Abstract
Huntington’s disease (HD) is a dominantly inherited neurodegenerative disorder resulting from a CAG expansion in the huntingtin (HTT) gene, which leads to the production and accumulation of mutant huntingtin (mHTT). While primarily considered a disorder of the central nervous system, multiple changes have been described to occur throughout the body, including activation of the immune system. In other neurodegenerative disorders, activation of the immune system has been shown to include the production of antibodies against disease-associated pathological proteins. However, the existence of mHTT-targeted antibodies has never been reported. In this study, we assessed the presence and titer of antibodies recognizing HTT/mHTT in patients with HD (n = 66) and age- and gender-matched healthy controls (n = 66) using a combination of Western blotting and ELISA. Together, these analyses revealed that antibodies capable of recognizing HTT/mHTT were detectable in the plasma samples of all participants, including healthy controls. When antibody levels were monitored at different disease stages, it was observed that antibodies against full-length mHTT were highest in patients with severe disease while antibodies against HTTExon1 were elevated in patients with mild disease. Combined, these results suggest that antibodies detecting different forms of mHTT peak at different disease stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder that is defined by a pathological CAG expansion exceeding 35 repeats in Exon1 of the huntingtin (HTT) gene. This leads to the production of a protein containing an expanded polyglutamine (polyQ) stretch referred to as mutant huntingtin (mHTT) [15, 27]. mHTT can be found in the body as a full-length protein or an Exon1 fragment [42]. While the exact contribution of different mHTT forms to the pathology is unclear, it is known that over time, the presence of mHTT within cells causes dysfunction and death, all of which leads to the clinical expression and development of overt HD features. While mHTT is classically described to drive pathology from within the central nervous system (CNS), it is expressed in all cell types and can be found in the cerebrospinal fluid (CSF), the plasma as well as within the extracellular space/matrix [12, 28, 33, 50].
Several systemic abnormalities have been described in HD, including early changes within the immune system [5]. In the CNS, this is observed by a prominent glial response [37] while in the periphery, increases in circulating levels of pro-inflammatory cytokines and chemokines associated with monocyte and macrophage alterations are measurable [8, 41, 49]. The presence of mHTT within peripheral monocytes and T lymphocytes has been suggested to correlate with disease stage and progression of caudate atrophy in patients [48]. Further evidence of interactions between mHTT and the immune system comes from a recent study which demonstrated that prolonged injection of either synthetic mHTT or HTTExon1 fibrils (HD-relevant assemblies of human HTTExon1 fragment protein, aa. 1–90) into the tail vein of mice resulted in the production of antibodies against the injected form of HTT [34]. The generated antibody response was robust, particularly after inoculation of mHTTExon1 fibrils. These findings indicate that the immune system can recognize mHTT as immunogenic and mount an antibody-targeted response against it, at least in mice.
Such antibodies—generally termed autoantibodies—are produced when the immune system targets and reacts to the individual’s own tissues, organs or specific proteins leading to inflammation, damage and dysfunction [19]. In the context of neurodegenerative diseases, there is compelling evidence to suggest that autoantibodies are present in both blood and CSF [17]. For example, autoantibodies directed against amyloid-beta [46], tau [3, 4, 29, 30, 39], α-synuclein [1, 7, 10, 22, 25, 51] and superoxide dismutase [9] have been detected in the CSF or plasma of patients with Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) as well as healthy controls. While such antibodies are consistently found, there is less consensus regarding how levels change during disease with some studies indicating higher levels at later stages [1, 4, 25, 39] while others report a decrease in autoantibody production as the disease progresses [3, 7, 10]. Variability is consistent with findings obtained in traditional autoimmune disorders and it is speculated that factors such as the subtype of antibodies detected, the affinity/avidity of antibodies for the substrate, the various bodily fluid used in antibody screens, the range of age or disease severity of patients included in the studies as well as the age/gender of the control participants could all account for discrepancies in results [2, 13, 20, 31, 44]. In contrast to the detrimental role of autoantibodies in classic autoimmune disorders, such as induction of myelin damage in multiple sclerosis (MS) [18, 21], autoantibodies against pathological proteins have also been speculated to contribute to their clearance [36]. This interpretation is strengthened by a correlation between reduced disease severity and higher autoantibody titer in patients with sporadic ALS [9].
Given the immune dysfunction associated with HD and the production of antibodies against mHTT after peripheral injection in wild-type mice [34], we developed a sensitive plasma screening platform including an indirect enzyme-linked immunosorbent and Western blot assays using multiple forms of HTT/mHTT to determine if an autoantibody response occurs in HD.
Methods
Study cohort
HD patients and age-/gender-matched healthy controls were recruited from HD clinics in Quebec City, Montreal and Cambridge. Each site received approval from the relevant ethical committee (CHU de Québec, #A13-2-1096; CHU of Montreal, #2015-5705; Cambridge Central Regional Motor Ethics Committee, REC #03/303 and #08/H0306/26; Cambridge University Hospitals Foundation Trust Research and Development department, R&D #A085170 and #A091246) and all participants provided informed written consent. In total, blood samples were collected from 66 gene carriers and 66 age- and gender-matched healthy controls (CTRL) (Table 1). The HD group included premanifest gene carriers (n = 18, 9 women and 9 men) and manifest patients with early disease (stage 1–2; n = 24, 11 women and 13 men) or advanced disease (stage 3–5; n = 24, 12 women and 12 men) according to clinical evaluations [5, 40]. A questionnaire related to health issues and medication was completed by the participant and/or their caregiver on the day the blood was collected. Comorbidities were obtained from medical information provided by the questionnaire (Table 1). A routine blood count was concurrently performed for each individual (Table 1) from blood retrieved in dipotassium ethylenediaminetetraacetic acid coated tubes (BD Vacutainer, Cat#367861). According to the reference range [16], no subject with abnormal blood counts (i.e., blood cell count, haematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean platelet volume) was recruited in the study. The mean pathological CAG repeat length within HD patients was 42 ± 2. Clinical evaluations, including the total score of the Unified Huntington’s Disease Rating Scale (UHDRS) [26] and total functional capacity (TFC) [26] were conducted within 6 months of blood collection, with the majority having been performed on the same day as blood sampling (Table 1).
Human plasma isolation
Two citrate blood collection tubes (BD Vacutainer, Cat#363083) per patient were centrifuged 2 × 15 min (min) at 2500g at room temperature (RT). The supernatant was harvested, aliquoted and stored at − 80 °C within 2 hours (h) of sampling.
Production of HTT proteins
All HTT protein samples (wild-type full-length HTT(Q23), full-length mHTT(Q54), Exon1-deleted HTT spanning residues 80–3144 (HTTΔExon1)) were purified, as previously described [23, 24]. Briefly, proteins were produced by baculoviral transduction in Sf9 insect cell culture and purified by FLAG-affinity chromatography and size-exclusion chromatography. pET32a-HD16Q and pET32a-HD46Q were gifts from Pamela Bjorkman (Addgene plasmid # 11487; http://n2t.net/addgene:11487; RRID: Addgene_11487 and Addgene plasmid # 11515; http://n2t.net/addgene:11515; RRID: Addgene_11515 respectively). HTTExon1(Q16) and mHTTExon1(Q46) were expressed in E. coli and purified by Ni-affinity and size-exclusion chromatography, as previously described [6]. The cDNA corresponding to full-length Ataxin3 with 80 glutamine repeats (RRID: Addgene_22129, a gift from Henry Paulson) was subcloned into pFBOH-SBP-TEV and verified by DNA sequencing. Ataxin3 Q80 was produced by baculoviral transduction of this construct in Sf9 insect cell culture. Cells were resuspended in 20 mM HEPES pH 7.4, 300 mM NaCl, 5% (v/v) glycerol, 1 mM TCEP supplemented with protease inhibitors and benzonase. The cell suspension was lysed by sonication and the clarified lysate was incubated with Talon resin (Cytiva). Resin was washed with a purification buffer supplemented with 5 mM imidazole and protein eluted with a purification buffer supplemented with 300 mM imidazole. Eluted protein was further purified by gel filtration using a S200 16/60 column equilibrated in the purification buffer. All samples were aliquoted and flash frozen in liquid nitrogen prior to use. Protein purity was confirmed by SDS-PAGE and sample concentration was measured using a nano drop.
Western blot assays
Quantification of HTT/mHTT-reactive antibodies
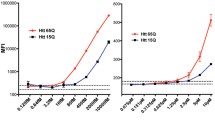
Detection of denatured HTT/mHTT was assessed using Western blotting. To test this, each HTT sample (HTT/mHTT/ HTTΔExon1/HTTExon1/mHTTExon1), as well as commercial Ataxin3 (GenWay, Cat#GWB-P1694D) and Ataxin 3 Q80, were diluted in 1X Laemmli buffer and boiled at 95 °C for 5 min. Five nmol of each protein was then run on an 8% SDS-PAGE gel for 1 h 20 min at 100 V. After migration, proteins were transferred onto Millipore Immobilon-fluorescent low 0.45 μm PVDF membranes (Sigma Millipore, Cat# IPFL00005) O/N at 20 V. Membranes were removed after the transfer and total protein staining was performed using a REVERT kit (LI-COR Biotechnology, Cat#926-11011) according to the manufacturer’s instructions (Supplementary Fig. 1i). Membranes were then blocked for 45 min in 5% milk powder (Bioshop, Cat#SKI400) in phosphate buffered saline with 0.1% Tween 20 (PBST) and incubated O/N with processed plasma (dilution 1/250) from human samples (Supplementary Fig. 1k) or injected mice (Supplementary Fig. 1j) diluted in the same blocking buffer. Membranes were washed with PBST and incubated with HRP-goat anti-human Fc-gamma fragment specific secondary antibody (1/20,000, Jackson ImmunoResearch, Cat#109-035-098) or HRP-horse anti-mouse IgG (1/20,000, Cell Signaling, Cat#7076) diluted in the same blocking solution for 45 min at RT. They were then washed and HRP signal was revealed after a 2-min incubation in chemiluminescence with Immobilon Forte western HRP Substrate (Merck-Millipore, Cat#WBLUF0500) using a myECL imager (ThermoFisher, #G2236X). Band intensity was evaluated with the Odyssey Imaging System (Odyssey, Li-Cor). Human plasma IgG signals against each protein were corrected to REVERT signal intensity (Supplementary Fig. 1i) and to total IgG plasma content (Supplementary Fig. 1d–e).
ELISA
Antibodies against HTT, mHTT and HTTΔExon1 deletion were titered using an indirect homemade ELISA and purified plasma (Supplementary Fig. 1f). A 96-well plate (Nunc MaxiSorp™ Flat-bottom plate, Invitrogen, Cat#44-2404-21) was coated by incubation with 100 µL of 0.1 M PBS solution containing 10 µg/mL of HTT proteins of various lengths or Ataxin3 O/N at 4 °C. The plate was then washed twice with 0.1 M PBS and blocked with 5% milk powder (Bioshop, Cat#SKI400) in PBST for 2 h at RT and washed twice with 0.1 M PBS. To detect the antibody, 100 µl of purified plasma diluted in blocking solution (human: dilution factor from 80 to 2000, mice: dilution factor from 50 to 10,000) was added to the plate O/N at 4 °C. The plate was then washed 6 times with 0.1 M PBS and incubated for 45 min at RT with HRP-goat anti-human Fc-gamma fragment secondary antibody (1/4000, Jackson ImmunoResearch, Cat#109-035-098) or HRP-horse anti-mouse IgG (1/4 000, Cell Signaling, Cat#7076) diluted in blocking solution. After incubation with the secondary antibody, the plate was washed 6 times with PBST and the 3,3′,5,5′-tetramethylbenzidine (e-Bioscience, Cat# 00-4201-56) substrate was added for 8 min at RT. The reaction was stopped by addition of 0.18 M sulfuric acid and signal intensity at 450 nm was measured using a multi-detection microplate reader (Synergy HT, BioTek).
Statistical analyses
For Table 1, comparisons between groups were obtained using Unpaired t-tests or Mann–Whitney tests. Differences between plasma and purified plasma were determined using Wilcoxon matched pairs signed rank test (Supplementary Fig. 1b). For analyses pertaining to Supplementary Fig. 1c, data were compared to a theoretical mean ratio of one using a One-sample t-test. A theoretical ratio of one was used as it corresponds to an equal concentration of IgG between plasma and purified plasma. Correlation between IgG concentration and age were assessed using simple linear regressions (Supplementary Fig. 1e).
Effects of the demographic variables age and sex on IgG concentration were evaluated using a Two-way ANOVA (Table 2). This model failed the test of normality. To produce both a normally distributed data set and a linear model for regression analyses, ELISA data underwent a natural logarithmic transformation and Western data a square root transformation. After the two respective data transformations, the residuals were both randomly and normally distributed for linear regression analyses and normally distributed according to a Shapiro–Wilk test for ANOVA. Therefore, all data shown in Table 2 and Fig. 2 were performed with transformed data. When the entire data set was considered, age affected several of the investigated proteins and therefore comparisons between controls and patients were performed using student’s unpaired t-test following a weight adjustment for age. Given the increased antibody level in mHTT and HTT as measured by Western blotting, the relationship between protein levels in HD gene carriers and CAG repeat length/DBS was evaluated using a simple linear regression. Finally, the relationship between disease stage and antibody levels in HD patients was tested using a simple linear regression. In Fig. 1, the relationship between age and antibody levels in HD patients was found to be absent, so graphs were generated using absolute values. Group differences in the antibody responses obtained by ELISA and Western blot assays were then evaluated to ensure normality of the data sets using Agostino-Pearson Omnibus k2. As unequal variances were detected between groups, a nonparametric test was used (Krustal-Wallis test). Frequency analysis of detectable antibody signal for mHTT/HTTExon1 Western blot was performed using a chi-square test. For Fig. 2, simple linear regressions were performed to evaluate potential relationships between disease metrics and antibody levels. Simple linear regressions were performed using the lm function in R. Unadjusted R2 values were reported. Outliers were removed for one-way ANOVA analyses but were included for all regression analyses except for HTT and mHTT Western blotting data for which they were eliminated as their inclusion resulted in a non-linear model. Outliers were defined as data points that are more than 1.5 interquartile ranges below the first quartile, or 1.5 interquartile ranges above the third quartile. All statistical analyses were completed using Prism®9.3.1 (GraphPad Software) or R Studio®1.3.1093.
HTT and mHTT-reactive antibody response. Detection of antibodies against mHTT(Q54) (a, f), HTT(Q23) (b, g) HTT with Exon1 deletion (HTTΔExon1) (c, h), HTTExon1 (d, i), mHTTExon1 (e, j) was performed by ELISA (a–e) or Western blot (f–j) assays. Purified plasma from premanifest patients (n = 18) and age/gender-matched healthy controls (n = 18) as well as HD patients with early disease (stage 1-2, n = 24) or advanced clinical features (stage 3–5, n = 24) and age-gender matched healthy controls (n = 24/24) were tested. For western blot detection, signal was absent from 21 (HTT with Exon1 deletion), 100 (HTTExon1) and 93 (mHTTExon1) of the 132 participants. Statistical analyses: Kruskal–Wallis test followed by Dunn’s multiple comparisons test (*p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001). Abbreviations: HTT Huntingtin protein, IgG Immunoglobulin gamma, mHTT mutant huntingtin protein
Correlations with disease features. To evaluate the relationship between the antibody response and disease metrics, we performed simple linear regression (a–c) for both ELISA and Western blot data. All HD gene carriers were included in the analysis. P-values: as indicated in the figure. Abbreviations: HTT Huntingtin protein, IgG Immunoglobulin gamma, mHTT mutant huntingtin protein, TFC Total Functional Capacity, UHDRS Unified Huntington’s Disease Rating Scale
Results
Characterization of HTT/mHTT recognizing antibodies
For this study, we recruited premanifest and manifest HD gene carriers (n = 66) along with age/gender-matched healthy controls (n = 66) (Table 1) for a total of 132 participants. Both premanifest and manifest patients were selected to determine if antibodies could be detected at different stages of disease. Manifest patients displayed significantly higher clinical scores, and a higher proportion of subjects were prescribed medication for anxiety and depression, than age/gender-matched healthy controls and pre-manifest gene carriers. All other parameters were equivalent between groups aside from hypercholesterolemia, which was more prevalent in controls (Table 1). To determine if hypercholesteroleamia altered antibody levels in age/gender-matched healthy controls, the nine individuals taking medication for this condition, were compared to the remaining cohort (57 individuals) using an unpaired t-test and no significant differences were observed. Routine haematological measures were similar between HD patients and healthy controls (Table 1).
Detection of HTT/mHTT recognizing antibodies in human plasma
Prior to commencement of the analysis of our clinical cohort, our two detection methods were optimized and validated. Details can be found in Supplementary materials. Once this validation was complete, we performed Western blot and ELISA assays on all samples. These tests revealed that antibodies were detectable in the plasma of all participants using the ELISA method and in both controls and HD gene carriers for Western blot analysis. To further explore these findings, we analyzed our complete data set to determine if demographic factors such as age and gender, which have previously been reported to impact IgG concentrations, affected the overall levels of antibodies in our experimental cohort (Table 2). A two-way ANOVA revealed that age had a significant effect on antibody levels measured by ELISA for HTTExon1 and mHTTExon1 while both age and gender affected the titer of HTT-detecting antibodies. Antibody levels detected by Western blot were not significantly affected by either factor.
After determining that antibodies are present in all participants and that age may significantly affect the titer of antibodies detected by ELISA, we next compared manifest HD patients (n = 48) to controls (n = 66) for all proteins measured by ELISA after correcting for age (Table 2). No significant differences were found. Manifest patients and age/gender matched healthy controls were compared using a student’s unpaired t-test after correcting for age, which indicated that antibody levels were significantly different between patients and controls for both HTT and mHTT (Table 2). Given this significant increase in manifest patients, we next explored the relationship between the detected antibodies and CAG repeat length, a critical determinant of disease as well as a predictor of age of onset (Table 2). However, no correlation between antibody levels and CAG repeat expansion were observed for any of the tested proteins using either detection method. Our previous analyses have highlighted the importance of age as a modifier of antibody levels, so we extended our analysis of CAG repeat length to include disease burden score, a metric which reflects CAG repeat length and age [38, 52]. This measure yielded significant correlations between antibodies recognizing mHTTExon1 and HTTΔExon1 (Table 2). This effect was weaker than the relationship between mHTTExon1 and age alone, and therefore does not seem to indicate that CAG repeat length is an important modifier of antibody levels.
To further explore the relationship between antibody levels and disease, we next evaluated the relationship between antibody levels and disease stage (Table 2). This analysis highlighted a modest but significant correlation between disease stage and mHTT antibody levels as measured by Western blot, as well as a trend towards a significant relationship for antibodies measured by ELISA (Table 2).
Assessment of antibody level progression across disease
To better understand how antibody levels changed across disease progression, we performed a one-way ANOVA analysis of antibody levels in controls, pre-manifest gene carriers and manifest patients with mild or severe disease (Fig. 1). Due to the lack of relationship between age and antibody levels in HD patients, absolute values were used for this portion of the study. This analysis demonstrated a complex relationship between disease severity and antibody titers for mHTT and HTT/mHTTExon1. For mHTT, the one-way ANOVA analysis showed that pre-manifest gene carriers did not significantly differ from controls, while patients at early disease stages displayed a significant decrease in antibody levels which was lost at later disease stages (Fig. 1a). This fluctuation across disease stage was absent for the two disease unrelated proteins, namely HTT and HTTΔExon1 (Fig. 1b, c). In fact, antibodies recognizing these two proteins were found at identical levels in all groups. This contrasted with the antibody levels against the mHTT/HTTExon1 proteins which, when compared to controls, were significantly decreased in pre-manifest patients and increased in patients with early manifest disease. Oddly, manifest patients with advanced disease had antibody levels that were significantly lower than patients with early manifest disease, such that the advanced manifest group did not significantly differ from controls (Fig. 1d, e).
In contrast, we observed a progressive increase in mHTT antibodies measured by Western blotting as disease progressed, with premanifest gene carriers having significantly lower levels than patients at advanced stages of disease (Fig. 1f; Controls vs. patients with stage 3–5 disease p = 0.08). For antibodies detecting HTT, a slightly different pattern was observed with both manifest groups showing similar levels, although only early manifest patients reached statistical significance when compared to premanifest gene carries (Fig. 1g). Finally, no changes in antibody signal against HTTΔExon1 measured by Western blot was observed between any groups (Fig. 1h).
The rarity of participants with detectable levels of mHTT/HTTExon1 antibodies using Western blot techniques made statistical comparison difficult. However, significant increases were observed in patients with early manifest disease as compared to controls (Fig. 1i, j). This finding was supported by a frequency analysis performed using a chi-square test, which showed that individuals with detectable antibody levels were more common in the early manifest group than in the control group (p = 0.02).
Taken together, these results suggest that the specificity of stage 1–2 patients’ antibodies is possibly more directed to mHTTExon1 fragments than mHTT full-length. By contrast, samples from patients with advanced disease (stage 3–5) have more antibodies against the full-length mHTT.
Assessment of the relationship between antibody levels and disease features
Combined, our findings demonstrate that all individuals express antibodies that are capable of recognizing multiple forms of HTT. Furthermore, the degree of detection of full-length HTT/mHTT antibodies is elevated in manifest HD patients and mHTT antibody levels correlate with disease stage. To better understand the relationship between antibody levels and disease features, we performed simple linear regression analyses to compare all five different protein forms to disease metrics (Fig. 2a–c). Of all the protein forms assessed by ELISA, only full-length mHTT showed a statistically significant, but very weak, association with disease symptom severity, as measured by TFC (Fig. 2a).
Correlations between antibody levels detected by Western blot and disease features yielded similar but slightly more robust findings to those of the antibodies measured by ELISA (Fig. 2b, c). Specifically, mHTT significantly correlated with both TFC and UHDRS. While significant correlations were observed, all were relatively weak, indicating that the severity of disease features only explained a small percentage of the change in antibody levels.
Detection of antibodies against another polyQ protein
Overall, our analyses suggest that manifest HD patients, either stage 1–2 or stage 3–5 depending on the protein used to detect antibodies, have more antibodies detecting mHTTExon1 than healthy controls which could indicate a polyQ-specific autoantibody response in this population. Multiple proteins contain polyglutamine tracts, so it is therefore possible that some of the autoantibodies detecting mHTT also recognize other polyglutamine-containing proteins. We, therefore, evaluated if the increased mHTT-recognizing antibody titer present in HD patients was also present for another polyQ-containing protein, namely Ataxin3 [45]. We first identified the optimal Ataxin3 coating concentration for the ELISA plate using a commercial antibody (Supplementary Table 1). Detection of Ataxin3 with either a normal (Supplementary Fig. 2a) or expanded polyQ stretch (Supplementary Fig. 2b) was not increased in stage 3–5 HD patients, as measured by either ELISA or Western blot, which indicates that the heightened response against HTT is not primarily against the polyQ repeat. However, it should be noted that a strong trend towards an increase was observed for Ataxin3 with an expanded polyQ stretch, which could indicate that at least some of the antibodies measured in patients may bind directly to the polyQ stretch.
Discussion
This study provides evidence that antibodies recognizing different forms of HTT/mHTT are present in human plasma. With the use of multiple forms of HTT/mHTT, we were additionally able to establish and characterize the presence of antibodies in purified human plasma using both ELISA and Western blot assays. We have identified a previously unreported aspect of HD pathology by demonstrating that there is an antibody response against HTT/mHTT in samples from 66 HD gene carriers and 66 age- and gender-matched healthy controls and that this response is amplified at certain stages of disease.
Data presented in this manuscript pertain to auto-HTT IgG antibodies. In humans, autoantibodies are of the IgM and IgG isotypes [2]. IgM autoantibodies play a housekeeping role while IgG autoantibodies are usually high-affinity, somatically mutated antibodies of which the expression reflects a pathologic process [19]. It was previously thought that most autoantibodies were IgM, but additional study has highlighted the prominence of IgG isotypes and indicated that their number is influenced by age, gender and disease [35]. In light of these findings, we focused our study on high affinity antibodies by quantifying the presence of auto-IgG antibodies in HD.
Our analyses revealed an increase in the number of antibodies present in manifest patients as compared to healthy age/gender-matched controls. While certain populations of antibodies were solely elevated in patients with stage 3–5 HD, others were additionally raised in stage 1–2 patients, indicating that increased production of mHTT recognizing antibodies is an early feature of disease which continues to change throughout the course of disease. This elevation is consistent with previous studies reporting that there is a general increase in the production and release of antibodies against autoantigens with age and disease [47], presumably as cells die and release more immunogenic antigen. The previously reported increase in inflammatory markers throughout disease progression is also likely to influence the changes in antibody levels [14]. However, the differing patterns found for the various HTT forms could indicate that antibody levels are impacted by other factors. From the current study, it is not possible to determine if the antibodies present in HD patients were initially raised against HTT, mHTT or both. However, the lack of increase in HTT-reactive antibodies (Fig. 1), as well as the absence of correlation between HTT-reactive antibody titers and clinical scores (i.e., TFC) (Fig. 2) could suggest that at least a subset of the antibodies recognizing mHTT are not the same as those detecting HTT, or that the affinity of these antibodies is stronger for mHTT than for HTT. The idea that the expanded CAG repeat is important for detection of the full-length protein is further supported by the Ataxin 3 data which showed a trend towards an increase in severe manifest patients that mirrored the results from the full-length mHTT protein (Supplementary Fig. 2). Further work with additional CAG repeat containing proteins would be required to confirm this hypothesis, especially since the length of the CAG repeat in the ataxin protein was longer than that observed for mHTT.
According to our data, the epitopes favoured by antibodies changed with disease severity and age. Specifically, our results show a transient increase in antibodies against mHTT/HTTExon1 in stage 1–2 patients which was contrasted by a steady increase in mHTT detecting antibodies measured by western blot across disease (Fig. 1). This transient detection of Exon1 is intriguing and would suggest that Exon1 fragments are more prevalent in the blood earlier in disease, with full-length forms more predominant in later disease. This hypothesis would be consistent with the idea that levels of soluble HTT decrease with disease progression and that small N-terminal fragments are more prone to aggregation than the full-length protein [32, 43]. While the presence of the alternatively spliced Exon1 fragment could explain this transient increase in early manifest HD patients, it does not explain the presence of HTT-recognizing antibodies in the plasma of healthy controls. For HTT-directed antibodies to be present, HTT release into the plasma must occur under physiological conditions. Recent work supports this idea through the demonstration that both forms of the protein are actively secreted by neurons [11]. Therefore, this process is likely to be a contributing factor to the generation of HTT autoantibodies in HD patients as well as in healthy individuals. One question that remains to be answered is how HTT/mHTT species may differ between controls and patients at various stages of disease. To date, there is no consensus regarding the type of HTT/mHTT forms present in different bodily fluids, and it is quite possible that there is a large spectrum of HTT/mHTT epitopes present in HD patients and healthy controls, including some outside of Exon1. It is additionally probable that the relative concentrations of these different HTT proteoforms would influence the HTT autoantibody titer. Therefore, further work will be needed to both characterize the HTT proteoforms measurable in plasma as well as to better delineate the epitopes recognized by circulating HTT-reactive antibodies as they arise during the disease process.
Conclusion
Using two complementary methods, we show that HTT/mHTT detecting antibodies are present within the plasma of all tested participants, including healthy controls. We further demonstrate that their levels change in HD patients as disease progresses. Specifically, HTT/mHTTExon1 recognizing antibodies are predominant in early manifest HD followed by higher levels of mHTT antibodies later in disease. Additional work is waranted to evaluate the relationships between the levels of different forms of plasma HTT and antibody levels.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akhtar RS, Licata JP, Luk KC, Shaw LM, Trojanowski JQ, Lee VM-Y (2018) Measurements of auto-antibodies to α-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease. J Neurochem 145:489–503. https://doi.org/10.1111/jnc.14330
Avrameas S, Ternynck T, Tsonis IA, Lymberi P (2007) Naturally occurring B-cell autoreactivity: a critical overview. J Autoimmun 29:213–218. https://doi.org/10.1016/j.jaut.2007.07.010
Bartos A, Fialová L, Švarcová J (2018) Lower serum antibodies against tau protein and heavy neurofilament in Alzheimer’s disease. J Alzheimers Dis 64:751–760. https://doi.org/10.3233/JAD-180039
Bartos A, Fialová L, Svarcová J, Ripova D (2012) Patients with Alzheimer disease have elevated intrathecal synthesis of antibodies against tau protein and heavy neurofilament. J Neuroimmunol 252:100–105. https://doi.org/10.1016/j.jneuroim.2012.08.001
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ (2015) Huntington disease. Nat Rev Dis Primers 1:15005. https://doi.org/10.1038/nrdp.2015.5
Bennett MJ, Huey-Tubman KE, Herr AB, West AP, Ross SA, Bjorkman PJ (2002) A linear lattice model for polyglutamine in CAG-expansion diseases. Proc Natl Acad Sci USA 99:11634–11639. https://doi.org/10.1073/pnas.182393899
Besong-Agbo D, Wolf E, Jessen F, Oechsner M, Hametner E, Poewe W, Reindl M, Oertel WH, Noelker C, Bacher M, Dodel R (2013) Naturally occurring α-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology 80:169–175. https://doi.org/10.1212/WNL.0b013e31827b90d1
Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, Leavitt BR, Möller T, Tabrizi SJ (2008) A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med 205:1869–1877. https://doi.org/10.1084/jem.20080178
van Blitterswijk M, Gulati S, Smoot E, Jaffa M, Maher N, Hyman BT, Ivinson AJ, Scherzer CR, Schoenfeld DA, Cudkowicz ME, Brown RH, Bosco DA (2011) Anti-superoxide dismutase antibodies are associated with survival in patients with sporadic amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12:430–438. https://doi.org/10.3109/17482968.2011.585163
Brudek T, Winge K, Folke J, Christensen S, Fog K, Pakkenberg B, Pedersen LØ (2017) Autoimmune antibody decline in Parkinson’s disease and Multiple System Atrophy; a step towards immunotherapeutic strategies. Mol Neurodegener 12:44. https://doi.org/10.1186/s13024-017-0187-7
Caron NS, Banos R, Yanick C, Aly AE, Byrne LM, Smith ED, Xie Y, Smith SEP, Potluri N, Findlay Black H, Casal L, Ko S, Cheung D, Kim H, Seong IS, Wild EJ, Song J-J, Hayden MR, Southwell AL (2021) Mutant huntingtin is cleared from the brain via active mechanisms in Huntington disease. J Neurosci 41:780–796. https://doi.org/10.1523/JNEUROSCI.1865-20.2020
Cicchetti F, Lacroix S, Cisbani G, Vallières N, Saint-Pierre M, St-Amour I, Tolouei R, Skepper JN, Hauser RA, Mantovani D, Barker RA, Freeman TB (2014) Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann Neurol 76:31–42. https://doi.org/10.1002/ana.24174
Cohen IR (2007) Biomarkers, self-antigens and the immunological homunculus. J Autoimmun 29:246–249. https://doi.org/10.1016/j.jaut.2007.07.016
Crotti A, Glass CK (2015) The choreography of neuroinflammation in Huntington’s disease. Trends Immunol 36:364–373. https://doi.org/10.1016/j.it.2015.04.007
Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90:537–548. https://doi.org/10.1016/S0092-8674(00)80513-9
Dean L (2005) Blood and the cells it contains. NCBI Bookshelf 1:1–41
DeMarshall C, Sarkar A, Nagele EP, Goldwaser E, Godsey G, Acharya NK, Nagele RG (2015) Utility of autoantibodies as biomarkers for diagnosis and staging of neurodegenerative diseases. Int Rev Neurobiol 122:1–51. https://doi.org/10.1016/bs.irn.2015.05.005
Egg R, Reindl M, Deisenhammer F, Linington C, Berger T (2001) Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult Scler 7:285–289. https://doi.org/10.1177/135245850100700503
Elkon K, Casali P (2008) Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 4:491–498. https://doi.org/10.1038/ncprheum0895
Folke J, Rydbirk R, Løkkegaard A, Salvesen L, Hejl A-M, Starhof C, Bech S, Winge K, Christensen S, Pedersen LØ, Aznar S, Pakkenberg B, Brudek T (2019) Distinct autoimmune anti-α-synuclein antibody patterns in multiple system atrophy and Parkinson’s disease. Front Immunol 10:2253. https://doi.org/10.3389/fimmu.2019.02253
Genain CP, Cannella B, Hauser SL, Raine CS (1999) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5:170–175. https://doi.org/10.1038/5532
Gruden MA, Sewell RDE, Yanamandra K, Davidova TV, Kucheryanu VG, Bocharov EV, Bocharova OA, Bocharova OR, Polyschuk VV, Sherstnev VV, Morozova-Roche LA (2011) Immunoprotection against toxic biomarkers is retained during Parkinson’s disease progression. J Neuroimmunol 233:221–227. https://doi.org/10.1016/j.jneuroim.2010.12.001
Harding RJ, Deme JC, Hevler JF, Tamara S, Lemak A, Cantle JP, Szewczyk MM, Begeja N, Goss S, Zuo X, Loppnau P, Seitova A, Hutchinson A, Fan L, Truant R, Schapira M, Carroll JB, Heck AJR, Lea SM, Arrowsmith CH (2021) Huntingtin structure is orchestrated by HAP40 and shows a polyglutamine expansion-specific interaction with exon 1. Commun Biol 4:1–16. https://doi.org/10.1038/s42003-021-02895-4
Harding RJ, Loppnau P, Ackloo S, Lemak A, Hutchinson A, Hunt B, Holehouse AS, Ho JC, Fan L, Toledo-Sherman L, Seitova A, Arrowsmith CH (2019) Design and characterization of mutant and wildtype huntingtin proteins produced from a toolkit of scalable eukaryotic expression systems. J Biol Chem 294:6986–7001. https://doi.org/10.1074/jbc.RA118.007204
Horvath I, Iashchishyn IA, Forsgren L, Morozova-Roche LA (2017) Immunochemical detection of α-synuclein autoantibodies in Parkinson’s disease: correlation between plasma and cerebrospinal fluid levels. ACS Chem Neurosci 8:1170–1176. https://doi.org/10.1021/acschemneuro.7b00063
Huntington Study Group (1996) Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord 11:136–142. https://doi.org/10.1002/mds.870110204
Jana NR, Zemskov EA, Wang Gh, Nukina N (2001) Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 10:1049–1059. https://doi.org/10.1093/hmg/10.10.1049
Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J, Lee N, Li L, Im W, Kim M, Kim HS, Oh S-H, Kim T-A, Ko JJ, Aubé B, Oueslati A, Kim YJ, Song J (2016) Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol 132:577–592. https://doi.org/10.1007/s00401-016-1582-9
Klaver AC, Coffey MP, Bennett DA, Loeffler DA (2017) Specific serum antibody binding to phosphorylated and non-phosphorylated tau in non-cognitively impaired, mildly cognitively impaired, and Alzheimer’s disease subjects: an exploratory study. Transl Neurodegener 6:32. https://doi.org/10.1186/s40035-017-0100-x
Krestova M, Ricny J, Bartos A (2018) Changes in concentrations of tau-reactive antibodies are dependent on sex in Alzheimer’s disease patients. J Neuroimmunol 322:1–8. https://doi.org/10.1016/j.jneuroim.2018.05.004
Kronimus Y, Dodel R, Neumann S (2018) A Quantitative view on naturally occurring autoantibodies in neurodegenerative diseases. J Neurol Neuromed 3:4. https://doi.org/10.29245/2572.942X/2018/4.1189
Landles C, Milton RE, Jean A, McLarnon S, McAteer SJ, Taxy BA, Osborne GF, Zhang C, Duan W, Howland D, Bates GP (2021) Development of novel bioassays to detect soluble and aggregated Huntingtin proteins on three technology platforms. Brain Commun 3:fcaa231. https://doi.org/10.1093/braincomms/fcaa231
Liu X, Valentine SJ, Plasencia MD, Trimpin S, Naylor S, Clemmer DE (2007) Mapping the human plasma proteome by SCX-LC-IMS-MS. J Am Soc Mass Spectrom 18:1249–1264. https://doi.org/10.1016/j.jasms.2007.04.012
Masnata M, Sciacca G, Maxan A, Bousset L, Denis HL, Lauruol F, David L, Saint-Pierre M, Kordower JH, Melki R, Alpaugh M, Cicchetti F (2019) Demonstration of prion-like properties of mutant huntingtin fibrils in both in vitro and in vivo paradigms. Acta Neuropathol. https://doi.org/10.1007/s00401-019-01973-6
Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG (2013) Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS ONE 8:e60726. https://doi.org/10.1371/journal.pone.0060726
Neff F, Wei X, Nölker C, Bacher M, Du Y, Dodel R (2008) Immunotherapy and naturally occurring autoantibodies in neurodegenerative disorders. Autoimmun Rev 7:501–507. https://doi.org/10.1016/j.autrev.2008.04.010
Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A (2019) The role of microglia and astrocytes in Huntington’s disease. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2019.00258
Penney JB Jr, Vonsattel J-P, Macdonald ME, Gusella JF, Myers RH (1997) CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol 41:689–692. https://doi.org/10.1002/ana.410410521
Rosenmann H, Meiner Z, Geylis V, Abramsky O, Steinitz M (2006) Detection of circulating antibodies against tau protein in its unphosphorylated and in its neurofibrillary tangles-related phosphorylated state in Alzheimer’s disease and healthy subjects. Neurosci Lett 410:90–93. https://doi.org/10.1016/j.neulet.2006.01.072
Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ (2014) Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10:204–216. https://doi.org/10.1038/nrneurol.2014.24
Sassone J, Colciago C, Cislaghi G, Silani V, Ciammola A (2009) Huntington’s disease: the current state of research with peripheral tissues. Exp Neurol 219:385–397. https://doi.org/10.1016/j.expneurol.2009.05.012
Saudou F, Humbert S (2016) The biology of Huntingtin. Neuron 89:910–926. https://doi.org/10.1016/j.neuron.2016.02.003
Schindler F, Praedel N, Neuendorf N, Kunz S, Schnoegl S, Mason MA, Taxy BA, Bates GP, Khoshnan A, Priller J, Grimm J, Maier M, Boeddrich A, Wanker EE (2021) Small, seeding-competent huntingtin fibrils are prominent aggregate species in brains of zQ175 Huntington’s disease knock-in mice. Front Neurosci 15:682172. https://doi.org/10.3389/fnins.2021.682172
Sim K-Y, Im KC, Park S-G (2020) The functional roles and applications of immunoglobulins in neurodegenerative disease. Int J Mol Sci. https://doi.org/10.3390/ijms21155295
Sullivan R, Yau WY, O’Connor E, Houlden H (2019) Spinocerebellar ataxia: an update. J Neurol 266:533–544. https://doi.org/10.1007/s00415-018-9076-4
Szabo P, Relkin N, Weksler ME (2008) Natural human antibodies to amyloid beta peptide. Autoimmun Rev 7:415–420. https://doi.org/10.1016/j.autrev.2008.03.007
Watad A, Bragazzi NL, Adawi M, Amital H, Toubi E, Porat B-S, Shoenfeld Y (2017) Autoimmunity in the elderly: insights from basic science and clinics—a mini-review. GER 63:515–523. https://doi.org/10.1159/000478012
Weiss A, Träger U, Wild EJ, Grueninger S, Farmer R, Landles C, Scahill RI, Lahiri N, Haider S, Macdonald D, Frost C, Bates GP, Bilbe G, Kuhn R, Andre R, Tabrizi SJ (2012) Mutant huntingtin fragmentation in immune cells tracks Huntington’s disease progression. J Clin Invest 122:3731–3736. https://doi.org/10.1172/JCI64565
Wild E, Magnusson A, Lahiri N, Krus U, Orth M, Tabrizi SJ, Björkqvist M (2011) Abnormal peripheral chemokine profile in Huntington’s disease. PLoS Curr 3:RRN1231. https://doi.org/10.1371/currents.RRN1231
Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S, Miller JRC, Zetterberg H, Leavitt BR, Kuhn R, Tabrizi SJ, Macdonald D, Weiss A (2015) Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington’s disease patients. J Clin Invest 125:1979–1986. https://doi.org/10.1172/JCI80743
Yanamandra K, Gruden MA, Casaite V, Meskys R, Forsgren L, Morozova-Roche LA (2011) α-synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson’s disease patients. PLoS ONE 6:e18513. https://doi.org/10.1371/journal.pone.0018513
Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS, PREDICT-HD Investigators and Coordinators of the Huntington Study Group (2011) Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet 156B:751–763. https://doi.org/10.1002/ajmg.b.31232
Acknowledgements
The authors would like to thank all the students and staff who helped with the blood drives in Quebec City, Montreal and Cambridge, and in particular the patients and their families who generously gave their time to participate in this study. We would also like to thank the biotechnology team at SGC Toronto (Peter Loppnau, Alma Seitova, Ashley Hutchinson) for helping generate the full-length HTT protein samples and Renu Chandrasekaran for purification of ataxin3 samples. Special thanks to Dr. Ray Truant of McMaster University, Canada for valuable discussions. This study was funded by the Canadian Institutes of Health Research (CIHR) -FC, the Fonds de recherche du Québec (FRQS) en santé-FC. Partial funding was received from the Huntington Society of Canada-RJH and CHA, NIHR -RAB. The Structural Genomics Consortium is a registered charity (No: 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], the EU and EFPIA through the Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN Grant 875510], Janssen, Merck KGaA (aka EMD in Canada and US), Pfizer, Takeda and the Wellcome Trust [106169/ZZ14/Z]. RM received support from the EC Joint Programme on Neurodegenerative Diseases and Agence Nationale pour la Recherche (TransPathND, ANR-17-JPCD-0002-02). HLD was supported by FRQS doctoral research award and MA by a post-doctoral fellowship from CIHR. MA currently receives salary support from the Huntington’s Disease Society of America (HDSA) and is now affiliated with the University of Guelph. RJH is the recipient of the HDSA Berman Topper Career Development Fellowship.
Funding
This study was funded by the Canadian Institutes of Health Research (CIHR; Grant Number PJT-168865) to FC who is also a recipient of a Researcher Chair from the Fonds de recherche du Québec (FRQS; Grant Number 28941) en santé providing salary support and operating funds. This research was also partly supported by a grant from the Huntington Society of Canada to RJH and CHA and an NIHR grant funding the Cambridge Biomedical Research Centre (RAB). The Structural Genomics Consortium is a registered charity (No: 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], the EU and EFPIA through the Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN Grant 875510], Janssen, Merck KGaA (aka EMD in Canada and US), Pfizer, Takeda and the Wellcome Trust [106169/ZZ14/Z]. RM received support from the EC Joint Programme on Neurodegenerative Diseases and Agence Nationale pour la Recherche (TransPathND, ANR-17-JPCD-0002–02). HLD was supported by FRQS doctoral research award and MA by a post-doctoral fellowship from CIHR. MA currently receives salary support from the Huntington’s Disease Society of America (HDSA) and is now affiliated with the University of Guelph. RJH is the recipient of the HDSA Berman Topper Career Development Fellowship.
Author information
Authors and Affiliations
Contributions
HLD: designed the experiments and participated in the blood drives. She conducted human/mice experiments, analyzed and interpretated data. She wrote the first version of the manuscript and prepared figures and table. MA: was involved in the experimental design, discussion and took part in data analysis and interpretation. She also helped write the manuscript. CPA: contributed to the design of the ELISA experiments, performed some of the mice analyses and revised the manuscript. LB: generated mHTT fibrils for the mice study. RAB: recruited patients-Cambridge, UK. He revised the manuscript. SC: recruited patients-Montreal, Canada. RM: provided the mHTT fibrils used in the mice study and was involved in the data interpretation of the related experiments. CHA: helped supervise the project and contributed to study discussions. RL: provided advice regarding all statistical analyses and revised the manuscript. RJH: produced and purified HTT/mHTT proteins. She was involved in discussions pertaining to the study and revised the manuscript. FC: initiated the study and was involved in the experimental design. She supervised the project and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
HD patients and age-/gender-matched healthy controls were recruited from HD clinics in Quebec City, Montreal and Cambridge. Each site received approval from the relevant ethical committee (CHU de Québec, #A13-2-1096; CHU of Montreal, #2015-5705; Cambridge Central Regional Motor Ethics Committee, REC #03/303 & #08/H0306/26; Cambridge University Hospitals Foundation Trust Research and Development department, R&D #A085170 & #A091246).
Consent to participate
All participants provided informed written consent.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Denis, H.L., Alpaugh, M., Alvarez, C.P. et al. Detection of antibodies against the huntingtin protein in human plasma. Cell. Mol. Life Sci. 80, 45 (2023). https://doi.org/10.1007/s00018-023-04687-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04687-x