Abstract
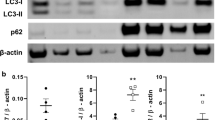
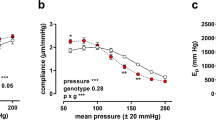
Klotho is an anti-aging gene that shortens the life span when disrupted and extends the lifespan when overexpressed. This study investigated whether autophagy plays a role in Klotho gene deficiency-induced arterial stiffening and hypertension. Klotho mutant heterozygous (KL+/−) mice and age- and sex-matched wild-type (WT) mice were used. Arteries were examined for autophagy using Western blot assays. Pulse wave velocity (PWV), a direct measure of arterial stiffness, and blood pressure (BP) increased significantly in KL (+/−) mice. The autophagy level, as measured by LC3-II expression and autophagy flux, increased in aortas of KL (+/−) mice, indicating that Klotho gene deficiency upregulated autophagy. Chloroquine diminished Klotho gene deficiency-induced increases in PWV and BP and eliminated the upregulation of autophagic flux in KL (+/−) mice. Klotho gene deficiency-induced arterial stiffness was accompanied by upregulation of MMP9, TGFβ-1, TGFβ-3, RUNX2, and ALP, but these changes were effectively mitigated by chloroquine. Chloroquine also halted an increase in scleraxis expression in aortas of Klotho (+/−) mice. In cultured mouse aortic smooth muscle cells, Klotho gene deficiency increased autophagy, leading to upregulation of scleraxis, a key transcription factor of collagen synthesis. Klotho gene deficiency failed to upregulate scleraxis expression when autophagy was inhibited, suggesting that autophagy is a critical mediator of Klotho gene deficiency-induced upregulation of scleraxis. Suppression of enhanced autophagy by chloroquine lessens Klotho gene deficiency-induced arterial stiffening and hypertension by stopping upregulation of MMP9 and scleraxis. The enhanced autophagic activity plays a crucial role in Klotho gene deficiency-induced arterial stiffening and hypertension.
Key messages
-
Klotho gene deficiency upregulates autophagy.
-
Upregulation of autophagy plays a role in the pathogenesis of arterial stiffening.
-
Autophagy regulates MMP9 activity and scleraxis expression.






Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- BFA:
-
Bafilomycin A1
- bHLH:
-
Basic helix-loop-helix
- KL (−):
-
Klotho free medium
- KL (+/−):
-
Klotho mutant heterozygous
- LC3-II:
-
Microtubule-associated proteins 1A/1B light chain 3B
- MMP:
-
Matrix metalloproteinases
- MOVAS:
-
Mouse vascular aortic smooth muscle cell
- P62/SQSTM1:
-
Sequestosome 1
- PBS:
-
Phosphate-buffered saline
- PWV:
-
Pulse wave velocity
- Runx2:
-
Runt-related transcription factor 2
- SCX:
-
Scleraxis
- sKL:
-
Recombinant secreted Klotho
- TGFβ:
-
Transforming growth factor beta
- TIMP:
-
Tissue inhibitors of metalloproteinases
- VSMC:
-
Vascular smooth muscle cells
- WT:
-
Wild type
References
Kobayashi S, Liang Q (2015) Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852:252–261
Poillet-Perez L, Xie X, Zhan L, Yang Y, Sharp DW, Hu ZS, Su X, Maganti A, Jiang C, Lu W, Zheng H, Bosenberg MW, Mehnert JM, Guo JY, Lattime E, Rabinowitz JD, White E (2018) Autophagy maintains tumour growth through circulating arginine. Nature 563:569–573
Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, Okuda S, Lee HC, Ikeda K, Kanegae Y, Saito I, Auwerx J, Motohashi H, Suematsu M, Soga T, Yokomizo T, Waguri S, Mizushima N, Komatsu M (2019) Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat Commun 10:1567
Kang C, Avery L (2008) To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy 4:82–84
Fernandez AF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, He C, Ting T, Liu Y, Chiang WC, Marciano DK, Schiattarella GG, Bhagat G, Moe OW, Hu MC, Levine B (2018) Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558:136–140
Savini M, Wang MC (2019) Does autophagy promote longevity? It depends. Cell 177:221–222
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222
Yeganeh B, Lee J, Ermini L, Lok I, Ackerley C, Post M (2019) Autophagy is required for lung development and morphogenesis. J Clin Invest 130:2904–2919
Loos B, du Toit A, Hofmeyr JH (2014) Defining and measuring autophagosome flux-concept and reality. Autophagy 10:2087–2096
Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC (2008) Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4:849–850
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Kuro-o M (2012) Klotho in health and disease. Curr Opin Nephrol Hypertens 21:362–368
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833
Zhou L, Li Y, Zhou D, Tan RJ, Liu Y (2013) Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol 24:771–785
Lin Y, Sun Z (2015) In vivo pancreatic beta-cell-specific expression of antiaging gene Klotho: a novel approach for preserving beta-cells in type 2 diabetes. Diabetes 64:1444–1458
Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286:8655–8665
Moe SM (2012) Klotho: a master regulator of cardiovascular disease? Circulation 125:2181–2183
Xiao NM, Zhang YM, Zheng Q, Gu J (2004) Klotho is a serum factor related to human aging. Chin Med J (Engl) 117:742–747
Kotsis V, Stabouli S (2011) Arterial stiffness, vascular aging, and intracranial large artery disease. Am J Hypertens 24:252
Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H (2013) A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 8:e56695
Chen K, Zhou X, Sun Z (2015) Haplodeficiency of Klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension 66:1006–1013
Hashimoto J, Ito S (2011) Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58:839–846
Hashimoto J, Ito S (2013) Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension 62:542–549
Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M, Nephro Test Study G (2012) Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 60:1451–1457
Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ (2013) Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension 61:112–119
Sun Z (2015) Aging, arterial stiffness, and hypertension. Hypertension 65:252–256
Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E (2011) Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 17:654–666
Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW (2013) Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112:1159–1170
Hartley CJ, Taffet GE, Michael LH, Pham TT, Entman ML (1997) Noninvasive determination of pulse-wave velocity in mice. Am J Physiol 273:H494–H500
Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG (2004) Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286:H2408–H2415
Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z (2016) Activation of SIRT1 attenuates Klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension 68:1191–1199
Lin Y, Chen J, Sun Z (2016) Antiaging gene Klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension 67:564–573
Zhou X, Chen K, Lei H, Sun Z (2015) Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26:121–132
Chen GF, Sun Z (2006) Effects of chronic cold exposure on the endothelin system. J Appl Physiol (1985) 100:1719–1726
Chen K, Kobayashi S, Xu X, Viollet B, Liang Q (2013) AMP activated protein kinase is indispensable for myocardial adaptation to caloric restriction in mice. PloS one 8:e59682
Capelli A, Lusuardi M, Cerutti CG, Donner CF (1997) Lung alkaline phosphatase as a marker of fibrosis in chronic interstitial disorders. Am J Respir Crit Care Med 155:249–253
Lin ME, Chen T, Leaf EM, Speer MY, Giachelli CM (2015) Runx2 expression in smooth muscle cells is required for arterial medial calcification in mice. Am J Pathol 185:1958–1969
Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN (1995) Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121:1099–1110
Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ (2001) Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128:3855–3866
Brent AE, Schweitzer R, Tabin CJ (2003) A somitic compartment of tendon progenitors. Cell 113:235–248
Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP (2009) The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol 47:188–195
Bagchi RA, Czubryt MP (1823) Synergistic roles of scleraxis and Smads in the regulation of collagen 1alpha2 gene expression. Biochim Biophys Acta 2012:1936–1944
Xu Y, Sun Z (2015) Molecular basis of Klotho: from gene to function in aging. Endocr Rev 36:174–193
Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T (2008) Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience 152:924–941
Iida RH, Kanko S, Suga T, Morito M, Yamane A (2011) Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Mol Cell Biochem 348:89–98
De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM, Martinet W (2015) Autophagy in vascular disease. Circ Res 116:468–479
Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS (1999) Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 205:158–170
Johnson C, Galis ZS (2004) Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscl Throm Vas. 24:54–60
Ishikawa J, Kario K, Matsui Y, Shibasaki S, Morinari M, Kaneda R, Hoshide S, Eguchi K, Hojo Y, Shimada K (2005) Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res 28:995–1001
Acknowledgments
We would like to thank Dr. Nathan Tipton for his assistance in editing the manuscript.
Support and funding
This work was supported by NIH R01 HL118558, AG049780, HL122166, HL116863, DK093403, AG062375, HL102074, and HL105302.
Author information
Authors and Affiliations
Contributions
Z.S. created the experimental design, provided management for the funding, and contributed to the writing and editing of the manuscript. K.C. executed the experiments, analyzed the data, and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 461 kb)
Rights and permissions
About this article
Cite this article
Chen, K., Sun, Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J Mol Med 97, 1615–1625 (2019). https://doi.org/10.1007/s00109-019-01841-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01841-6