and deacetylase sirtuin 1 (SIRT1) and controls the acetylation status of the p65 subunit of NF-κB. In VCM, the detrimental impact of RGS7 could be mitigated by inhibiting Tip60 or activating SIRT1, indicating that the ability of RGS7 to modulate cellular acetylation capacity is critical for its pro-inflammatory actions. Further, RGS7-driven, Tip60/SIRT1-dependent cytokines released from ventricular cardiac myocytes and transplanted onto cardiac fibroblasts increased oxidative stress, markers of transdifferentiation, and activity of extracellular matrix remodelers emphasizing the importance of the RGS7–Tip60–SIRT1 complex in paracrine signaling in the myocardium. Importantly, while RGS7 overexpression in heart resulted in sterile inflammation, fibrotic remodeling, and compromised left-ventricular function, activation of SIRT1 counteracted the detrimental impact of RGS7 in heart confirming that RGS7 increases acetylation of SIRT1 substrates and thereby drives cardiac dysfunction. Together, our data identify RGS7 as an amplifier of inflammatory signaling in heart and possible therapeutic target in chemotherapeutic drug-induced cardiotoxicity.








Similar content being viewed by others
Availability of data and materials
All the data are mentioned either in the manuscript or in the supplement.
References
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97(11):2869–2879
Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJ, Shankar S, Sieswerda E, Skinner R, Steinberger J, van Dalen EC, van der Pal H, Wallace WH, Levitt G, Kremer LC, G International Late Effects of Childhood Cancer Guideline Harmonization (2015) Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16(3):e123–e136
Bures J, Jirkovska A, Sestak V, Jansova H, Karabanovich G, Roh J, Sterba M, Simunek T, Kovarikova P (2017) Investigation of novel dexrazoxane analogue JR-311 shows significant cardioprotective effects through topoisomerase IIbeta but not its iron chelating metabolite. Toxicology 392:1–10
Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL (2007) Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol 25(5):493–500
Henriksen PA (2018) Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104(12):971–977
Cardinale D, Iacopo F, Cipolla CM (2020) Cardiotoxicity of anthracyclines. Front Cardiovasc Med 7:26
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229
Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C (2002) Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res 62(16):4592–4598
Davies KJ, Doroshow JH (1986) Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261(7):3060–3067
Doroshow JH, Davies KJ (1986) Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261(7):3068–3074
Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G (2004) Doxorubicin cardiotoxicity and the control of iron metabolism: quinone-dependent and independent mechanisms. Methods Enzymol 378:340–361
Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, McDermott BJ, Grieve DJ (2010) Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res 70(22):9287–9297
Ma ZG, Kong CY, Wu HM, Song P, Zhang X, Yuan YP, Deng W, Tang QZ (2020) Toll-like receptor 5 deficiency diminishes doxorubicin-induced acute cardiotoxicity in mice. Theranostics 10(24):11013–11025
Guo Z, Tang N, Liu FY, Yang Z, Ma SQ, An P, Wu HM, Fan D, Tang QZ (2020) TLR9 deficiency alleviates doxorubicin-induced cardiotoxicity via the regulation of autophagy. J Cell Mol Med 24(18):10913–10923
Nozaki N, Shishido T, Takeishi Y, Kubota I (2004) Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 110(18):2869–2874
Riad A, Bien S, Gratz M, Escher F, Westermann D, Heimesaat MM, Bereswill S, Krieg T, Felix SB, Schultheiss HP, Kroemer HK, Tschope C (2008) Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail 10(3):233–243
Krysko DV, Kaczmarek A, Krysko O, Heyndrickx L, Woznicki J, Bogaert P, Cauwels A, Takahashi N, Magez S, Bachert C, Vandenabeele P (2011) TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ 18(8):1316–1325
Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR (2006) Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res 72(3):384–393
Kaczmarek A, Krysko O, Heyndrickx L, Love-Aaes T, Delvaeye T, Bachert C, Leybaert L, Vandenabeele P, Krysko DV (2013) TNF/TNF-R1 pathway is involved in doxorubicin-induced acute sterile inflammation. Cell Death Dis 4(12):e961
Bhagat A, Shrestha P, Jeyabal P, Peng Z, Watowich SS, Kleinerman ES (2022) Doxorubicin-induced cardiotoxicity is mediated by neutrophils through release of neutrophil elastase. Front Oncol 12:947604
Zhang H, Xu A, Sun X, Yang Y, Zhang L, Bai H, Ben J, Zhu X, Li X, Yang Q, Wang Z, Wu W, Yang D, Zhang Y, Xu Y, Chen Q (2020) Self-maintenance of cardiac resident reparative macrophages attenuates doxorubicin-induced cardiomyopathy through the SR-A1-c-Myc axis. Circ Res 127(5):610–627
Clayton ZS, Brunt VE, Hutton DA, Casso AG, Ziemba BP, Melov S, Campisi J, Seals DR (2021) Tumor necrosis factor alpha-mediated inflammation and remodeling of the extracellular matrix underlies aortic stiffening induced by the common chemotherapeutic agent doxorubicin. Hypertension 77(5):1581–1590
Basak M, Sengar AS, Das K, Mahata T, Kumar M, Kumar D, Biswas S, Sarkar S, Kumar P, Das P, Stewart A, Maity B (2023) A RGS7-CaMKII complex drives myocyte-intrinsic and myocyte-extrinsic mechanisms of chemotherapy-induced cardiotoxicity. Proc Natl Acad Sci U S A 120(1):e2213537120
Basak M, Das K, Mahata T, Sengar AS, Verma SK, Biswas S, Bhadra K, Stewart A, Maity B (2023) RGS7-ATF3-Tip60 complex promotes hepatic steatosis and fibrosis by directly inducing TNFalpha. Antioxid Redox Signal 38(1–3):137–159
Benzing T, Brandes R, Sellin L, Schermer B, Lecker S, Walz G, Kim E (1999) Upregulation of RGS7 may contribute to tumor necrosis factor-induced changes in central nervous function. Nat Med 5(8):913–918
Benzing T, Kottgen M, Johnson M, Schermer B, Zentgraf H, Walz G, Kim E (2002) Interaction of 14-3-3 protein with regulator of G protein signaling 7 is dynamically regulated by tumor necrosis factor-alpha. J Biol Chem 277(36):32954–32962
Zhang JH, Barr VA, Mo Y, Rojkova AM, Liu S, Simonds WF (2001) Nuclear localization of G protein beta 5 and regulator of G protein signaling 7 in neurons and brain. J Biol Chem 276(13):10284–10289
Kim JW, Jang SM, Kim CH, An JH, Kang EJ, Choi KH (2012) New molecular bridge between RelA/p65 and NF-kappaB target genes via histone acetyltransferase TIP60 cofactor. J Biol Chem 287(10):7780–7791
Greene WC, Chen LF (2004) Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp 259:208–217 (discussion 218–25)
Kuno A, Hosoda R, Tsukamoto M, Sato T, Sakuragi H, Ajima N, Saga Y, Tada K, Taniguchi Y, Iwahara N, Horio Y (2023) SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc Res 118(17):3360–3373
Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, Deng W, Tang QZ (2018) CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol 114:38–47
Wang S, Wang Y, Zhang Z, Liu Q, Gu J (2017) Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis 8(8):e3018
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23(12):2369–2380
Moulin M, Piquereau J, Mateo P, Fortin D, Rucker-Martin C, Gressette M, Lefebvre F, Gresikova M, Solgadi A, Veksler V, Garnier A, Ventura-Clapier R (2015) Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circ Heart Fail 8(1):98–108
Nair A, Morsy MA, Jacob S (2018) Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res 79(8):373–382
Nagai K, Nogami S, Egusa H, Konishi H (2014) Pharmacokinetic evaluation of intraperitoneal doxorubicin in rats. Pharmazie 69(2):125–127
Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X (2011) Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res 90(3):538–545
Cappetta D, Esposito G, Piegari E, Russo R, Ciuffreda LP, Rivellino A, Berrino L, Rossi F, De Angelis A, Urbanek K (2016) SIRT1 activation attenuates diastolic dysfunction by reducing cardiac fibrosis in a model of anthracycline cardiomyopathy. Int J Cardiol 205:99–110
Ruan Y, Dong C, Patel J, Duan C, Wang X, Wu X, Cao Y, Pu L, Lu D, Shen T, Li J (2015) SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell Physiol Biochem 35(3):1116–1124
Pramanick A, Chakraborti S, Mahata T, Basak M, Das K, Verma SK, Sengar AS, Singh PK, Kumar P, Bhattacharya B, Biswas S, Pal PB, Sarkar S, Agrawal V, Saha S, Nath D, Chatterjee S, Stewart A, Maity B (2021) G protein beta5-ATM complexes drive acetaminophen-induced hepatotoxicity. Redox Biol 43:101965
Long CS, Henrich CJ, Simpson PC (1991) A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul 2(12):1081–1095
Maillet A, Tan K, Chai X, Sadananda SN, Mehta A, Ooi J, Hayden MR, Pouladi MA, Ghosh S, Shim W, Brunham LR (2016) Modeling doxorubicin-induced cardiotoxicity in human pluripotent stem cell derived-cardiomyocytes. Sci Rep 6:25333
Das K, Basak M, Mahata T, Kumar M, Kumar D, Biswas S, Chatterjee S, Moniruzzaman M, Saha NC, Mondal K, Kumar P, Das P, Stewart A, Maity B (2022) RGS11-CaMKII complex mediated redox control attenuates chemotherapy-induced cardiac fibrosis. Redox Biol 57:102487
Liu MH, Shan J, Li J, Zhang Y, Lin XL (2016) Resveratrol inhibits doxorubicin-induced cardiotoxicity via sirtuin 1 activation in H9c2 cardiomyocytes. Exp Ther Med 12(2):1113–1118
Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS (2009) Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med 46(12):1589–1597
Coffey K, Blackburn TJ, Cook S, Golding BT, Griffin RJ, Hardcastle IR, Hewitt L, Huberman K, McNeill HV, Newell DR, Roche C, Ryan-Munden CA, Watson A, Robson CN (2012) Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS ONE 7(10):e45539
Pourtaghi-Anvarian S, Mohammadi S, Hamzeh-Mivehroud M, Alizadeh AA, Dastmalchi S (2019) Characterization of the novel anti-TNF-alpha single-chain fragment antibodies using experimental and computational approaches. Prep Biochem Biotechnol 49(1):38–47
Chakraborti S, Pramanick A, Saha S, Roy SS, Chaudhuri AR, Das M, Ghosh S, Stewart A, Maity B (2018) Atypical G protein beta5 promotes cardiac oxidative stress, apoptosis, and fibrotic remodeling in response to multiple cancer chemotherapeutics. Cancer Res 78(2):528–541
Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ 2nd, Ischiropoulos H (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52(1):1–6
Pierce BG, Hourai Y, Weng Z (2011) Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS ONE 6(9):e24657
Negi SS, Schein CH, Oezguen N, Power TD, Braun W (2007) InterProSurf: a web server for predicting interacting sites on protein surfaces. Bioinformatics 23(24):3397–3399
Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, Anker SD, Atherton J, Bohm M, Butler J, Drazner MH, Michael Felker G, Filippatos G, Fiuzat M, Fonarow GC, Gomez-Mesa JE, Heidenreich P, Imamura T, Jankowska EA, Januzzi J, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, Seferovic P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S (2021) Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 23(3):352–380
Bhagat A, Shrestha P, Kleinerman ES (2022) The innate immune system in cardiovascular diseases and its role in doxorubicin-induced cardiotoxicity. Int J Mol Sci 23(23):14649
Xiao Y, Nagai Y, Deng G, Ohtani T, Zhu Z, Zhou Z, Zhang H, Ji MQ, Lough JW, Samanta A, Hancock WW, Greene MI (2014) Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Rep 7(5):1471–1480
Wang J, Chen J (2010) SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J Biol Chem 285(15):11458–11464
Yamagata K, Kitabayashi I (2009) Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX. Biochem Biophys Res Commun 390(4):1355–1360
Wang X, Wan TC, Kulik KR, Lauth A, Smith BC, Lough JW, Auchampach JA (2023) Pharmacological inhibition of the acetyltransferase Tip60 mitigates myocardial infarction injury. Dis Model Mech 16(5):049786
Wang X, Wan TC, Lauth A, Purdy AL, Kulik KR, Patterson M, Lough JW, Auchampach JA (2022) Conditional depletion of the acetyltransferase Tip60 protects against the damaging effects of myocardial infarction. J Mol Cell Cardiol 163:9–19
Wang AJ, Zhang J, Xiao M, Wang S, Wang BJ, Guo Y, Tang Y, Gu J (2021) Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways. Cell Mol Life Sci 78(7):3105–3125
Viatour P, Merville MP, Bours V, Chariot A (2005) Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci 30(1):43–52
Chen LF, Mu Y, Greene WC (2002) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21(23):6539–6548
Goren N, Cuenca J, Martin-Sanz P, Bosca L (2004) Attenuation of NF-kappaB signalling in rat cardiomyocytes at birth restricts the induction of inflammatory genes. Cardiovasc Res 64(2):289–297
Kraut B, Maier HJ, Kokai E, Fiedler K, Boettger T, Illing A, Kostin S, Walther P, Braun T, Wirth T (2015) Cardiac-specific activation of IKK2 leads to defects in heart development and embryonic lethality. PLoS ONE 10(11):e0141591
Maier HJ, Schips TG, Wietelmann A, Kruger M, Brunner C, Sauter M, Klingel K, Bottger T, Braun T, Wirth T (2012) Cardiomyocyte-specific IkappaB kinase (IKK)/NF-kappaB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci U S A 109(29):11794–11799
Cheng W, Cui C, Liu G, Ye C, Shao F, Bagchi AK, Mehta JL, Wang X (2023) NF-kappaB, a potential therapeutic target in cardiovascular diseases. Cardiovasc Drugs Ther 37(3):571–584
Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T (1997) Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem 272(49):31092–31099
Hausmann ON, Hu WH, Keren-Raifman T, Witherow DS, Wang Q, Levay K, Frydel B, Slepak ZV, Bethea RJ (2002) Spinal cord injury induces expression of RGS7 in microglia/macrophages in rats. Eur J Neurosci 15(4):602–612
Lin B, Zhao H, Li L, Zhang Z, Jiang N, Yang X, Zhang T, Lian B, Liu Y, Zhang C, Wang J, Wang F, Feng D, Xu J (2020) Sirt1 improves heart failure through modulating the NF-kappaB p65/microRNA-155/BNDF signaling cascade. Aging (Albany NY) 13(10):14482–14498
Wang AJ, Tang Y, Zhang J, Wang BJ, Xiao M, Lu G, Li J, Liu Q, Guo Y, Gu J (2022) Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol 52:102310
Sin TK, Tam BT, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Rudd JA, Siu PM (2015) Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J Physiol 593(8):1887–1899
Xiao M, Tang Y, Wang J, Lu G, Niu J, Wang J, Li J, Liu Q, Wang Z, Huang Z, Guo Y, Gao T, Zhang X, Yue S, Gu J (2022) A new FGF1 variant protects against adriamycin-induced cardiotoxicity via modulating p53 activity. Redox Biol 49:102219
Drenan RM, Doupnik CA, Boyle MP, Muglia LJ, Huettner JE, Linder ME, Blumer KJ (2005) Palmitoylation regulates plasma membrane-nuclear shuttling of R7BP, a novel membrane anchor for the RGS7 family. J Cell Biol 169(4):623–633
Panicker LM, Zhang JH, Posokhova E, Gastinger MJ, Martemyanov KA, Simonds WF (2010) Nuclear localization of the G protein beta 5/R7-regulator of G protein signaling protein complex is dependent on R7 binding protein. J Neurochem 113(5):1101–1112
Jia L, Linder ME, Blumer KJ (2011) Gi/o signaling and the palmitoyltransferase DHHC2 regulate palmitate cycling and shuttling of RGS7 family-binding protein. J Biol Chem 286(15):13695–13703
Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY (2005) R7BP, a novel neuronal protein interacting with RGS proteins of the R7 family. J Biol Chem 280(7):5133–5136
Larminie C, Murdock P, Walhin JP, Duckworth M, Blumer KJ, Scheideler MA, Garnier M (2004) Selective expression of regulators of G-protein signaling (RGS) in the human central nervous system. Brain Res Mol Brain Res 122(1):24–34
Kardestuncer T, Wu H, Lim AL, Neer EJ (1998) Cardiac myocytes express mRNA for ten RGS proteins: changes in RGS mRNA expression in ventricular myocytes and cultured atria. FEBS Lett 438(3):285–288
Yang J, Maity B, Huang J, Gao Z, Stewart A, Weiss RM, Anderson ME, Fisher RA (2013) G-protein inactivator RGS6 mediates myocardial cell apoptosis and cardiomyopathy caused by doxorubicin. Cancer Res 73(6):1662–1667
Mahata T, Sengar AS, Basak M, Das K, Pramanick A, Verma SK, Singh PK, Biswas S, Sarkar S, Saha S, Chatterjee S, Das M, Stewart A, Maity B (2021) Hepatic regulator of G protein signaling 6 (RGS6) drives non-alcoholic fatty liver disease by promoting oxidative stress and ATM-dependent cell death. Redox Biol 46:102105
Chen CK, Eversole-Cire P, Zhang H, Mancino V, Chen YJ, He W, Wensel TG, Simon MI (2003) Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5. Proc Natl Acad Sci U S A 100(11):6604–6609
Huang J, Stewart A, Maity B, Hagen J, Fagan RL, Yang J, Quelle DE, Brenner C, Fisher RA (2014) RGS6 suppresses Ras-induced cellular transformation by facilitating Tip60-mediated Dnmt1 degradation and promoting apoptosis. Oncogene 33(27):3604–3611
Huang J, Yang J, Maity B, Mayuzumi D, Fisher RA (2011) Regulator of G protein signaling 6 mediates doxorubicin-induced ATM and p53 activation by a reactive oxygen species-dependent mechanism. Cancer Res 71(20):6310–6319
Maity B, Yang J, Huang J, Askeland RW, Bera S, Fisher RA (2011) Regulator of G protein signaling 6 (RGS6) induces apoptosis via a mitochondrial-dependent pathway not involving its GTPase-activating protein activity. J Biol Chem 286(2):1409–1419
Maity B, Stewart A, O’Malley Y, Askeland RW, Sugg SL, Fisher RA (2013) Regulator of G protein signaling 6 is a novel suppressor of breast tumor initiation and progression. Carcinogenesis 34(8):1747–1755
Yang J, Platt LT, Maity B, Ahlers KE, Luo Z, Lin Z, Chakravarti B, Ibeawuchi SR, Askeland RW, Bondaruk J, Czerniak BA, Fisher RA (2016) RGS6 is an essential tumor suppressor that prevents bladder carcinogenesis by promoting p53 activation and DNMT1 downregulation. Oncotarget 7(43):69159–69172
Yang J, Huang J, Maity B, Gao Z, Lorca RA, Gudmundsson H, Li J, Stewart A, Swaminathan PD, Ibeawuchi SR, Shepherd A, Chen CK, Kutschke W, Mohler PJ, Mohapatra DP, Anderson ME, Fisher RA (2010) RGS6, a modulator of parasympathetic activation in heart. Circ Res 107(11):1345–1349
Posokhova E, Wydeven N, Allen KL, Wickman K, Martemyanov KA (2010) RGS6/Gbeta5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ Res 107(11):1350–1354
Posokhova E, Ng D, Opel A, Masuho I, Tinker A, Biesecker LG, Wickman K, Martemyanov KA (2013) Essential role of the m2R-RGS6-IKACh pathway in controlling intrinsic heart rate variability. PLoS ONE 8(10):e76973
Nolte IM, Munoz ML, Tragante V, Amare AT, Jansen R, Vaez A, von der Heyde B, Avery CL, Bis JC, Dierckx B, van Dongen J, Gogarten SM, Goyette P, Hernesniemi J, Huikari V, Hwang SJ, Jaju D, Kerr KF, Kluttig A, Krijthe BP, Kumar J, van der Laan SW, Lyytikainen LP, Maihofer AX, Minassian A, van der Most PJ, Muller-Nurasyid M, Nivard M, Salvi E, Stewart JD, Thayer JF, Verweij N, Wong A, Zabaneh D, Zafarmand MH, Abdellaoui A, Albarwani S, Albert C, Alonso A, Ashar F, Auvinen J, Axelsson T, Baker DG, de Bakker PIW, Barcella M, Bayoumi R, Bieringa RJ, Boomsma D, Boucher G, Britton AR, Christophersen I, Dietrich A, Ehret GB, Ellinor PT, Eskola M, Felix JF, Floras JS, Franco OH, Friberg P, Gademan MGJ, Geyer MA, Giedraitis V, Hartman CA, Hemerich D, Hofman A, Hottenga JJ, Huikuri H, Hutri-Kahonen N, Jouven X, Junttila J, Juonala M, Kiviniemi AM, Kors JA, Kumari M, Kuznetsova T, Laurie CC, Lefrandt JD, Li Y, Li Y, Liao D, Limacher MC, Lin HJ, Lindgren CM, Lubitz SA, Mahajan A, McKnight B, Zu Schwabedissen HM, Milaneschi Y, Mononen N, Morris AP, Nalls MA, Navis G, Neijts M, Nikus K, North KE, O’Connor DT, Ormel J, Perz S, Peters A, Psaty BM, Raitakari OT, Risbrough VB, Sinner MF, Siscovick D, Smit JH, Smith NL, Soliman EZ, Sotoodehnia N, Staessen JA, Stein PK, Stilp AM, Stolarz-Skrzypek K, Strauch K, Sundstrom J, Swenne CA, Syvanen AC, Tardif JC, Taylor KD, Teumer A, Thornton TA, Tinker LE, Uitterlinden AG, van Setten J, Voss A, Waldenberger M, Wilhelmsen KC, Willemsen G, Wong Q, Zhang ZM, Zonderman AB, Cusi D, Evans MK, Greiser HK, van der Harst P, Hassan M, Ingelsson E, Jarvelin MR, Kaab S, Kahonen M, Kivimaki M, Kooperberg C, Kuh D, Lehtimaki T, Lind L, Nievergelt CM, O’Donnell CJ, Oldehinkel AJ, Penninx B, Reiner AP, Riese H, van Roon AM, Rioux JD, Rotter JI, Sofer T, Stricker BH, Tiemeier H, Vrijkotte TGM, Asselbergs FW, Brundel B, Heckbert SR, Whitsel EA, den Hoed M, Snieder H, de Geus EJC (2017) Genetic loci associated with heart rate variability and their effects on cardiac disease risk. Nat Commun 8:15805
Acknowledgements
The authors convey our sincere gratitude to DR. Sayan Biswas, MD, Assistant Professor, Department of Forensic Medicine, College of Medicine and Sagore Dutta Hospital, Kolkata, West Bengal for providing the human autopsy samples. The authors are thankful to Aryakul College of Pharmacy & Research, Lucknow for the help in mice experiments. Dr. Pranesh Kumar was appointed as Assistant Professor there before he moved to University of Lucknow.
Funding
We acknowledge funds from Indian Council of Medical Research (ICMR-5/4/1-26/2020-NCD-I and 5/4/1-22/CVD/2022-NCD-I) to BM.
Author information
Authors and Affiliations
Contributions
Conception and design: MB, AS, and BM. Acquisition of data: MB, KD, TM, DK, PK, NN, and KMP. Analysis and interpretation of data: MB, KD, TM, NN, KMP, PK, PD, and AS, BM. Writing or revision of the manuscript: MB, AS, and BM. Study supervision: AS and BM.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval and consent to participate
Mouse experiments were performed at the Aryakul College of Pharmacy & Research, Lucknow, India under the guidance of Dr. Pranesh Kumar. Animals were procured after obtaining clearance from the college Animal Ethics Committee (1896/PO/Re/S/16/CPCSEA/2021/5). Post-mortem human tissue samples were acquired from the Department of Forensic Medicine, Sagore Dutta Medical College & Hospital, Kolkata, West Bengal after obtaining the ethical clearance from the Centre of Biomedical Research Ethics Committee (Ref: IEC/CBMR/Corr/2020/16/6).
Consent for publication
All the authors give their consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Madhuri Basak and Kiran Das have equally contributed this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Basak, M., Das, K., Mahata, T. et al. RGS7 balances acetylation/de-acetylation of p65 to control chemotherapy-dependent cardiac inflammation. Cell. Mol. Life Sci. 80, 255 (2023). https://doi.org/10.1007/s00018-023-04895-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04895-5