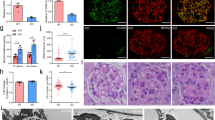
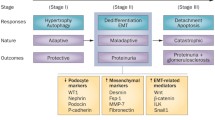
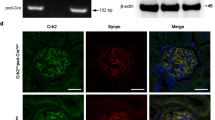
Abstract
Glomerular diseases afflict millions of people and impose an enormous burden on public healthcare costs worldwide. Identification of potential therapeutic targets for preventing glomerular diseases is of considerable clinical importance. CHILKBP is a focal adhesion protein and modulates a wide array of biological functions. However, little is known about the role of CHILKBP in glomerular diseases. To investigate the function of CHILKBP in maintaining the structure and function of podocytes in a physiologic setting, a mouse model (CHILKBP cKO) was generated in which CHILKBP gene was conditionally deleted in podocytes using the Cre-LoxP system. Ablation of CHILKBP in podocytes resulted in massive proteinuria and kidney failure in mice. Histologically, typical podocyte injury including podocyte loss, foot process effacement, and glomerulosclerosis was observed in CHILKBP cKO mice. Mechanistically, we identified ZO-1 as a key junctional protein that interacted with CHILKBP. Loss of CHILKBP in podocytes exhibited a significant reduction of ZO-1 expression, leading to abnormal actin organization, aberrant slit diaphragm protein expression and compromised podocyte filtration capacity. Restoration of CHILKBP or ZO-1 in CHILKBP-deficient podocytes effectively alleviated podocyte injury induced by the loss of CHILKBP in vitro and in vivo. Finally, we showed the glomerular expression of CHILKBP and ZO-1 was decreased in patients with proteinuric kidney diseases. Our findings reveal a novel signaling pathway consisting of CHILKBP and ZO-1 that plays an essential role in maintaining podocyte homeostasis and suggest novel therapeutic approaches to alleviate glomerular diseases.









Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary files. Requests for materials should be addressed to YS.
References
Mortality GBD (2016) Causes of Death. C Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388:1459–544
Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V (2017) Chronic kidney disease and the global NCDs agenda. BMJ Glob Health 2:e000380
Collaboration GBDCKD (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 395:709–33.
Liu ZH (2013) Nephrology in China. Nat Rev Nephrol 9:523–528
Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J (2005) Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16:1733–1741
Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R (2010) Targeting podocyte-associated diseases. Adv Drug Deliv Rev 62:1325–1336
Mundel P, Shankland SJ (2002) Podocyte biology and response to injury. J Am Soc Nephrol 13:3005–3015
Brinkkoetter PT, Ising C, Benzing T (2013) The role of the podocyte in albumin filtration. Nat Rev Nephrol 9:328–336
Tu Y, Huang Y, Zhang Y, Hua Y, Wu C (2001) A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 153:585–598
Montanez E, Wickstrom SA, Altstatter J, Chu H, Fassler R (2009) Alpha-parvin controls vascular mural cell recruitment to vessel wall by regulating RhoA/ROCK signalling. EMBO J 28:3132–3144
Fraccaroli A, Pitter B, Taha AA, Seebach J, Huveneers S, Kirsch J et al (2015) Endothelial alpha-parvin controls integrity of developing vasculature and is required for maintenance of cell-cell junctions. Circ Res 117:29–40
Altstatter J, Hess MW, Costell M, Montanez E (2020) alpha-parvin is required for epidermal morphogenesis, hair follicle development and basal keratinocyte polarity. PLoS ONE 15:e0230380
Sun Y, Ding Y, Guo C, Liu C, Ma P, Ma S et al (2019) alpha-Parvin promotes breast cancer progression and metastasis through interaction with G3BP2 and regulation of TWIST1 signaling. Oncogene 38:4856–4874
Ito M, Hagiyama M, Mimae T, Inoue T, Kato T, Yoneshige A et al (2014) alpha-Parvin, a pseudopodial constituent, promotes cell motility and is associated with lymph node metastasis of lobular breast carcinoma. Breast Cancer Res Treat 144:59–69
Pignatelli J, LaLonde SE, LaLonde DP, Clarke D, Turner CE (2012) Actopaxin (alpha-parvin) phosphorylation is required for matrix degradation and cancer cell invasion. J Biol Chem 287:37309–37320
Itoh M, Nakadate K, Horibata Y, Matsusaka T, Xu J, Hunziker W et al (2014) The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS ONE 9:e106621
Itoh M, Nakadate K, Matsusaka T, Hunziker W, Sugimoto H (2018) Effects of the differential expression of ZO-1 and ZO-2 on podocyte structure and function. Genes Cells 23:546–556
Schnabel E, Anderson JM, Farquhar MG (1990) The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111:1255–1263
Odenwald MA, Choi W, Buckley A, Shashikanth N, Joseph NE, Wang Y et al (2017) ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J Cell Sci 130:243–259
Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM et al (2015) ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 208:821–838
Itoh M, Nagafuchi A, Moroi S, Tsukita S (1997) Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 138:181–192
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273:29745–29753
Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K (2011) Transcriptome analysis of human diabetic kidney disease. Diabetes 60:2354–2369
Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S et al (2010) A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol 177:1674–1686
Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S et al (2006) Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17:2164–2175
Kim JH, Xie J, Hwang KH, Wu YL, Oliver N, Eom M et al (2017) Klotho may ameliorate proteinuria by targeting TRPC6 channels in podocytes. J Am Soc Nephrol 28:140–151
Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J (2004) Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci U S A 101:14883–14888
Liu F, Song Y, Liu D (1999) Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 6:1258–1266
Song YK, Liu F, Zhang G, Liu D (2002) Hydrodynamics-based transfection: simple and efficient method for introducing and expressing transgenes in animals by intravenous injection of DNA. Methods Enzymol 346:92–105
Okuda S, Languino LR, Ruoslahti E, Border WA (1990) Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 86:453–62
Schaier M, Lehrke I, Schade K, Morath C, Shimizu F, Kawachi H et al (2001) Isotretinoin alleviates renal damage in rat chronic glomerulonephritis. Kidney Int 60:2222–2234
Yamamoto T, Noble NA, Miller DE, Border WA (1994) Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int 45:916–927
Schwayer C, Shamipour S, Pranjic-Ferscha K, Schauer A, Balda M, Tada M et al (2019) Mechanosensation of tight junctions depends on ZO-1 phase separation and flow. Cell 179(937–52):e18
Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C, Honigmann A (2019) Phase separation of zonula occludens proteins drives formation of tight junctions. Cell 179(923–36):e11
Sun Y, Guo C, Ma P, Lai Y, Yang F, Cai J et al (2017) Kindlin-2 association with Rho GDP-dissociation inhibitor alpha suppresses Rac1 activation and podocyte injury. J Am Soc Nephrol 28:3545–3562
Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K et al (2014) Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124:1098–1113
Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y (2009) Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20:1997–2008
Sun Y, Duan Y, Eisenstein AS, Hu W, Quintana A, Lam WK et al (2012) A novel mechanism of control of NFkappaB activation and inflammation involving A2B adenosine receptors. J Cell Sci 125:4507–4517
Qian T, Liu C, Ding Y, Guo C, Cai R, Wang X et al (2020) PINCH-1 interacts with myoferlin to promote breast cancer progression and metastasis. Oncogene 39:2069–2087
Li M, Corbelli A, Watanabe S, Armelloni S, Ikehata M, Parazzi V et al (2016) Three-dimensional podocyte-endothelial cell co-cultures: assembly, validation, and application to drug testing and intercellular signaling studies. Eur J Pharm Sci 86:1–12
Honda S, Shirotani-Ikejima H, Tadokoro S, Tomiyama Y, Miyata T (2013) The integrin-linked kinase-PINCH-parvin complex supports integrin alphaIIbbeta3 activation. PLoS ONE 8:e85498
Wu C (2004) The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta 1692:55–62
Sepulveda JL, Wu C (2006) The parvins. Cell Mol Life Sci 63:25–35
Legate KR, Montanez E, Kudlacek O, Fassler R (2006) ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7:20–31
Lu CC, Wang GH, Lu J, Chen PP, Zhang Y, Hu ZB et al (2019) Role of podocyte injury in glomerulosclerosis. Adv Exp Med Biol 1165:195–232
Chen W, Wang S, Adhikari S, Deng Z, Wang L, Chen L et al (2016) Simple and integrated spintip-based technology applied for deep proteome profiling. Anal Chem 88:4864–4871
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O et al (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589
Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS (1988) Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol 106:1141–1149
Kawachi H, Fukusumi Y (2020) New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol 24:193–204
Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B et al (2003) The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem 278:13417–13421
Chen VC, Li X, Perreault H, Nagy JI (2006) Interaction of zonula occludens-1 (ZO-1) with alpha-actinin-4: application of functional proteomics for identification of PDZ domain-associated proteins. J Proteome Res 5:2123–2134
Soler MJ, Riera M, Batlle D (2012) New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy. Exp Diabetes Res 2012:616313
Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J et al (2009) Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296:F213–F229
Menon MC, Chuang PY, He CJ (2012) The glomerular filtration barrier: components and crosstalk. Int J Nephrol 2012:749010
Schlondorff D, Wyatt CM, Campbell KN (2017) Revisiting the determinants of the glomerular filtration barrier: what goes round must come round. Kidney Int 92:533–536
El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G et al (2006) Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17:1334–1344
Acknowledgements
We thank Dr. Ruijun Tian of the Department of Chemistry, Southern University of Science and Technology, for help with nano-LC-MS/MS analysis; Jin Menglang in the Sun Yat-sen University for help with scanning electron microscopy and transmission electron microscopy characterization; Keyu Chen and Haiyang Liu, Shenzhen Bay Laboratory, for technical assistance. We thank SUSTech Animal Facility for the maintenance of mice and the SUSTech Core Facility for supports on the use of the confocal microscopy and transmission electron microscopy.
Funding
This work was supported, in part, by grants from the National Natural Science Foundation of China (82070728, 81900632, and 81772983); the Natural Science Foundation of Guangdong Province (2020A1515011305, 2121B1515120063 and 2017B030301018); the Shenzhen Innovation Committee of Science and Technology, China (JCYJ20190809141003834, JCYJ20200109141212325); the Stable Support Plan Program of Shenzhen Natural Science Fund (Grant No. GXWD20201230110313001 (Program Contract No.20200925153241002)).
Author information
Authors and Affiliations
Contributions
Y.S. designed the study, supervised the project and wrote the manuscript; C.W. provided advice on some experiments and wrote the manuscript; C.G., Y.D., A.Y.,Y.G., C.L., L.Z., L.M., Z.Y., F.H., K.J., R.C., and P.B. performed the experiments and data analysis; Y.D. and M.Q. advised on some experiments; C.G., Y.D., A.Y., Y.G. and Y.S. take the responsibility for the integrity of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Ethics approval
All animal experiments were performed in accordance with the guidelines and regulations and approved by the Institutional Animal Care and Use Committee at the Southern University of Science and Technology of China (Approval Number: SUSTC-JY2020015). All studies for human kidney samples complied the Ethics Committee of the First Affiliated Hospital of Kunming Medical University, and informed consent was obtained from all participants in this research.
Consent to participate
Written informed consent was obtained from individual or guardian participants.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 8B.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, C., Ding, Y., Yang, A. et al. CHILKBP protects against podocyte injury by preserving ZO-1 expression. Cell. Mol. Life Sci. 80, 18 (2023). https://doi.org/10.1007/s00018-022-04661-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04661-z