Abstract
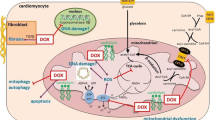
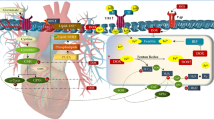
The anti-cancer agent doxorubicin (DOX) has high cardiotoxicity that is linked to DOX-mediated increase in oxidative stress, mitochondrial iron overload, DNA damage, autophagy, necrosis, and apoptosis, all of which are also associated with secondary tumorigenicity. This limits the clinical application of DOX therapies. Previous studies have attributed DOX-mediated cardiotoxicity to mitochondrial iron accumulation and the production of reactive oxygen species (ROS), which seem to be independent of its anti-tumor DNA damaging effects. Chemo-sensitization of soluble guanylate cyclase (sGC) in the cyclic guanosine monophosphate (cGMP) pathway induces tumor cell death despite the cardiotoxicity associated with DOX treatment. However, sGC–cGMP signaling must be activated during heart failure to facilitate myocardial cell survival. The sGC pathway is dependent on nitric oxide and signal transduction via the nitric oxide–sGC–cGMP pathway and is attenuated in various cardiovascular diseases. Additionally, cGMP signaling is regulated by the action of certain phosphodiesterases (PDEs) that protect the heart by inhibiting PDE, an enzyme that hydrolyses cGMP to GMP activity. In this review, we discuss the studies describing the interactions between cGMP regulation and DOX-mediated cardiotoxicity and their application in improving DOX therapeutic outcomes. The results provide novel avenues for the reduction of DOX-induced secondary tumorigenicity and improve cellular autonomy during DOX-mediated cardiotoxicity.

Similar content being viewed by others
References
Sawyer DB. Anthracyclines and heart failure. N Engl J Med. 2013;368(12):1154–6.
Torres VM, Simic VD. Doxorubicin-induced oxidative injury of cardiomyocytes—do we have right strategies for prevention. Cardiotoxic Oncol Treat. 2012:1–43.
Salvatorelli E, Menna P, Chello M, Covino E, Minotti G. Modeling human myocardium exposure to doxorubicin defines the risk of heart failure from low-dose doxorubicin. J Pharmacol Exp Ther. 2017;362(2):263–70.
Jean SR, Tulumello DV, Riganti C, Liyanage SU, Schimmer AD, Kelley SO. Mitochondrial targeting of doxorubicin eliminates nuclear effects associated with cardiotoxicity. ACS Chem Biol. 2015;10(9):2007–15.
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Prasad SVN, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Investig. 2014;124(2):617–30.
Bian K, Murad F. What is next in nitric oxide research? From cardiovascular system to cancer biology. Nitric Oxide. 2014;43:3–7.
Lee DI, Kass DA. Phosphodiesterases and cyclic GMP regulation in heart muscle. Physiology. 2012;27(4):248–58.
Takimoto E. Cyclic GMP-dependent signaling in cardiac myocytes. Circ J. 2012:CJ-12-0664.
Vandenwijngaert S, Swinnen M, Walravens A-S, Beerens M, Gillijns H, Caluwé E, et al. Decreased soluble guanylate cyclase contributes to cardiac dysfunction induced by chronic doxorubicin treatment in mice. Antioxid Redox Signal. 2017;26(4):153–64.
McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75.
Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC Jr, et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc. 2013;2(6):
Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352–80.
Dong J, Chen H. Cardiotoxicity of anticancer therapeutics. Front Cardiovasc Med. 2018;5:9.
Wallace KB, Sardão VA, Oliveira PJJCR. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126(7):926–41.
Han X, Zhou Y, Liu W. Precision cardio-oncology: understanding the cardiotoxicity of cancer therapy. NPJ Precis Oncol. 2017;1(1):1–11.
Mitry MA, Edwards JG. Doxorubicin induced heart failure: phenotype and molecular mechanisms. IJC Heart Vasc. 2016;10:17–24.
Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012;8(4):647–70.
Mouli S, Nanayakkara G, AlAlasmari A, Eldoumani H, Fu X, Berlin A, et al. The role of frataxin in doxorubicin-mediated cardiac hypertrophy. Ame J Physiol Heart Circ Physiol. 2015;309(5):H844–59.
Yadav N, Kumar S, Marlowe T, Chaudhary A, Kumar R, Wang J, et al. Oxidative phosphorylation-dependent regulation of cancer cell apoptosis in response to anticancer agents. Cell Death Dis. 2015;6(11):e1969-e.
Maccarinelli F, Gammella E, Asperti M, Regoni M, Biasiotto G, Turco E, et al. Mice lacking mitochondrial ferritin are more sensitive to doxorubicin-mediated cardiotoxicity. J Mol Med. 2014;92(8):859–69.
Huang ML-H, Becker EM, Whitnall M, Rahmanto YS, Ponka P, Richardson DR. Elucidation of the mechanism of mitochondrial iron loading in Friedreich’s ataxia by analysis of a mouse mutant. Proc Natl Acad Sci. 2009;106(38):16381–6.
Bencze KZ, Yoon T, Millán-Pacheco C, Bradley PB, Pastor N, Cowan J, et al. Human frataxin: iron and ferrochelatase binding surface. Chem Commun. 2007;18:1798–800.
Shimizu R, Lan NN, Tai TT, Adachi Y, Kawazoe A, Mu A, et al. p53 directly regulates the transcription of the human frataxin gene and its lack of regulation in tumor cells decreases the utilization of mitochondrial iron. Gene. 2014;551(1):79–85.
Panjrath GS, Patel V, Valdiviezo CI, Narula N, Narula J, Jain D. Potentiation of doxorubicin cardiotoxicity by iron loading in a rodent model. J Am Coll Cardiol. 2007;49(25):2457–64.
Nie G, Chen G, Sheftel AD, Pantopoulos K, Ponka P. In vivo tumor growth is inhibited by cytosolic iron deprivation caused by the expression of mitochondrial ferritin. Blood. 2006;108(7):2428–34.
Bartesaghi S, Graziano V, Galavotti S, Henriquez NV, Betts J, Saxena J, et al. Inhibition of oxidative metabolism leads to p53 genetic inactivation and transformation in neural stem cells. Proc Natl Acad Sci. 2015;112(4):1059–64.
Galluzzi L, Morselli E, Kepp O, Vitale I, Rigoni A, Vacchelli E, et al. Mitochondrial gateways to cancer. Mol Aspects Med. 2010;31(1):1–20.
Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res. 2012;96(3):456–65.
White E, Mehnert JM, Chan CS. Autophagy, metabolism, and cancer. AACR; 2015.
Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16(11):1069–79.
Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113(18):2221.
Sandner P, Stasch JP. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Respir Med. 2017;122:S1–9.
Morbidelli L, Pyriochou A, Filippi S, Vasileiadis I, Roussos C, Zhou Z, et al. The soluble guanylyl cyclase inhibitor NS-2028 reduces vascular endothelial growth factor-induced angiogenesis and permeability. 2010;298(3):R824–R32.
Hall KC, Bernier SG, Jacobson S, Liu G, Zhang PY, Sarno R, et al. sGC stimulator praliciguat suppresses stellate cell fibrotic transformation and inhibits fibrosis and inflammation in models of NASH. Proc Natl Acad Sci. 2019;116(22):11057–62.
Michalak M, Armstrong PW. Exploring new cardiovascular pathways: are soluble guanylate cyclase stimulators the right direction?: Am Heart Assoc; 2018.
Boerrigter G, Lapp H, Burnett JC. Modulation of cGMP in heart failure: a new therapeutic paradigm. cGMP: generators, effectors and therapeutic implications. Springer; 2009. p. 485-506.
Irvine JC, Ganthavee V, Love JE, Alexander AE, Horowitz JD, Stasch J-P, et al. The soluble guanylyl cyclase activator BAY 58-2667 selectively limits cardiomyocyte hypertrophy. PLoS One. 2012;7(11).
Xiao-Xiao Z, Cho H, Lee S, Woo JS, Song M-Y, Cheng XW, et al. BAY60-2770 attenuates doxorubicin-induced cardiotoxicity by decreased oxidative stress and enhanced autophagy. Chemico Biol Interact. 2020:109190.
Zhang M, Kass DA. Phosphodiesterases and cardiac cGMP: evolving roles and controversies. Trends Pharmacol Sci. 2011;32(6):360–5.
Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540(2):457–67.
Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovasc Res. 1999;41(3):514–23.
Kukreja RC, Salloum FN, Das A. Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J Am Coll Cardiol. 2012;59(22):1921–7.
Booth L, Roberts JL, Poklepovic A, Gordon S, Dent P. PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget. 2017;8(1):1449.
Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci. 2010;107(42):18202–7.
Lee KH, Lee S-R, Cho H, Woo JS, Kang JH, Jeong Y-M, et al. Cardioprotective effects of PKG activation by soluble GC activator, BAY 60-2770, in ischemia-reperfusion-injured rat hearts. PloS One. 2017;12(7).
Nakamura T, Zhu G, Ranek MJ, Kokkonen-Simon K, Zhang M, Kim GE, et al. Prevention of PKG-1α oxidation suppresses antihypertrophic/antifibrotic effects from PDE5 inhibition but not sGC stimulation. Circ Heart Fail. 2018;11(3):e004740.
Ghosh A, Stasch J-P, Papapetropoulos A, Stuehr DJ. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J Biol Chem. 2014;289(22):15259–71.
Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013;280(6):1381–96.
Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16(7):393–405.
Park SJ, Kostic M, Dyson HJ. Dynamic interaction of Hsp90 with its client protein p53. J Mol Biol. 2011;411(1):158–73.
Knorr A, Hirth-Dietrich C, Alonso-Alija C, Härter M, Hahn M, Keim Y, et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittelforschung. 2008;58(02):71–80.
Purohit R, Fritz BG, The J, Issaian A, Weichsel A, David CL, et al. YC-1 binding to the β subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the α subunit. Biochemistry. 2014;53(1):101–14.
Mattern M, Nambi P, Bartus J, Mirabelli C, Crooke S, Johnson R. Regulation of topoisomerase I and II activities by cyclic nucleotide-and phospholipid-dependent protein kinases. Effects of interactions between the two transduction pathways. Receptor. 1991;1(3):181–90.
Skourti-Stathaki K, Proudfoot NJ. A double-edged sword: r loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014;28(13):1384–96.
Popanda O, Thielmann HW. The function of DNA topoisomerases in UV-induced DNA excision repair: studies with specific inhibitors in permeabilized human fibroblasts. Carcinogenesis. 1992;13(12):2321–8.
Lee KH, Cho H, Lee S, Woo JS, Cho BH, Kang JH, et al. Enhanced-autophagy by exenatide mitigates doxorubicin-induced cardiotoxicity. Int J Cardiol. 2017;232:40–7.
Broz DK, Mello SS, Bieging KT, Jiang D, Dusek RL, Brady CA, et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013;27(9):1016–31.
Acknowledgements
We would like to acknowledge the contributions of each of our team members, as without their individual contributions, this article would not have reached fruition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (2017R1C1B5075748) with additional funding from the Ministry of Education (2020R1F1A1076495).
Competing interests
Haneul Cho, Xiao–Xiao Zhao, Sora Lee, Jong Shin Woo, Min-Young Song, Xian Wu Cheng, Kyung Hye Lee, and Weon Kim declare no relationship with industry or other relevant entities that might pose a conflict of interest in connection with this article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
No other person’s work, such as a figure or table, was used in this article. We do not have any content requiring a request for permission.
Authors’ contributions
HC and XXZ participated in drafting the manuscript. SL participated in acquisition of data and table creation. JSW supported research funding. MYS and KHL participated in concept design and analysis. XWC participated in critical revision of the manuscript for important intellectual content. WK participated in analysis as a supervisor. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Cho, H., Zhao, XX., Lee, S. et al. The sGC-cGMP Signaling Pathway as a Potential Therapeutic Target in Doxorubicin-Induced Heart Failure: A Narrative Review. Am J Cardiovasc Drugs 22, 117–125 (2022). https://doi.org/10.1007/s40256-021-00487-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00487-5