Abstract
Objective
Aging is associated with compromised immune function and arterial remodeling and stiffness. The purpose of this study is to investigate whether in vivo AAV-based delivery of secreted Klotho (SKL) gene (AAV-SKL) improves aging- and senescence-associated immune dysfunction and arterial stiffness.
Methods and results
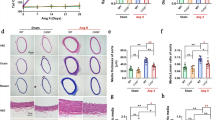
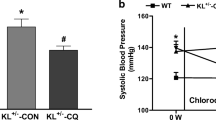
Senescence-accelerated mice prone strain 1 (SAMP1, 10 months) and old mice (20 months) were used. Serum SKL levels, B-cell population and serum IgG levels were markedly decreased in SAMP1 and old mice. Rescue of downregulation of serum SKL levels by in vivo AAV2-based delivery of SKL gene (AAV-SKL) increased B-cell population and serum IgG levels and attenuated arterial stiffness in SAMP1 and old mice. Thus, Klotho deficiency may play a role in senescence- and aging-associated humoral immune dysfunction and arterial stiffness. Vascular infiltration of inflammatory cells and expression of TGFβ1, collagen 1, scleraxis, MMP-2 and MMP-9 were increased while the elastin level was decreased in aortas of SAMP1 and old mice which can be rescued by AAV-SKL. Interestingly, treatment with IgG effectively rescued arterial inflammation and remodeling and attenuated arterial stiffness and hypertension in aging mice. In cultured B-lymphoblast cells, we further showed that SKL regulates B-cell proliferation and maturation partly via the NFkB pathway.
Conclusion
Aging-associated arterial stiffening may be largely attributed to downregulation of B-cell population and serum IgG levels. AAV-SKL attenuates arterial stiffness in aging mice partly via restoring B-cell population and serum IgG levels which attenuates aging-associated vascular inflammation and arterial remodeling.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Simon AK, Hollander GA, McMichael A (2015) Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. https://doi.org/10.1098/rspb.2014.3085
Lock RJ, Unsworth DJ (2003) Immunoglobulins and immunoglobulin subclasses in the elderly. Ann Clin Biochem 40:143–148. https://doi.org/10.1258/000456303763046067
Buckley CE 3rd, Dorsey FC (1970) The effect of aging on human serum immunoglobulin concentrations. J Immunol 105:964–972
Haruna H, Inaba M, Inaba K, Taketani S, Sugiura K, Fukuba Y, Doi H, Toki J, Tokunaga R, Ikehara S (1995) Abnormalities of B cells and dendritic cells in SAMP1 mice. Eur J Immunol 25:1319–1325. https://doi.org/10.1002/eji.1830250528
Han J, Hosokawa M, Umezawa M, Yagi H, Matsushita T, Higuchi K, Takeda T (1998) Age-related changes in blood pressure in the senescence-accelerated mouse (SAM): aged SAMP1 mice manifest hypertensive vascular disease. Lab Anim Sci 48:256–263
De Salvo C, Wang XM, Pastorelli L, Mattioli B, Omenetti S, Buela KA, Chowdhry S, Garg RR, Goodman WA, Rodriguez-Palacios A, Smith DE, Abbott DW, Cominelli F et al (2016) IL-33 drives eosinophil infiltration and pathogenic type 2 helper T-cell immune responses leading to chronic experimental ileitis. Am J Pathol. https://doi.org/10.1016/j.ajpath.2015.11.028
Chen J, Fan J, Wang S, Sun Z (2018) Secreted klotho attenuates inflammation-associated aortic valve fibrosis in senescence-accelerated mice P1. Hypertension 71:877–885. https://doi.org/10.1161/hypertensionaha.117.10560
Nishimura Y, Hosokawa T, Hosono M, Baba M, Hosokawa M (2002) Insufficient interleukin-2 production from splenic CD4+ T cells causes impaired cell proliferation and early apoptosis in SAMP1, a strain of senescence-accelerated mouse. Immunology 107:190–198. https://doi.org/10.1046/j.1365-2567.2002.01496.x
Iwai H, Baba S, Omae M, Lee S, Yamashita T, Ikehara S (2008) Maintenance of systemic immune functions prevents accelerated presbycusis. Brain Res 1208:8–16. https://doi.org/10.1016/j.brainres.2008.02.069
Sun Z (2015) Aging, arterial stiffness, and hypertension. Hypertension 65:252–256. https://doi.org/10.1161/HYPERTENSIONAHA.114.03617
Chen K, Sun Z (2018) Activation of DNA demethylases attenuates aging-associated arterial stiffening and hypertension. Aging Cell. https://doi.org/10.1111/acel.12762
Chen K, Sun Z (2019) Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J Mol Med (Berl) 97:1615–1625. https://doi.org/10.1007/s00109-019-01841-6
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121:505–511. https://doi.org/10.1161/circulationaha.109.886655
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833. https://doi.org/10.1126/science.1112766
Xu Y, Sun Z (2015) Molecular basis of klotho: from gene to function in aging. Endocr Rev 36:174–193. https://doi.org/10.1210/er.2013-1079
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Ong KL, Cheung BM, Man YB, Lau CP, Lam KS (2007) Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 49:69–75. https://doi.org/10.1161/01.HYP.0000252676.46043.18
Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D (1995) Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 25:305–313. https://doi.org/10.1161/01.hyp.25.3.305
Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N et al (2010) Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398:513–518. https://doi.org/10.1016/j.bbrc.2010.06.110
Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM (2013) Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46:1079–1083. https://doi.org/10.1016/j.clinbiochem.2013.05.046
Wang X, Skelley L, Wang B, Mejia A, Sapozhnikov V, Sun Z (2012) AAV-based RNAi silencing of NADPH oxidase gp91(phox) attenuates cold-induced cardiovascular dysfunction. Hum Gene Ther 23:1016–1026. https://doi.org/10.1089/hum.2012.078
Chen K, Wang S, Sun QW, Zhang B, Ullah M, Sun Z (2021) Klotho deficiency causes heart aging via impairing the Nrf2-GR pathway. Circ Res 128:492–507. https://doi.org/10.1161/circresaha.120.317348
Gao D, Wang S, Lin Y, Sun Z (2020) In vivo AAV delivery of glutathione reductase gene attenuates anti-aging gene klotho deficiency-induced kidney damage. Redox Biol 37:101692. https://doi.org/10.1016/j.redox.2020.101692
Chen K, Wang S, Sun Z (2022) In vivo cardiac-specific expression of adenylyl cyclase 4 gene protects against klotho deficiency-induced heart failure. Transl Res. https://doi.org/10.1016/j.trsl.2022.01.006
Dimayuga PC, Cesena FH, Chyu KY, Yano J, Amorn A, Fishbein MC, Shah PK, Cercek B (2009) Natural antibodies and complement modulate intimal thickening after arterial injury. Am J Physiol Regul Integr Comp Physiol 297:R1593–R1600. https://doi.org/10.1152/ajpregu.00114.2009
Hartley CJ, Taffet GE, Michael LH, Pham TT, Entman ML (1997) Noninvasive determination of pulse-wave velocity in mice. Am J Physiol 273:H494-500
Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE (2003) Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol 285:H1464–H1470. https://doi.org/10.1152/ajpheart.00004.2003
Chen J, Lin Y, Sun Z (2016) Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKalpha-mediated activation of RUNX2. Aging Cell 15:853–860. https://doi.org/10.1111/acel.12494
Crosswhite P, Sun Z (2010) Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 55:1484–1491. https://doi.org/10.1161/HYPERTENSIONAHA.109.146902
Lin Y, Chen J, Sun Z (2016) Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension 67:564–573. https://doi.org/10.1161/hypertensionaha.115.06825
Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG (2004) Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286:H2408–H2415. https://doi.org/10.1152/ajpheart.01089.2003
Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K (2008) Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21:1288–1291. https://doi.org/10.1038/ajh.2008.301
Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z (2016) Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension 68:1191–1199. https://doi.org/10.1161/hypertensionaha.116.07709
Wang X, Sun Z (2010) RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol Genom 41:120–126. https://doi.org/10.1152/physiolgenomics.00192.2009
Wang Y, Sun Z (2009) Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54:810–817. https://doi.org/10.1161/hypertensionaha.109.134320
Chen K, Sun Z (2021) Estrogen inhibits renal Na-Pi Co-transporters and improves klotho deficiency-induced acute heart failure. Redox Biol 47:102173. https://doi.org/10.1016/j.redox.2021.102173
Lin Y, Sun Z (2021) Klotho deficiency-induced arterial calcification involves osteoblastic transition of VSMCs and activation of BMP signaling. J Cell Physiol. https://doi.org/10.1002/jcp.30541
Chen K, Zhou X, Sun Z (2015) Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension 66:1006–1013. https://doi.org/10.1161/hypertensionaha.115.06033
Wang Q, Wang S, Sun Z (2021) Kidney-specific klotho gene deletion causes aortic aneurysm via hyperphosphatemia. Hypertension 78:308–319. https://doi.org/10.1161/hypertensionaha.121.17299
Zhou X, Chen K, Lei H, Sun Z (2015) Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26:121–132. https://doi.org/10.1681/asn.2013101033
Chen K, Kobayashi S, Xu X, Viollet B, Liang Q (2013) AMP activated protein kinase is indispensable for myocardial adaptation to caloric restriction in mice. PLoS ONE 8:e59682. https://doi.org/10.1371/journal.pone.0059682
Ullah M, Sun Z (2018) Klotho deficiency accelerates stem cells aging by impairing telomerase activity. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly261
Lin Y, Sun Z (2015) Antiaging gene klotho attenuates pancreatic beta-cell apoptosis in type 1 diabetes. Diabetes 64:4298–4311. https://doi.org/10.2337/db15-0066
Lin Y, Sun Z (2015) In vivo pancreatic beta-cell-specific expression of antiaging gene Klotho: a novel approach for preserving beta-cells in type 2 diabetes. Diabetes 64:1444–1458. https://doi.org/10.2337/db14-0632
Chen K, Zhang B, Sun Z (2021) MicroRNA 379 regulates klotho deficiency-induced cardiomyocyte apoptosis via repression of Smurf1. Hypertension 78:342–352. https://doi.org/10.1161/hypertensionaha.120.16888
Han X, Sun Z (2020) Epigenetic regulation of KL (Klotho) via H3K27me3 (Histone 3 Lysine [K] 27 Trimethylation) in renal tubule cells. Hypertension 75:1233–1241. https://doi.org/10.1161/hypertensionaha.120.14642
Han X, Sun Z (2022) Adult mouse kidney stem cells orchestrate the de novo assembly of a nephron via Sirt2-modulated canonical Wnt/β-catenin signaling. Adv Sci (Weinh). https://doi.org/10.1002/advs.202104034
Varshney R, Ali Q, Wu C, Sun Z (2016) Monocrotaline-induced pulmonary hypertension involves downregulation of antiaging protein klotho and eNOS activity. Hypertension 68:1255–1263. https://doi.org/10.1161/hypertensionaha.116.08184
Wang Y, Sun Z (2009) Current understanding of klotho. Ageing Res Rev 8:43–51. https://doi.org/10.1016/j.arr.2008.10.002
Shipley JM, Doyle GA, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, Welgus HG, Senior RM (1996) The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J Biol Chem 271:4335–4341. https://doi.org/10.1074/jbc.271.8.4335
Wang X, Skelley L, Cade R, Sun Z (2006) AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther 13:1097–1103. https://doi.org/10.1038/sj.gt.3302768
Huang YJ, Zhao JH, Yang TJ, Zhang JH, Cai WQ (2004) Construction and expression of adeno-associated virus vectors of Smad 6 and Smad 7 genes in human renal tubule epithelial cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 20:274–277
Crosswhite P, Chen K, Sun Z (2014) AAV delivery of tumor necrosis factor-alpha short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension 64:1141–1150. https://doi.org/10.1161/HYPERTENSIONAHA.114.03791
Kimura B, Mohuczy D, Tang X, Phillips MI (2001) Attenuation of hypertension and heart hypertrophy by adeno-associated virus delivering angiotensinogen antisense. Hypertension 37:376–380. https://doi.org/10.1161/01.hyp.37.2.376
Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR (2010) Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588:3971–3982. https://doi.org/10.1113/jphysiol.2010.194753
Santana AB, de Souza Oliveira TC, Bianconi BL, Barauna VG, Santos EW, Alves TP, Silva JC, Fiorino P, Borelli P, Irigoyen MC, Krieger JE, Lacchini S (2014) Effect of high-fat diet upon inflammatory markers and aortic stiffening in mice. BioMed Res Int. https://doi.org/10.1155/2014/914102
Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K (2008) Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol 295:H69-76. https://doi.org/10.1152/ajpheart.00341.2008
Fan J, Sun Z (2016) The anti-aging gene klotho regulates proliferation and differentiation of adipose-derived stem cells. Stem Cells. https://doi.org/10.1002/stem.2305
Bagchi RA, Wang R, Jahan F, Wigle JT, Czubryt MP (2016) Regulation of scleraxis transcriptional activity by serine phosphorylation. J Mol Cell Cardiol 92:140–148. https://doi.org/10.1016/j.yjmcc.2016.02.013
Zeglinski MR, Roche P, Hnatowich M, Jassal DS, Wigle JT, Czubryt MP, Dixon IM (2016) TGFbeta1 regulates Scleraxis expression in primary cardiac myofibroblasts by a Smad-independent mechanism. Am J Physiol Heart Circ Physiol 310:H239–H249. https://doi.org/10.1152/ajpheart.00584.2015
Yasmin A, McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB (2005) Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25:372. https://doi.org/10.1161/01.atv.0000151373.33830.41
Yan YW, Fan J, Bai SL, Hou WJ, Li X, Tong H (2016) Zinc prevents abdominal aortic aneurysm formation by induction of A20-mediated suppression of NF-kappaB pathway. PLoS ONE 11:e0148536. https://doi.org/10.1371/journal.pone.0148536
Hosokawa T, Hosono M, Hanada K, Aoike A, Kawai K, Takeda T (1987) Immune responses in newly developed short-lived SAM mice. Selectively impaired T-helper cell activity in in vitro antibody response. Immunology 62:425–429
Blomberg BB, Frasca D (2013) Age effects on mouse and human B cells. Immunol Res 57:354–360. https://doi.org/10.1007/s12026-013-8440-9
Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J (2008) Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol 29:608–615. https://doi.org/10.1016/j.it.2008.08.004
Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, Magy L, Balaji KN, Kaveri SV, Bayry J (2013) Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood 122:1419–1427. https://doi.org/10.1182/blood-2012-11-468264
Bayry J, Negi VS, Kaveri SV (2011) Intravenous immunoglobulin therapy in rheumatic diseases. Nat Rev Rheumatol 7:349–359. https://doi.org/10.1038/nrrheum.2011.61
Galeotti C, Kaveri SV, Bayry J (2017) IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol 29:491–498. https://doi.org/10.1093/intimm/dxx039
Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J (2011) Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin Exp Immunol 164(Suppl 2):2–5. https://doi.org/10.1111/j.1365-2249.2011.04387.x
Kaveri SV, Lacroix-Desmazes S, Bayry J (2008) The antiinflammatory IgG. N Engl J Med 359:307–309. https://doi.org/10.1056/NEJMcibr0803649
Bayry J, Bansal K, Kazatchkine MD, Kaveri SV (2009) DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc Natl Acad Sci USA 106:E24. https://doi.org/10.1073/pnas.0900016106 (author reply E5)
Elluru S, Duong Van Huyen JP, Prost F, Delignat S, Bayry J, Ephrem A, Siberil S, Misra N, Lacroix-Desmzes S, Kazatchkine MD, Kaveri SV (2006) Comparative study of the anti-inflammatory effect of two intravenous immunoglobulin preparations manufactured by different processes. Immunol Lett 107:58–62. https://doi.org/10.1016/j.imlet.2006.07.009
Bayry J, Misra N, Latry V, Prost F, Delignat S, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV (2003) Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases. Transfus Clin Biol 10:165–169. https://doi.org/10.1016/s1246-7820(03)00035-1
Ebringer A, Doyle AE (1970) Raised serum IgG levels in hypertension. Br Med J 2:146–148. https://doi.org/10.1136/bmj.2.5702.146
Suryaprabha P, Padma T, Rao UB (1984) Increased serum IgG levels in essential hypertension. Immunol Lett 8:143–145. https://doi.org/10.1016/0165-2478(84)90067-1
Hilme E, Herlitz H, Soderstrom T, Hansson L (1989) Increased secretion of immunoglobulins in malignant hypertension. J Hypertens 7:91–95
Gudbrandsson T, Hansson L, Herlitz H, Lindholm L, Nilsson LA (1981) Immunological changes in patients with previous malignant essential hypertension. Lancet 1:406–408. https://doi.org/10.1016/s0140-6736(81)91790-6
Chan CT, Lieu M, Toh BH, Kyaw TS, Bobik A, Sobey CG, Drummond GR (2014) Antibodies in the pathogenesis of hypertension. Biomed Res Int. https://doi.org/10.1155/2014/504045
Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS et al (2015) Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66:1023–1033. https://doi.org/10.1161/hypertensionaha.115.05779
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H et al (2011) Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124:1370–1381. https://doi.org/10.1161/circulationaha.111.034470
Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, Knorr M, Hu H, Kroller-Schon S, Schonfelder T, Grabbe S, Oelze M, Daiber A et al (2013) Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol 33:1313–1319. https://doi.org/10.1161/atvbaha.113.301437
Mikolajczyk TP, Guzik TJ (2019) Adaptive immunity in hypertension. Curr Hypertens Rep 21:68. https://doi.org/10.1007/s11906-019-0971-6
Taylor EB, Barati MT, Powell DW, Turbeville HR, Ryan MJ (2018) Plasma cell depletion attenuates hypertension in an experimental model of autoimmune disease. Hypertension 71:719–728. https://doi.org/10.1161/hypertensionaha.117.10473
Oh YS, Berkowitz DE, Cohen RA, Figueroa CA, Harrison DG, Humphrey JD, Larson DF, Leopold JA, Mecham RP, Ruiz-Opazo N, Santhanam L, Seta F, Shyy JYJ et al (2017) A special report on the NHLBI initiative to study cellular and molecular mechanisms of arterial stiffness and its association with hypertension. Circ Res 121:1216–1218. https://doi.org/10.1161/circresaha.117.311703
Sun ZJ, Zhang ZE (2005) Historic perspectives and recent advances in major animal models of hypertension. Acta Pharmacol Sin 26:295–301. https://doi.org/10.1111/j.1745-7254.2005.00054.x
Sun Z, Cade JR, Fregly MJ (1997) Cold-induced hypertension. A model of mineralocorticoid-induced hypertension. Ann N Y Acad Sci 813:682–688. https://doi.org/10.1111/j.1749-6632.1997.tb51767.x
Stortz JA, Hollen MK, Nacionales DC, Horiguchi H, Ungaro R, Dirain ML, Wang Z, Wu Q, Wu KK, Kumar A, Foster TC, Stewart BD, Ross JA et al (2019) Old mice demonstrate organ dysfunction as well as prolonged inflammation, immunosuppression, and weight loss in a modified surgical sepsis model. Crit Care Med. https://doi.org/10.1097/ccm.0000000000003926
El Assar M, Angulo J, Rodriguez-Manas L (2013) Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65:380–401. https://doi.org/10.1016/j.freeradbiomed.2013.07.003
Guarner V, Rubio-Ruiz ME (2015) Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol 40:99–106. https://doi.org/10.1159/000364934
Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD et al (2016) Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 31:1–8. https://doi.org/10.1016/j.arr.2016.08.006
Mikhed Y, Daiber A, Steven S (2015) Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int J Mol Sci 16:15918–15953. https://doi.org/10.3390/ijms160715918
Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, Wahle KW, Calder PC (2008) Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis 196:298–305. https://doi.org/10.1016/j.atherosclerosis.2006.11.002
Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL (2005) The origins of age-related proinflammatory state. Blood 105:2294–2299. https://doi.org/10.1182/blood-2004-07-2599
Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG (2014) Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 114:616–625. https://doi.org/10.1161/CIRCRESAHA.114.302157
Saha C, Kothapalli P, Patil V, ManjunathaReddy GB, Kaveri SV, Bayry J (2019) Intravenous immunoglobulin suppresses the polarization of both classically and alternatively activated macrophages. Hum Vaccin Immunother. https://doi.org/10.1080/21645515.2019.1602434
Araujo LM, Chauvineau A, Zhu R, Diem S, Bourgeois EA, Levescot A, Huerre M, Gombert JM, Bayry J, Daeron M, Bruhns P, Kaveri SV, Herbelin A (2011) Cutting edge: intravenous Ig inhibits invariant NKT cell-mediated allergic airway inflammation through FcgammaRIIIA-dependent mechanisms. J Immunol 186:3289–3293. https://doi.org/10.4049/jimmunol.1003076
Othy S, Hegde P, Topcu S, Sharma M, Maddur MS, Lacroix-Desmazes S, Bayry J, Kaveri SV (2013) Intravenous gammaglobulin inhibits encephalitogenic potential of pathogenic T cells and interferes with their trafficking to the central nervous system, implicating sphingosine-1 phosphate receptor 1-mammalian target of rapamycin axis. J Immunol 190:4535–4541. https://doi.org/10.4049/jimmunol.1201965
Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y et al (2014) Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64:1108–1115. https://doi.org/10.1161/hypertensionaha.114.04147
Marvar PJ, Harrison DG (2012) Stress-dependent hypertension and the role of T lymphocytes. Exp Physiol 97:1161–1167. https://doi.org/10.1113/expphysiol.2011.061507
Wang X, Wang Q, Sun Z (2012) Normal IgG downregulates the intracellular superoxide level and attenuates migration and permeability in human aortic endothelial cells isolated from a hypertensive patient. Hypertension 60:818–826. https://doi.org/10.1161/HYPERTENSIONAHA.112.199281
Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR, Chieppa M, Arseneau KO, Ley K, Cominelli F (2011) SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis 17:2566–2584. https://doi.org/10.1002/ibd.21638
Ishikawa D, Okazawa A, Corridoni D, Jia LG, Wang XM, Guanzon M, Xin W, Arseneau KO, Pizarro TT, Cominelli F (2013) Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol 6:267–275. https://doi.org/10.1038/mi.2012.67
Li Z, Butto LF, Buela KA, Jia LG, Lam M, Ward JD, Pizarro TT, Cominelli F (2018) Death receptor 3 signaling controls the balance between regulatory and effector lymphocytes in SAMP1/YitFc mice with Crohn’s disease-like ileitis. Front Immunol 9:362. https://doi.org/10.3389/fimmu.2018.00362
Mikulski Z, Johnson R, Shaked I, Kim G, Nowyhed H, Goodman W, Chodaczek G, Pizarro TT, Cominelli F, Ley K (2015) SAMP1/YitFc mice develop ileitis via loss of CCL21 and defects in dendritic cell migration. Gastroenterology 148:783–93.e5. https://doi.org/10.1053/j.gastro.2015.01.027
Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F (2005) Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology 128:654–666. https://doi.org/10.1053/j.gastro.2004.11.053
Zanoli L, Rastelli S, Granata A, Inserra G, Empana JP, Boutouyrie P, Laurent S, Castellino P (2016) Arterial stiffness in inflammatory bowel disease: a systematic review and meta-analysis. J Hypertens 34:822–829. https://doi.org/10.1097/hjh.0000000000000867
Funding
This work was supported by NIH R01 AG062375, AG049780, HL118558, and HL154147.
Author information
Authors and Affiliations
Contributions
Zhongjie Sun contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jun Fan, Shirley Wang and Kai Chen. The first draft of the manuscript was written by Jun Fan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. The authors declare no competing financial interests.
Ethics approval
This study was carried out according to the Guidelines of the National Institutes of Health on the Care and Use of Laboratory Animals. The study complies with the Declaration of Helsinki. This project was approved by Institutional Animal Care and Use Committee (IACUC) of the University of Oklahoma Health Sciences Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Current address of Jun Fan: Department of Tissue Engineering, School of Intelligent Medicine, China Medical University, Shenyang, 110122, China.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, J., Wang, S., Chen, K. et al. Aging impairs arterial compliance via Klotho-mediated downregulation of B-cell population and IgG levels. Cell. Mol. Life Sci. 79, 494 (2022). https://doi.org/10.1007/s00018-022-04512-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04512-x