Abstract
Ceramides are a heterogeneous group of bioactive membrane sphingolipids that play specialized regulatory roles in cellular metabolism depending on their characteristic fatty acyl chain lengths and subcellular distribution. As obesity progresses, certain ceramide molecular species accumulate in metabolic tissues and cause cell-type-specific lipotoxic reactions that disrupt metabolic homeostasis and lead to the development of cardiometabolic diseases. Several mechanisms for ceramide action have been inferred from studies in vitro, but only recently have we begun to better understand the acyl chain length specificity of ceramide-mediated signaling in the context of physiology and disease in vivo. New discoveries show that specific ceramides affect various metabolic pathways and that global or tissue-specific reduction in selected ceramide pools in obese rodents is sufficient to improve metabolic health. Here, we review the tissue-specific regulation and functions of ceramides in obesity, thus highlighting the emerging concept of selectively inhibiting production or action of ceramides with specific acyl chain lengths as novel therapeutic strategies to ameliorate obesity-associated diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Obesity rates have increased alarmingly over the past 50 years among both adults and children [1, 2], urging the WHO to describe obesity as “one of today’s most blatantly visible—yet most neglected—public health problems” [3]. Obesity is a complex, multifactorial disease of excess adiposity that can cause premature disability and death by increasing the risk of metabolic disorders such as type 2 diabetes mellitus, fatty liver disease, and cardiovascular impairment, mainly due to dyslipidemia and ectopic lipid deposition [4]. However, efforts to prevent or treat obesity and its comorbidities often fail in the long term, and available pharmacotherapeutics remain primarily ineffective and unspecific [5]. Thus, there is an urgent clinical need to better understand the physiological and molecular mechanisms linking obesity to metabolic deterioration in order to identify novel targets for future therapeutic interventions.
Many people with obesity show elevated levels of plasma free fatty acids (FFAs), which is partly attributable to unopposed lipolysis in adipocytes secondary to decreased insulin sensitivity and impaired adipose tissue function [6]. As a result, FFAs target organs such as the liver, muscle, pancreas, heart, and central nervous system, where they can be utilized, stored ectopically in an inert storage pool as triacylglycerols (TAG), or used for the production of other lipid species involved in regulating various metabolic processes [7]. However, when the maximal capacity for fatty acid oxidation or TAG deposition is reached, specific lipid metabolites accumulate that can cause cell-type-specific adverse reactions (referred to as lipotoxicity) and promote metabolic dysfunction such as local and systemic insulin resistance with far-reaching health consequences [7, 8].
In the early 1990s, it was found that specific sphingolipids, namely ceramides, accumulate in the liver and muscle of obese and diabetic rats [9]. Ceramides are composed of a sphingoid long-chain base attached to a single fatty acid. Later, ceramide levels in body tissue and plasma were correlated with diminished insulin sensitivity among obese and type 2 diabetic patients [10, 11]; and in 2007, increased endogenous ceramide synthesis came into the spotlight as to cause insulin resistance in vivo [12]. Meanwhile, it has been found that ceramides accrue in many other metabolic tissues in obesity, and numerous lipotoxic responses were attributed to ceramide action. Ceramides modulate cell membrane dynamics, endoplasmic reticulum (ER) and mitochondrial integrity, inflammation, and cell fate [13]. Notably, ceramides form a family of closely related but structurally and functionally diverse molecular species that differ in sphingoid base composition as well as length and saturation of the acyl chain [14, 15]. Depending on the acyl chain length, most commonly ranging from 14 to 26 carbons (C14-C26), ceramides produce distinct pathophysiological effects and accumulate differentially within each cell type and cell compartment, while causing a range of adverse consequences associated with obesity [16]. As a result, reducing selected pools of ceramides has proven sufficient for preventing the detrimental effects of fatty acid excess and—even more excitingly—for ameliorating metabolic homeostasis in obese murine models, with a significantly lower risk of adverse side effects than would ensue from the complete inhibition of global ceramide formation [16].
This review discusses the emerging concept of the ceramide species-specific regulation of metabolism in obesity, focusing on “classic ceramide species” that contain the typical sphingoid base sphingosine (d18:1) and a saturated acyl chain of defined length. We present the basic principles of mammalian ceramide turnover and highlight key aspects of their pathophysiological roles. We discuss a selection of pathways that employ ceramides as second messengers by controlling ceramide metabolic rate and thus contribute to ceramide accumulation when deregulated in obesity. Finally, we outline the tissue-specific regulation of ceramides in obesity and how this knowledge could be translated into clinics for the treatment of metabolic diseases. Thereby, we aim to provide an updated view of “the complex life of (these) simple sphingolipids”—as Hannun and Futerman once put it [17]—in the context of physiology, lipotoxicity, obesity-associated pathologies, and their treatment.
Metabolism of ceramides in mammals
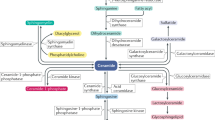
Ceramides are a family of ubiquitous, bioactive lipid molecules that serve as the structural unit of all more complex sphingolipid species. These comprise a set of aliphatic amino alcohols with a backbone of sphingoid long-chain bases. Ceramides consist of the sphingoid scaffold bound to a fatty acid via amide-linkage, and they vary in length and degree of unsaturation within both aliphatic components depending on the biological origin [18]. Three separate routes of endogenous ceramide formation can be distinguished, i.e., de novo synthesis, sphingomyelin hydrolysis, and sphingolipid salvage (Fig. 1). The canonical de novo synthesis pathway commences with the production of the long-chain base by condensation of serine and palmitoyl-CoA to form 3-ketosphinganine at the cytosolic surface of the ER. This reaction is catalyzed by serine palmitoyltransferase (SPT), an enzyme complex composed of two ubiquitously expressed large subunits, encoded by Sptlc1 and -2, and a small regulatory subunit [19]. Sptlc3 encodes an alternative large subunit forming a spectrum of straight and branched long-chain bases with distinct biophysico-chemical properties in restricted tissues [20]. The carbonyl group of 3-ketosphinganine is subsequently reduced by 3-ketodihydrosphingosine reductase (KDSR) to form sphinganine, which can become acylated with a fatty acyl-CoA of defined length (C14-C26) by one of six (dihydro)ceramide synthases (CerS1-6; see Box 1) to form dihydroceramide [21]. Ultimately, two distinct dihydroceramide desaturases (DES1 and DES2, encoded by Degs1 and Degs2) integrate a 4,5-trans-double bond into the sphingoid base to produce ceramides, with DES1 responsible for ceramide synthesis in most tissues [22]. Each ceramide species appears to contain a fixed acyl chain length, as there is currently no evidence for remodeling after ceramide formation. Studies in rodent models have indicated that the expression of ceramide biosynthetic genes increase in obesity and that interventions to reduce ceramide synthesis either by genetic modification (e.g., ablation of Sptlc2 [23, 24], Degs1 [12, 25], CerSs [26,27,28]) or pharmacological intervention (e.g., SPT inhibition using Myriocin [12, 29,30,31] or L-cycloserine [32], DES inhibition using Fenretinide [33], CerS1 inhibition using P053 [34], CerS6 depletion using antisense oligonucleotides (ASO) [35]) can ameliorate high-fat diet-induced metabolic dysfunction. These studies have identified the critical contribution of de novo ceramide formation to the development and progression of obesity-associated metabolic diseases.
Ceramide metabolism in mammals. Schematic representation of the ceramide metabolic pathway highlighting critical enzymes involved in ceramide turnover and their respective inhibitors. Six different ceramide synthases (CerS1-6) produce (dihydro)ceramides of varying acyl chain lengths by catalyzing the N-acylation of sphinganine (derived from the condensation of serine and palmitoyl-CoA; de novo pathway; highlighted in orange) or sphingosine (derived from sphingolipid breakdown; salvage pathway; highlighted in green) with a fatty acyl chain of defined length within the range C14–C26. Ceramides can also be derived from the hydrolysis of sphingomyelin (highlighted in purple). Ceramides serve as substrates for more complex sphingolipid species such as glucosylceramides and galactosylceramides, which can be further modified. Ceramides can also be converted to acylceramide species bearing an additional acyl chain at the 1-hydroxy position. ACSL5 Acyl-CoA synthetase long-chain family member 5, CDase ceramidase, CerS ceramide synthase, CGT ceramide UDP-galactosyltransferase, DEGS dihydroceramide desaturase, DGAT2 diacylglycerol O-acyltransferase 2, GALC galactosylceramidase, GCase glucocerebrosidase, GCS glucosylceramide synthase, KDSR 3-ketodihydrosphingosine reductase, ORMDL orosomucoid-like protein, R Fatty acyl chain moiety, SGPL1 sphingoine-1-phosphate lyase 1, SGPP1 sphingosine-1-phosphate phosphatase 1, SK sphingosine kinase, SMase sphingomyelinase, SMS sphingomyelin synthase, SPT serine palmitoyltransferase, UGCG UDP-glucose ceramide glucosyltransferase, UGT8 UDP glycosyltransferase
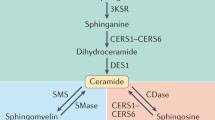
Once ceramides are produced, they can be transported within the cell and used for the generation of more complex sphingolipid species. On the ER-lipid droplet interface, long-chain-fatty-acid-CoA ligase (ACSL5) forms a multiprotein complex with the CerS enzymes and diacylglycerol acyltransferase (DGAT2) to catalyze ceramide acylation at the 1-hydroxy position [36]. This process appears to be relevant in the lipid-laden liver, possibly to divert ceramides from a bioactive- to a storage pool sequestered in lipid droplets in the form of less-toxic acylceramide species [36]. Ceramides in the ER are transported to the trans-Golgi apparatus at membrane contact sites through vesicular or non-vesicular pathways. Active transport involves the ceramide transport protein (CERT) that shuttles various types of species (C14:0–C20:0) to the Golgi apparatus for incorporation into sphingomyelin with lower efficacy for longer acyl chain ceramides [37,38,39]. Transport of ceramides to the cis-Golgi cisternae is required for glucosylceramide production, which are converted to more complex glycosphingolipids in trans-Golgi regions [40]. Complex sphingolipids can be enzymatically degraded to regenerate ceramides either via (a) sphingomyelin hydrolysis or (b) sphingolipid salvage [19]. These pathways involve (a) sphingomyelinases (SMase) to produce ceramides from sphingomyelin, and (b) ceramidases (CDase) that degrade ceramides obtained from sphingolipid catabolic breakdown to produce sphingosine that can be re-acylated to ceramides [41, 42]. Alternatively, sphingosine can be modified to sphingosine-1-phosphate (S1P), a potent sphingolipid signaling metabolite that is either dephosphorylated to regenerate sphingosine or irreversibly cleaved at the unique exit point of the sphingolipid-metabolic pathway [43]. Notably, targeted induction of ceramide degradation as achieved by tissue-specific overexpression of acid CDase or stimulation of ceramidase activity leads to beneficial metabolic outcomes in obese mice, similar to the inhibition of ceramide synthesis [44, 45]. Together, these studies have demonstrated the therapeutic potential of ceramide-lowering compounds in treating obesity-related metabolic diseases.
The enzymes involved in ceramide/sphingolipid turnover are active at distinct subcellular locations, with corresponding local differences in sphingolipid concentrations [19]. For example, members of the CerS family were detected in the ER, Golgi complex, mitochondria, and mitochondria-associated membranes (MAMs), while members of the SMase family show additional activity in the plasma membrane, nuclear envelope, and lysosome [16]. Critically, the subcellular localization of ceramides dictates their specific functions, and ceramide accumulation at spatially distinct sites in the cell produces specific metabolic outcomes (the reader is directed to a recent review on the organelle-specific regulation and function of ceramides [16]). Noteworthy is the complex regulation of endogenous ceramide turnover that depends on the availability of precursor substrates (amino acids and fatty acids) and is modulated by a number of intra- and extracellular stimuli (reviewed in [46]). In addition, three orosomucoid-like (ORMDL) proteins can sense ceramide levels in the ER membrane to cooperatively mediate feedback inhibition of de novo ceramide synthesis through interaction and modulation of SPT [47,48,49,50,51]. The complex regulatory network of ceramide turnover indicates that cellular ceramide levels need to be kept in a narrow range to maintain predetermined amounts for cellular integrity while ensuring rapid adaptation in ceramide concentrations in response to environmental changes and metabolic cues and preventing them from reaching cytotoxic levels.
Metabolic roles, modes of action, and toxic features of ceramides
Different biological functions have been attributed to ceramides, and current research aims at assigning them to individual ceramide molecular species. In this context, it has already been indicated that ceramides with specific acyl chain lengths (C16:0 and C18:0) have a metabolic impact [52]. In contrast, other ceramide molecular species (C24:0) do not, but what accounts for this specificity remains ill-defined [52]. Most conclusions about the physiological roles of ceramides have been drawn from studies aimed at inhibiting ceramide build-up or increasing ceramide degradation under conditions of fatty acid oversupply (e.g., fatty acid treatment in cells or high-fat diet feeding in mice). Few studies targeted the overexpression of ceramide biosynthetic genes associated with increased ceramide formation. Studies in which cells or animal models were treated with artificial short-chain ceramide analogs (C2 and C6 ceramide) provided additional evidence in this context; and although such analogs do not match the physicochemical properties of naturally occurring ceramides, their sphingoid backbone is rapidly recycled and re-acylated to long-chain species of functional relevance [53]. Together, these studies have provided compelling evidence that ceramides act as cell-autonomous nutrient sensors that accumulate with increasing fatty acid concentrations to adjust lipid and glucose homeostasis (this theory is discussed in detail elsewhere [54]). The hypothesis of ceramides acting as metabolic messengers upon fatty acid excess through cell-type-specific responses is based primarily on the following observations: when plasma FFA levels rise, acyl-CoA concentrations increase in oxidative tissues, which can be readily re-esterified to ceramides. Here, ceramides affect membrane dynamics, and they modulate transmembrane signaling at specific intracellular sites in part through direct interaction with regulatory proteins [55, 56]. Thereby, ceramides diminish insulin signaling, presumably to adjust metabolic substrate storage and utilization according to the degree of fatty acid flux [57]. Ceramides regulate mitochondrial plasticity, respiration, and the capacity for β-oxidation in adipocytes, hepatocytes, and myocytes [58]. Additionally, ceramides can stimulate the cellular uptake of fatty acids [44]. At the same time, a ceramide-induced increase in hepatic de novo lipogenesis may support the incorporation of incoming fatty acids into glycerolipid pools, e.g., for their intermediate storage esterified in TAGs [25]. Ceramides also block lipolysis in adipocytes, restricting the further supply of fatty acids from endogenous stores [25]. Thus, under physiological conditions, ceramides may promote the utilization of fatty acids or their storage in non-toxic molecules to limit their lipotoxic effects, characterized by detergent-like activities [54]. Lastly, when the amount of integrated fatty acids reaches a predetermined physiological maximum, excessive ceramide accrual also in mitochondrial membranes initiates programmed cell death [59], which may reflect an inherent ability of organisms to limit the toxic effects of compromised cells. In this regard, it is tempting to speculate that the adverse metabolic effects of ceramide accumulation result from an adaptive response to increased FFA flux that fails under the chronic metabolic burden in obesity through dysregulation of ceramide metabolism, rather than from ceramide function per se. The existence of regulated feedback loops and a wide array of metabolic pathways that cooperatively maintain tight control of ceramide levels under normal conditions support this theory. In practice, however, prolonged intake of foods high in fat leading to obesity can result in abnormal accumulation of ceramides in both plasma and tissues, and chronic ceramide actions may become deleterious, causing degenerative conditions. A selection of the key findings on the physiological relevance and lipotoxic properties of ceramides in obesity are summarized in (Fig. 2) and discussed in more detail in the following section.
Cellular and molecular mechanisms by which ceramides affect metabolic regulation. Excessive influx of free fatty acid (FFA) mediated by the fatty acid transporter CD36 drives the production of ceramides, which exert multifaceted effects to modulate cellular metabolic homeostasis. Ceramide-dependent effects are shown by black arrows, including regulatory proteins through which they act. The consequences of ceramide accumulation are highlighted in red, and the underlying mechanisms are highlighted in blue. Purple arrows depict conversion of lipids, and dashed lines indicate transport. AKT protein kinase B, BAX Bcl-2-associated X protein, CD1d cluster of differentiation 1d, CD36 cluster of differentiation 36 (fatty acid transporter), CerS ceramide synthase, DES dihydroceramide desaturase, eNOS endothelial NO synthase, ER endoplasmic reticulum, FA-CoA fatty acyl-coenzyme A, FFA free fatty acid, GalCer galactosylceramide, GLUT4 glucose transporter 4, HSL hormone-sensitive lipase, iNKT invariant natural killer T cell, IR insulin receptor, JNK c-Jun-N-terminal kinase, KDSR 3-ketodihydrosphingosine reductase, MFF mitochondrial fission factor, MOMP mitochondrial outer membrane permeabilization, NLRP3 NLR family pyrin domain-containing 3, NO nitric oxide, PC phosphatidylcholine, PERK protein kinase RNA-like ER kinase, PKCζ protein kinase C zeta, PKR protein kinase R, PM plasma membrane, PP2A protein phosphatase 2A, SPT serine palmitoyltransferase, SREBP1 sterol regulatory element-binding protein 1, STARD7 StAR-related lipid transfer protein 7, TAG triacylglycerol, VDAC2 voltage-dependent anion channel 2
Ceramides, biological membranes, and protein binding
Naturally occurring ceramides are of exceptional hydrophobicity and thus committed to cell membranes at low relative concentrations under basal conditions (< 1 mol%), but their levels significantly increase in response to fatty acid excess and other cellular stressors [55]. Hence, ceramide accumulation in the cell corresponds to alterations in membrane ceramide composition, and it is postulated that sustained ceramide excess in obesity impairs membrane dynamics [43]. Membrane ceramide enrichment may reduce membrane fluidity; this process is related to diabetes etiology [60, 61]. As mainly ascertained from monolayer- and bilayer-based model systems, ceramides can increase the molecular order of phospholipids, modulate the permeability of membranes to specific solutes, and promote lateral phase separation, transient nanodomain formation, and transmembrane (flip-flop) lipid motion (for more details, we refer the reader to [55]). Some of these properties stem from the ability of ceramides for intramolecular H-bonding in the polar region, which permits close ceramide packing in membranes [62]. Thereby, ceramides can assemble into raft-like ceramide-enriched membrane platforms (CEPs) to direct the recruitment, clustering, and activity of adaptors, receptors, and other signaling molecules [63]. Ceramides can also undergo polar headgroup interactions with other sphingolipid species [64]. A delicate balance of phase formation and transformation with mutual displacement of cholesterol by ceramides through interaction with sphingomyelin may exist, illustrating the interrelation of membrane sphingolipids and cholesterol and the multifaceted consequences resulting from imbalanced membrane ceramide plasticity [65, 66].
Several in vitro studies indicate that the length and saturation of the acyl chain define the biophysical characteristics of ceramide species, which may account for their differential effects on metabolic control [55]. For example, the membrane rigidifying and phase separation properties of ceramides are less pronounced for unsaturated compared to saturated molecular species, and the latter in particular have been linked to metabolic deregulation [67]. Interestingly, membranes isolated from tissues of CerS2-null mice show a variety of organ-specific changes in membrane fluidity, morphology, and trafficking, indicating that alterations at the level of selective ceramide synthesis in vivo can potently affect membrane dynamics [68]. These effects may underlie the detrimental phenotypes observed in CerS2-deficient mice, i.e., disturbed liver homeostasis, hepatopathy, hepatocarcinogenesis, and neurological abnormalities [69,70,71]. A follow-up study suggested that CerS2- versus CerS5-derived ceramides exert distinct effects on membranes [72]. Here, significant differences in the global order of the plasma membrane and CEP formation were observed in CerS2- versus CerS5-overexpressing HEK cells treated with bacterial SMase [72]. Thus, it has been concluded that ceramides with specific acyl chain lengths change the membrane properties to different extents. Still, the precise roles of the acyl chain ceramide distribution in membranes of eukaryotic cells and its consequences on metabolic regulation in obesity require further investigation. Another consideration is that cells exhibit a variety of different membranes, characterized by unique features through specific lipid and protein composition and specific interactions of the two [73, 74]. Furthermore, ceramide sub-compartmentalization and local changes in membrane ceramide concentrations are expected to be crucial factors in executing both their biological functions and pathological effects. This is also exemplified by a recent study on C16:0 ceramides, which impair mitochondrial dynamics in the mouse liver and systemic glucose metabolism when they accumulate in mitochondrial membranes and MAM in obesity [26].
In addition, ceramides act through direct interaction with and modulation of membrane proteins. Initial studies have identified proteins that target a mixture of ceramides, e.g., in protein-lipid overlay assays in vitro. Nevertheless, it must be considered that the physiological environment, the subcellular localization, and the acyl chain length confer specificity for certain sphingolipid–protein interactions in the cell [75]. We have recently demonstrated this using a sphingolipid precursor probe, i.e., a photoactivatable and clickable sphingosine molecule (Ref. [76]), which allowed co-precipitating sphingolipids with their protein-binding partners in cultured HeLa cells and those deficient in CerS5- or CerS6-dependent C16:0 ceramide synthesis [26]. These experiments revealed previously unknown protein targets of sphingolipids depending on C16:0 ceramide formation and yielded distinct protein targets of CerS5- versus CerS6-derived ceramides, a specificity likely due to differences in their spatial distributions [26]. In the future, it will be important to uncover the acyl chain length- and cell compartment-specific protein interactome of ceramides to provide a more detailed picture of the ceramide-dependent regulatory networks of cellular and systemic metabolism. Understanding the physicochemical properties of ceramides in relation to their biological functions is crucial for better understanding their pathological implications, which are likely to occur as a result of ceramide-dependent changes in membrane homeostasis and protein-ceramide interactions.
Ceramides, insulin signaling and glucose homeostasis
Obesity results in the deregulation of several cell-intrinsic pathways partly due to increased fatty acid influx and ceramide build-up, thus impairing insulin signal transmission [57]. Evidence indicates that ceramides directly interfere with the insulin receptor (IR) signaling cascade [57]. In insulin-responsive cells, insulin-binding triggers IR autophosphorylation that recruits IR substrates (IRS) to induce a downstream response leading to phosphorylation and activation of the protein kinase B/AKT [77]. AKT modulates various downstream regulatory proteins to confer a pro-survival signal while promoting nutrient uptake and anabolic metabolism [77]. The insulin-desensitizing properties of ceramides were first noted in cultured adipocytes, (cardio)myocytes, and hepatocytes, wherein the treatment with short-chain ceramide analogs led to inhibition of the insulin-stimulated phosphorylation of AKT, similar to the effect observed upon treatment with the saturated fatty acid palmitate that primarily fuels C16:0 ceramide de novo production [33, 78,79,80,81,82,83]. Indeed, ceramides induce a wide range of their metabolic effects at the level of AKT through distinct mechanisms involving the serine/threonine protein phosphatase 2A (PP2A) and the atypical protein kinase C zeta (PKCζ). It has been postulated that in adipocytes, myotubes, and vascular smooth muscle cells, ceramides direct PKCζ to caveolin-enriched microdomains (CEMs) to sequester AKT in a repressed state [84, 85]. In cells with a lower abundance of CEMs, ceramides within the plasma membrane promote dephosphorylation of AKT by activating PP2A [86, 87], but both pathways may co-exist within the same cell type [88]. By modulating AKT activity, ceramides also interfere with plasma membrane translocation and fusion of the GLUT4 glucose transporter in adipocytes and myocytes, suggesting that ceramides take critical roles in deregulating glycemic control by influencing insulin-dependent glucose uptake into adipose tissue and muscle [78, 89]. Furthermore, sustained ceramide action in cultured myocytes triggers JNK-dependent inhibitory phosphorylation of IRS1 via RNA-activated protein kinase (PKR) and may modify IR translocation into membrane lipid microdomains in control of insulin sensitivity [90, 91].
The transient influx of fatty acids into insulin-target tissues can diminish insulin signaling presumably as an adaptive response to adjust metabolic substrate handling, e.g., for the mobilization of energy stores in times of nutrient deficiency. Ceramides, formed from the incoming fatty acid sources, likely mediate these effects. Accordingly, it is interesting to postulate that the inhibitory effects of ceramides on insulin signal transmission are a relevant process in the insulin-dependent regulation of glucose and lipid metabolism through adaptive insulin resistance. However, such an adaptive response to transient fatty acid supply quickly becomes maladaptive upon prolonged fatty acid excess during obesity development, contributing to sustained reductions in IR signaling [92]. A critical role of ceramide accumulation in insulin-target tissues during obesity development and its link to the manifestation of insulin resistance in vivo has been mainly determined by the rate of insulin-stimulated AKT phosphorylation in key metabolic tissues of rodent models after ceramide-lowering interventions. For example, inhibition of SPT using Myriocin treatment to reduce global ceramide synthesis improved the ability of insulin to stimulate AKT in the liver and skeletal muscle of genetically obese (ob/ob) or diet-induced obese mice [29] and in the gastrocnemius muscle of obese and diabetic db/db mice [30]. Although this does not provide direct proof that ceramide accumulation alone is sufficient to attenuate insulin signal transmission, it indicates the insulin-sensitizing effects of limiting ceramide build-up in obesity.
More recently, a particular role of C16:0 ceramides in the regulation of insulin sensitivity and glucose homeostasis has been identified [26, 27, 35, 93]. As discussed in more detail below, CerS6-dependent C16:0 ceramide production is increased in specific tissues during obesity development, and transgenic expression of CerS6 in primary hepatocytes is sufficient to inhibit insulin-stimulated phosphorylation of AKT [27, 93]. Conversely, body-wide ablation of CerS6-derived C16:0 ceramide synthesis in mice fed a high-fat diet improved insulin-evoked AKT phosphorylation in the liver and in palmitate-treated primary hepatocytes isolated from mice with liver-specific CerS6 deficiency [27]. However, whether the effects on insulin sensitivity in these murine models are attributable to direct modulation of the IR signaling cascade, whether they occur secondary to changes in other cellular processes, or the combination of both, remains to be carefully differentiated. This distinction must also be considered in light of current discussions that simple, unitary defects in proximal insulin signaling may not be the primary cause of systemic insulin resistance in type 2 diabetes [94]. Undoubtedly, additional challenges that cooperatively compromise cellular homeostasis and trigger cellular stress with multifaceted effects on insulin sensing and signal transmission must be taken into account.
Ceramides and lipid homeostasis
Ceramides contribute to the homeostatic control of lipid metabolism by modulating the uptake, storage, and oxidation of fatty acids in adipocytes, hepatocytes, and myocytes. This appears to be attributable also to insulin-independent processes. Thus, blocking general ceramide synthesis in mouse liver through deletion of Degs1 improved hepatic insulin sensitivity but markedly reduced the expression of the sterol regulatory element-binding protein (Srebf1) mRNA and a variety of its downstream targets that control de novo lipogenesis [25]. It has recently been proposed that ceramides activate lipogenesis in the liver by modulating the activity of the SREBP1 protein and that CerS6-derived C16:0 ceramides are particularly relevant to this process [95]. In this way, (C16:0) ceramides may contribute to the selective insulin resistance paradox, wherein the insulin-resistant liver fails to suppress glucose production but continues to stimulate lipogenesis, which is a central mechanism in the pathophysiology of hepatic steatosis and type 2 diabetes [96, 97]. Similarly, ceramides stimulate the cellular uptake of fatty acids via PKCζ-mediated CD36 plasma membrane translocation in hepatocytes and adipocytes, where incoming fatty acids may be a direct source of both de novo lipogenesis of TAGs and ceramide synthesis once the former process is saturated [24, 25, 44]. In primary adipocytes, C2 ceramide treatment attenuated the stimulatory effects of β-adrenergic agonism on hormone-sensitive lipase (HSL) phosphorylation and activation, indicating that ceramide action can also inhibit lipolysis [25]. Furthermore, ceramides regulate the cellular capacity to oxidize incoming fatty acids in obesity [27, 34, 93]. Specifically, a reduction in C16:0 ceramide levels due to CerS6 deficiency in liver or BAT of mice increases mitochondrial β-oxidation capacity during high-fat diet feeding [27], whereas an increase in C16:0 ceramide levels in CerS2-haploinsufficient mice impairs hepatic β-oxidation [93]. Together, these studies revealed a critical inhibitory role of CerS6-derived C16:0 ceramides in the liver and BAT for β-oxidation. In contrast, in the skeletal muscle of obese mice, partial inhibition of CerS1-dependent C18:0 ceramide synthesis was sufficient to increase mitochondrial β-oxidation in myocytes, indicating the tissue-specific effects of ceramide species to control fatty acid turnover [34].
Ceramides and mitochondrial efficacy
Mitochondria play a central role in energy homeostasis, and defects in mitochondrial integrity are associated with obesity-related diseases such as heart failure, fatty liver disease, and diabetes [98, 99]. Obese individuals often exhibit altered mitochondrial morphology and diminished mitochondrial function in oxidative tissues, in part due to mitochondrial lipid accumulation [100, 101]. Ceramides can be detected in mitochondrial membranes, and it has become evident that certain ceramide species interfere with mitochondrial integrity [58].
The origin of mitochondrial ceramides is not entirely clear, but there is emerging evidence for mitochondria-autonomous ceramide production pathways [102]. Enzymes involved in ceramide turnover, including members of the CerS, SMase, and CDase families, co-localize with common mitochondrial markers or have been co-purified with mitochondria from cell or tissue extracts, suggesting that mitochondrial ceramides originate from different intraorganellar processes [103,104,105]. Furthermore, CerS activity has been detected in both inner and outer mitochondrial membranes, and CerS isoforms interact differentially with inner and outer membrane proteins, suggesting sub-organellar differences in ceramide synthesis [106, 107]. Efficient intramitochondrial de novo ceramide production has recently been corroborated by the observation that a subfraction of SPT localizes to the ER-mitochondrial interface to modulate mitochondrial ceramide content [108]. While a significant fraction of SPT is formed by SPTLC1 and SPTLC2 cis-assembly in the ER membrane, a portion of SPTLC2 is detectable in the mitochondrial outer membrane where it interacts in trans with ER-localized SPTLC1 at mitochondria-ER contact sites, possibly to provide 3-ketosphinganine for subsequent mitochondrial ceramide formation [108]. DES1 and KDSR also exhibited dual localization to the ER and mitochondria, arguing for a mitochondria-autonomous ceramide de novo synthesis machinery [108]. However, it cannot be excluded that the MAM is a major site for ceramide production to ensure mitochondrial ceramide supply. Enzymes required for ceramide biosynthesis can also be detected in MAM, and additional steps of lipid synthesis in MAM and MAM-to-mitochondria ceramide transport may co-exist [106].
Mitochondrial ceramides can modulate respiratory capacity in different oxidative tissues. As such, treating rat skeletal muscle mitochondria with different ceramide species impairs the ability for oxidative phosphorylation of ADP [109]. Conversely, reducing CerS6-derived mitochondrial C16:0 ceramides in the liver of obese mice increased ADP-stimulated mitochondrial respiration [26]. In some early seminal studies, ceramides were thought to directly interfere with components of the mitochondrial electron transport chain, modulating respiration and elevating the production of reactive oxygen species (ROS), with deleterious metabolic consequences [110,111,112]. These assumptions were based primarily on findings in cultured cells in which short-chain ceramide analog treatment inhibited mitochondrial respiratory chain complexes I and III [112, 113]. In addition, complex IV activity was inhibited upon incubation of mitochondria isolated from mouse liver with C16:0 ceramides but not upon incubation with C24:0 or C24:1 ceramides, arguing for ceramide species-specific effects on mitochondrial respiratory function [114]. A reduction in complex IV and II activity was also observed in the liver of mice with CerS2-haploinsuffiency, wherein C16:0 ceramides accumulate [93]. However, despite the evidence implicating C16:0 ceramides in the direct impairment of mitochondrial respiratory complex function, mice protected from the obesity-associated increase in C16:0 ceramides exhibited elevated mitochondrial respiration in the liver and BAT, albeit with a slight reduction in complex IV activity and without changes in other components of the respiratory machinery [27]. The above suggests that direct effects on electron transport chain components may not be the primary mechanism at work for (C16:0) ceramides to impair mitochondrial function in obesity, but alternative mechanisms may be at play through which ceramides secondarily alter mitochondrial respiration.
It has been found that ceramides can modulate mitochondrial morphology through direct interaction with the fusion/fission machinery of mitochondrial membranes [26]. Transient morphological changes are necessary for a dynamic adaptation of mitochondrial (respiratory) function to a variety of metabolic cues, also to balance intracellular fuel utilization and partitioning [115,116,117]. It was initially observed that fatty acid turnover in cultured cells triggers mitochondrial fragmentation through increased de novo ceramide synthesis [118]. Similarly, treatment with ceramide analogs promoted mitochondrial fragmentation in (cardio)myocytes [118, 119] and disrupted mitochondrial function in INS-1 β-cells [120]. Intriguingly, CerS6 and its derived C16:0 ceramides localize to and accumulate in hepatic mitochondria and MAM in obesity, promoting mitochondrial fragmentation in the liver of mice, diminished mitochondrial respiratory capacity, and defective glucose handling [26]. This occurs through a direct interaction of sphingolipids derived from CerS6-dependent ceramide formation with mitochondrial fission factor (MFF), an adaptor protein critical for mitochondrial membrane translocation of dynamin-related protein (DRP1) and the initiation of membrane fission [26]. Interestingly, MFF exhibits binding specificity towards CerS6-derived sphingolipids versus diacylglycerols (DAGs), which are also associated with mitochondrial fission events and are known to promote insulin resistance, but likely via a different mechanism [121,122,123]. Similar to CerS6, Sptlc2-deficient cells are protected from palmitate-induced mitochondrial fragmentation, which is partially restored upon re-expression of a mitochondria-directed but not an ER-directed SPTLC2 variant, suggesting a role of palmitate-driven de novo ceramide production at the ER-mitochondria junction in this process [108].
Moreover, it has been found that ceramides can bind STARD7, which acts as an intramitochondrial lipid transfer protein for phosphatidylcholine (PC) to shuttle PC between outer and inner membranes, and thus is involved in the dynamic regulation of mitochondrial lipid composition [56, 124]. PC concentrations in the inner membrane are important for the maintenance of respiration and cristae morphogenesis, and deficiencies in intramitochondrial PC transport can have profound effects on mitochondrial membrane homeostasis [124]. Changes in mitochondrial ceramide content could therefore lead to broader alterations in mitochondrial lipid plasticity to regulate mitochondrial respiration and alternative functions, a hypothesis that warrants further investigation.
The mitochondria-related effects of ceramides are likely driven not only by actions in mitochondrial membranes but also by impact within other cellular compartments such as MAMs, which are closely linked to the control of mitochondrial function. In the MAM, ceramides affect protein incorporation and MAM functionality [125, 126]. Disruption of MAM integrity, in turn, triggers metabolic inflexibility, insulin resistance, and cellular dysfunction in tissues [127], which may in part result from the obesity-related accumulation of ceramides at this particular subcellular site.
Ceramides and ER stress
The ER is the primary site for ceramide biogenesis involved in numerous metabolic processes, including calcium storage, lipid biosynthesis, and protein folding, while being vulnerable to lipotoxicity. Given the physicochemical properties of ceramides, it is likely that alterations in ceramide turnover affect ER ceramide content and subsequently ER membrane homeostasis, but not much is known about this relationship [128]. Ceramide-dependent control of ER proteostasis has been demonstrated in yeast, with specific acyl chain length ceramides regulating the sorting of GPI-anchored proteins into selective export sites of the secretory pathway [129]. Notably, any disturbance in ER proteostasis can induce the unfolded protein response (UPR) as a protective mechanism to restore internal homeostasis, but this response is insufficient to recover ER functionality in peripheral tissues and the brain in obesity leading to sustained ER stress and metabolic deterioration [130,131,132]. It is postulated that ceramides play a critical role in this process [133]. As such, ceramides promote ER stress and insulin resistance in the liver of mice with alcoholic [134] and non-alcoholic fatty liver disease [95], as well as in the hypothalamus during obesity development [135], associated with adverse metabolic consequences. In yeast, increased ceramide production through inhibition of the negative feedback regulation of SPT triggers chronic UPR activation and impairs ER-to-Golgi transport [136]. Moreover, in mammalian cultures, the UPR transducer ATF6 can be activated through direct interaction with two intermediates of ceramide synthesis, namely sphinganine and dihydroceramide, involved in physiological settings that show ER membrane expansion [137]. However, only few mechanisms have been proposed in vitro for ceramides to directly interfere with ER stress modulators, including C16:0 ceramide-dependent binding and activation of cathepsin B/D and activation of CD95-PERK signaling [133, 138, 139]. Still, the relevance of these pathways to ceramide-depended ER stress in the context of obesity remains elusive.
The availability of fatty acids for the synthesis of ceramides with specific acyl chain lengths determines the effects of ceramides on ER homeostasis. In mouse hepatocytes, palmitate (C16)-dependent increases in ceramide content were associated with increased expression of UPR marker genes, which was potentiated by the addition of myristate (C14), and reversed by inhibition of de novo ceramide synthesis [140]. The observation that myristate but not palmitate stimulated ER stress in intestinal epithelial cells through increased expression of CerS5 and CerS6 and increased C14:0 ceramide synthesis, supported the notion of cell-type-specific regulation and function for ceramides in this process [141]. Along these lines, in Hep3B cells, CerS6- but not CerS5-dependently formed C16:0 ceramides promoted ER stress, while CerS2-dependently formed longer-chain ceramides (C22:0–C24:0) elicited a protective effect [95].
The consequences of ceramide accumulation within the ER membrane are poorly understood. In yeast, it has been demonstrated that ceramide transfer out of the ER through increased ER-Golgi tethering during ER stress prevents the lipotoxic effects of ceramides on ER integrity [142]. From a different perspective, palmitate treatment impaired ceramide flow from the ER to the Golgi apparatus in insulinoma cells, promoting ER stress [143]. Similarly, blocking ER-to-Golgi ceramide traffic by inhibiting CERT in cultured myocytes potentiated the deleterious actions of lipotoxicity on insulin signaling [144]. However, it is unclear whether these effects result from ceramide accumulation in the ER membrane or insufficient availability of ceramides for the synthesis of complex sphingolipids in the Golgi apparatus, a matter that requires further investigation. Moreover, while most studies have linked ceramides to the induction of ER stress, evidence from in vitro experiments suggests that C16:0 ceramides may play a protective role in certain cell types. For example, it was reported that the generation of C16:0 ceramides by CerS6 protected human head and neck squamous cells from ER stress, whereas knockdown of CerS6 and a subsequent decrease of C16:0 ceramide content induced ATF6 expression via perturbation of ER Ca2+ homeostasis, which disrupted ER-Golgi networks leading to ER stress [145, 146].
Ceramides and inflammation
Obesity is accompanied by chronic inflammation in several tissues, which triggers adverse effects on insulin sensitivity. A mechanism of ceramide-induced lipotoxicity involves the NLRP3 inflammasome that can sense intracellular ceramides in adipose tissue and macrophages to induce inflammatory signaling and insulin resistance [147]. Nlrp3-deficient animals are protected from obesity-associated hepatic steatosis, adipose tissue inflammation, and glucose intolerance, supporting the notion that the effects of ceramides on NLRP3-dependent pathways may be relevant in the etiology of these metabolic disorders [147]. Further evidence indicates that ceramides affect signaling of tumor necrosis factor (TNFα) in control of inflammation and apoptosis, as disruption of membrane lipid microdomains in CerS2-null mice prevented the internalization and downstream signaling of the TNFα receptor (TNFR) [148].
Ceramides in antigen-presenting cells may further regulate the activity of particular immune cells. Invariant natural killer T (iNKT) cells, which are highly enriched in white adipose tissue, can react to lipid antigens presented in CD1d molecules with profound immunomodulatory potential [149]. In particular, glycosylated sphingolipids can be loaded onto CD1d and presented at the plasma membrane providing a potent ligand for iNKT cell activation [150]. α-galactosylceramide, a synthetic prototype iNKT cell lipid antigen derived from structure–activity relationship studies of its natural analog, was found to ameliorate the metabolic defects associated with a high-fat diet in mice [151]. Rates of endogenous ceramide turnover may thus result in alterations of endogenous glycosphingolipid pools, interfering with iNKT cell modulation and metabolic control. It is proposed that adipose tissue-resident iNKT cells exert protective roles in the development of obesity-associated diseases through regulatory cytokine production and stimulation of macrophage polarization [151, 152]. Yet, another study found that iNKT cells contributed to tissue inflammation, insulin resistance, and hepatic steatosis [153]. Notwithstanding these conflicting data, it is tempting to speculate that the pathophysiological properties of ceramides in immunometabolic diseases involve a role in providing iNKT cell lipid antigens.
Although several studies have uncovered cell-autonomous effects of ceramides on immune cell homeostasis, such a role in obesity-related diseases remains unclear [154]. De novo ceramide synthesis in cultured macrophages interferes with autophagosome formation, a process thought to play a critical role in regulating innate immunity [155, 156]. However, deleting either Sptlc2, Degs1, or CerS6 in the myeloid lineage in vivo did not result in metabolic alterations in high-fat diet-fed mice [23, 25, 27, 157]. These findings suggest that ceramide accumulation in myeloid cells, at least owing to increased de novo ceramide synthesis, may not be the primary mechanism in the manifestation of obesity-related metabolic diseases.
Ceramides and cell death
The role of ceramides in cell fate determination is probably the best-studied mechanism of ceramide action and has been extensively reviewed by experts in the field (e.g., [158,159,160]). Increased lipid-induced apoptosis (lipoapoptosis) often accompanies obesity and can be induced by ceramides in several cell types, leading to insulin resistance and metabolic dysfunction [161, 162]. Pro-apoptotic pathways that employ ceramides as second messengers appear to play an essential role in β-cell-, hepatocyte-, and cardiomyocyte death in the pathogenesis of type 2 diabetes mellitus, non-alcoholic fatty liver disease, and heart failure [163,164,165].
Ceramides have been linked to pro-apoptotic processes such as Fas-capping [166] and emerged as positive modulators of JNK- [167], kinase suppressor of Ras (KSR)- [168], and cathepsin D signaling [169] in stress-induced apoptosis. More recently, knockdown of selected CerSs revealed that a specific pool of C16:0 ceramides derived from CerS6 controls key events in the execution phase of apoptosis, such as the loss of Focal Adhesion Kinase (FAK) and permeabilization of the plasma membrane by regulating caspase-7 activity [170]. Ceramide-induced apoptosis has also been implicated in the activation of PKCδ, as the treatment with ceramide analogs induced PKCδ Golgi complex-translocation and apoptosis [171].
Moreover, ceramides evolved as important regulators of the mitochondria-intrinsic apoptotic pathway, with ceramide concentrations in mitochondria dictating apoptosis in cultured cells. Jain and colleagues have shown this in an elegant study where a mutated form of CERT (mitoCERT), which carries a mitochondrial anchor to facilitate ER-to-mitochondria ceramide transport, induced BAX-dependent mitochondrial outer membrane permeabilization (MOMP), cytochrome c release, and apoptosis [172]. This study confirmed earlier findings that BAX-dependent release of mitochondrial cytochrome c could be efficiently induced by ceramides, potentially in mitochondrial CEPs, and in particular by CerS6-derived C16:0 ceramides [173,174,175,176]. A proposed mechanism for ceramide-mediated apoptosis involves the interaction of ceramides with the voltage-dependent anion channel VDAC2 that provides a mitochondrial platform for BAX/BAK translocation [177]. Conversely, both disruption of ceramide synthesis and removal of ceramides from mitochondria via expression of a mitochondria-targeted CDase could prevent apoptotic processes [172]. Although still in debate, it has been suggested that MOMP also results from self-assembled ceramide pores (as shown for C2 and C16:0 ceramides) [178, 179]. The formation of such pores is inhibited by the incorporation of C22:0 ceramides that compete with C16:0 ceramides to form smaller channels to control the selective export of mitochondrial pro-apoptotic proteins and differential regulation of apoptosis [180].
In addition, CerS1-derived C18:0 ceramides bind LC3B-II at the outer mitochondrial membrane upon DRP1-mediated mitochondrial fission and direct autophagolysosomes to mitochondria to induce lethal mitophagy [181]. In the same study, exogenously applied C16:0 ceramides also localized to mitochondria, where they decreased mitochondrial oxygen-consumption rate and induced mitophagy [181]. However, it is unclear whether a specific threshold for ceramide concentration in mitochondrial membranes must be reached and what exactly determines the differential effects on mitochondrial respiratory function versus mitochondria-dependent death processes. Also, while most studies have shown that C16:0 ceramides act pro-apoptotic, it has been suggested that they trigger anti-apoptotic signals in certain other cell types [145]. More recently, it was found that C16:0 ceramides interact with RIP-kinase (RIPK1) in structures referred to as ceramidosomes, which assemble in the ER and translocate to the plasma membrane to trigger necroptotic signaling [182]. Together, these findings demonstrate the multifaceted effects of ceramides on different pathways leading to cell death.
Obesity-induced alterations to ceramide metabolism
In obesity, alterations in endogenous ceramide turnover due to increased substrate availability and deregulations in the metabolic pathways that fine-tune ceramide synthesis under healthy conditions lead to the accumulation of ceramides in body tissues and circulation, thereby disrupting cellular function and metabolic integrity. Some of the pathways associated with modulation of ceramide turnover thought to promote ceramide accumulation in obesity are discussed in the following section (Fig. 3).
Factors potentially contributing to ceramide accumulation in obesity. In conjunction with the increased availability of precursor fatty acids for ceramide production, several cell-extrinsic and -intrinsic factors have been linked to the control of ceramide turnover rate and may contribute to ceramide accumulation when deregulated in obesity. AMPK AMP-activated protein kinase, Asah N-acylsphingosine aminohydrolase (acid CDase), Acer2 alkaline ceramidase 2, CerS ceramide synthase, FFA free fatty acids, FGF21 fibroblast growth factor 21, FXR farnesoid X receptor, HIF2α hypoxia-induced factor 2α, MYC transcription factor MYC, Neu3 neuraminidase 3, Smpd3 sphingomyelin phosphodiesterase 3 (neutral SMase2), Sptlc serine palmitoyltransferase long-chain base subunit
Diet and substrate availability
A critical factor in tissue ceramide build-up is the diet providing precursor substrates, such as fatty acids, for endogenous ceramide formation. Dietary ceramides and complex sphingolipids are readily degraded in the intestinal tract, but their degradation into metabolites such as palmitate and serine can fuel tissue ceramide synthesis [183, 184]. Dietary sphingosine sources in turn can be directly used by the intestinal microbiota for the generation of sphingolipids, which can enter host circulation and routed to organs such as the liver for ceramide generation, thus impacting tissue ceramide content [183, 185]. The dietary fatty acid composition appears to determine the extent of ceramide formation and the related effects on body metabolism. In humans, it was found that diets rich in saturated fat increase plasma ceramide levels more than polyunsaturated fat, which has been associated with the development of liver steatosis and insulin resistance [186, 187]. Dietary effects on the gut microbiome also affect the levels of circulating ceramides linked to cardiovascular disease risk [188].
A large proportion of the fatty acids used for endogenous ceramide formation in obesity derives from adipose tissue lipid spillover. In obesity, increased adipocyte lipolysis as a result of inflammation and insulin resistance in adipose tissue promotes lipid mobilization from fat stores, increasing circulating FFAs and ectopic tissue influx that continuously supplies substrates for ceramide synthesis [189]. The ability of cells to import fatty acids and the availability of specific precursor fatty acids to promote the synthesis of ceramides with specific acyl chain lengths thus likely dictate the rates of endogenous ceramide production.
Inflammatory signaling
Ceramides can induce an inflammatory response, promoting ceramide biogenesis in a vicious cycle of ceramide production and inflammatory signaling that causes systemic defects in glucose handling [190]. This is mediated in part via the toll-like receptor (TLR)4, a pattern recognition receptor that modulates innate immune responses and insulin sensitivity [190, 191]. Stimulation of TLR4 was found to trigger increased expression of Sptlc1, -2, Degs1, and specific CerSs in myocytes following stimulation with lipopolysaccharide or palmitate, indicating that TLR4 induces ceramide synthesis upon inflammatory input and fatty acid excess [192]. Accordingly, infusion of lard oil in mice increased ceramide levels in the liver, muscle, and hypothalamus, depending on the TLR4/IKK-β pathway [192]. Intriguingly, while the increase in ceramide formation was not required for TLR4-dependent induction of inflammatory cytokines, it was essential for TLR4-dependent insulin resistance, linking lipid signaling induced by inflammatory stimulation to decreased insulin action [192]. Conversely, mice deficient in TLR4 are protected from the lipotoxic effects of ceramides on insulin sensitivity [192]. Upon activation, TLR4 recruits the innate immune signal transduction adaptor MyD88, which is also involved in the signaling pathway of the inflammatory cytokine Interleukin-1beta (IL-1β). Accordingly, MyD88 has been associated with increased ceramide production following IL-1β stimulation in cultured hypothalamic neurons, depending on the activation of neutral SMase [193]. Similar observations were made for alternative pro-inflammatory-and-death signals (e.g., TNFα, Fas, and TRAIL), which mediate their cellular effects in part by stimulating ceramide formation [194,195,196]. Previous studies found that TNFα induces ceramide accumulation via coordinated changes in the ceramide de novo and sphingomyelin hydrolysis pathways [197]. It was suggested that TNFR stimulation independently activates acid- and neutral SMase by different cytoplasmic domains, specifically coupled to selected pathways of TNFR signaling [198]. More recently, a pronounced effect of TNFα treatment on C16:0 ceramide formation was identified in MCF-7 cells, which was inhibited by silencing CerS6 but not CerS5 [170]. This finding suggests a putative mechanism affecting specific ceramide pools upon increased inflammatory input in obesity.
Adiponectin receptors
The adipokine adiponectin is predominantly secreted from mature white adipocytes and acts on several target tissues to exert anti-diabetic, anti-inflammatory, and cardioprotective actions [199]. Globular adiponectin expression in mouse models of obesity or atherosclerosis can ameliorate their detrimental cardiometabolic phenotypes by improving insulin sensitivity and inhibiting the progression of atherosclerotic lesions [200]. It has turned out that adiponectin exerts a large proportion of its beneficial properties via receptor-stimulated catabolism of tissue ceramides [45]. Adiponectin receptors (AdipoR1 and AdipoR2) possess intrinsic ceramidase activity, which is efficiently stimulated upon ligand-binding by 20-fold [45, 201]. AdipoR2 is capable of hydrolyzing shorter (C6) and longer (up to C24) ceramide substrates but appears to show a preference for C18 ceramide species [201]. In conjunction with these studies, inducible overexpression of AdipoR or oral administration of an AdipoR agonist (AdipoRon) can activate ceramidase activity to reduce tissue ceramide content and ameliorate diabetic phenotypes in mice, indicating its potential as a ceramide-lowering compound also in the treatment of obesity-associated metabolic diseases [202,203,204]. Similarly, the stress-inducible hormone fibroblast growth factor (FGF21) partly acts via a mechanism that involves adiponectin production and secretion, stimulating AdipoR-dependent ceramide degradation to enhance insulin sensitivity in multiple target tissues [205]. These findings further suggest that reduced circulating adiponectin levels in obesity may contribute to tissue ceramide accumulation by reduced stimulation of AdipoRs and insufficient ceramide degradation.
AMPK
AMP-activated protein kinase (AMPK) is an energy-sensing enzyme that controls a variety of physiological events to maintain energy homeostasis, including glucose and lipid metabolism [206]. When cellular energy levels are low, AMPK activity increases to induce catabolic pathways while inhibiting anabolic routes to replenish cellular ATP. Stimulation of AMPK activity improves insulin sensitivity, and sustained decreases in AMPK activity in obesity are associated with insulin resistance [207]. It is predicted that AMPK inhibits ceramide synthesis to modulate insulin sensitivity and glucose homeostasis. Specifically, it has been shown that AMPK activation attenuates the palmitate-dependent increase in Sptlc2 and CerS6 expression and cellular ceramide content in cultured myotubes, but the mechanisms of how AMPK activity would affect the expression of these genes have not been addressed [208]. In turn, chronic activation of AMPK decreases de novo ceramide formation and reduces ceramide content in soleus muscle of high-fat diet-fed rats and palmitate-treated cultured astrocytes [208, 209].
More recently, it was found that hyperthyroid rats exhibit reduced ceramide content in the hypothalamus associated with decreased hypothalamic ER stress [210]. These phenotypes were recapitulated by both T3 administration and the expression of a dominant-negative version of AMPK in the ventromedial hypothalamus [210]. Therefore, the authors proposed a model whereby AMPK activity is inhibited by thyroid hormone action to suppress ceramide production and ER stress in the hypothalamus, suggesting a role of AMPK in regulating ceramide levels in cellular stress and metabolic control [210]. Since AMPK activity is stimulated within the AdipoR signaling cascade, additional ceramidase-independent effects of AdipoRs on ceramide metabolism could be related to adiponectin-stimulated changes in AMPK activity. Together, these findings support the notion that the metabolic actions of AMPK are partly mediated by reducing cellular ceramide levels. As a result, decreased AMPK action in obesity may have causal roles in ceramide accumulation, thereby decreasing insulin sensitivity in multiple organs.
Endocannabinoids
Endocannabinoids are endogenous lipid-based retrograde neurotransmitters that act via cannabinoid receptors, including CB1R, expressed in the central nervous system and peripheral tissues to regulate various metabolic processes [211, 212]. In obesity, the endocannabinoid system is often highly active, while its ablation or inhibition reduces body weight and improves insulin sensitivity [213,214,215]. Studies in human glioma cells have revealed that cannabinoid action triggers ceramide accumulation either acutely through sphingomyelin hydrolysis or sustainedly through de novo synthesis via regulation of SPT activity [216]. In diet-induced obese mice, blockage of CB1R by chronic treatment with a peripherally restricted inverse agonist (JD5037) attenuated the diet-induced increases in hepatic C14:0, C16:0, C18:0, and C20:0 ceramides and improved glucose tolerance and insulin sensitivity [217]. From a mechanistic point of view, CB1R inverse agonism reversed the high-fat diet-dependent increase in SPT activity, decreased the expression of ceramide biosynthetic genes (Sptlc3, CerS1, CerS6), and increased the expression of ceramidases (Asah1, Asah2) [217]. These observations have led the authors to conclude that the ceramide-lowering effects and beneficial metabolic outcomes of CB1R inhibition are due to both reduced ceramide de novo synthesis and increased ceramide degradation [217]. Accordingly, it is tempting to speculate that increased CB1R signaling during obesity development contributes to tissue ceramide accumulation.
Intestinal transcription factors
In the intestine, specific transcriptional regulators have been associated with ceramide production and secretion in the pathophysiology of obesity-related metabolic pathologies [218]. Convincing evidence is provided by a series of studies from the Gonzalez group showing that the intestinal farnesoid X receptor (FXR) promotes ceramide synthesis in the gut, leading to systemic increases in ceramide content that can trigger liver steatosis and systemic metabolic defects [219,220,221]. In turn, inhibition of intestinal FXR in obese mice decreases ceramide levels both in the intestine and circulation, which resolves hepatic steatosis and enhances the thermogenic capacity of adipose tissue, in part through increased mitochondrial uncoupling and adipose tissue browning to ameliorate obesity and insulin resistance [219,220,221]. Neutral sphingomyelinase (Smpd3), encoding for nSMase2, was recently identified as an FXR target gene mediating the effects on intestinal ceramide (mainly C16:0) production and secretion, also in the pathophysiology of atherosclerosis [222]. The gut microbiota has been implicated as an environmental factor that modulates obesity and its related diseases through FXR [223]. Ceramides may thus be critical determinants of a subject’s susceptibility to developing metabolic diseases in obesity related to specific alterations of the intestinal microbiome.
In addition, intestinal ceramide levels appear to be under the control of HIF2α, a transcription factor stabilized under hypoxic conditions [224]. HIF2α was found to govern transcriptional control over neuraminidase 3 (Neu3), encoding a key enzyme in the ceramide salvage pathway [224]. In this study, disruption of intestinal HIF2α in mice reduced intestinal and circulating ceramide levels during high-fat diet feeding (most notably C16:0 ceramides), accompanied by reductions in body weight gain and hepatic steatosis, and improvements in systemic insulin sensitivity [224]. HIF2α-dependent effects on ceramide turnover also occur in hypoxic WAT, but through a distinct mechanism that involves transcriptional regulation of alkaline CDase (Acer2), and this process has been linked to the pathophysiology of atherosclerosis [225].
It was recently found that the transcriptional regulator MYC also interferes with ceramide production, thus modulating intestinal and systemic ceramide levels in obesity [226]. Similar to Fxr and Hif2a, Myc expression in the intestine is increased in obesity [226]. In turn, disruption of Myc in intestinal epithelial cells led to a reduction in serum ceramide levels in mice and ameliorated HFD-induced obesity and hepatic steatosis [226]. The changes in ceramide content following MYC disruption were attributed to changes in the expression of CerS4, which turned out to be a MYC target gene increased in the intestine of obese subjects [226]. However, proof of a casual relationship between intestinal CerS4-dependent ceramide synthesis and obesity-related metabolic diseases is pending. Nevertheless, the studies collectively indicate that altered regulation of specific transcription factors in the intestine affects endogenous ceramide production. Although FXR, HIF2a, and MYC alter ceramide formation through unique processes, each system promotes the delivery of ceramides from the intestine to other tissues, including the liver, thereby impairing systemic metabolic integrity in obesity.
Other factors
Additional pathways that may contribute to ceramide accumulation in obesity by modulating ceramide metabolic rate are currently discussed. For example, β-adrenergic signaling was found to efficiently shut down ceramide synthesis in primary adipocytes [25]. However, the molecular targets downstream of the β-adrenergic receptor involved in this process and its implication in the obesity-related accrual of ceramides in adipose tissue are still unclear. In addition, studies in yeast point toward a role of the Target of Rapamycin (TOR) complex 2 (TORC2), which is closely related to obesity and metabolic control, in regulating SPT-dependent ceramide formation and CerS phosphorylation [227, 228].
The mechanisms by which the pathways presented here modulate ceramide content in obesity often remain vaguely defined, and the vast majority of studies report correlative changes in the expression of proteins involved in general ceramide turnover, such as SPT, DES, or CDase, regardless of whether their alteration is the cause or consequence of altered metabolic control. In addition, it is assumed that these enzymes do not have substrate specificity or preference for certain acyl chain length molecular species and can thus be attributed to the obesity-related changes in specific ceramides only upon differential availability of precursor substrates driving selective ceramide synthesis. At the same time, altered regulation of CerSs is thought to promote the obesity-related increases in acyl chain length-specific de novo ceramide formation. However, the CerS-modifying signaling pathways involved in this process remain largely unknown. Indeed, there is a vast opportunity embedded in understanding how CerS activity is regulated in obesity (e.g., at the transcriptional and post-translational level) to change the content of specific ceramide species and influence systemic glucose homeostasis, and this needs to be an intensive area of investigation in the near future.
Relevant tissues of ceramide metabolism and action in obesity
Ceramide accumulation and the associated metabolic effects in obesity are highly organ-specific (Fig. 4). This has been demonstrated by the use of model organisms together with (sphingo)lipidomic analyses in human tissue biopsies and rodents with obesity and/or dyslipidemia. Inhibition or overexpression of specific CerSs in murine models has started to provide evidence about the molecular nature of the specific ceramide species eliciting lipotoxic responses in obesity and further demonstrated that inhibiting chain length-specific ceramide synthesis in individual tissues can substantially improve metabolic homeostasis. In this context, challenges in interpreting data obtained from sphingolipidomic analyses and CerS interference should be noted, as we discuss in (Box 2). Below, we present a selection of key findings that have contributed to our current understanding of the tissue-specific regulation and functional roles of ceramides in the pathophysiology of obesity-related metabolic diseases.
Tissue-specific effects of ceramide accumulation and the related health consequences in obesity. Most conclusive observations have been demonstrated in rodent models of obesity or dyslipidemia. Although ceramides have been associated with obesity-related metabolic dysfunction and disease development in all tissues shown, the exact ceramide molecular species involved in these processes often remain undefined. If there is evidence of the ceramide species promoting tissue-specific lipotoxicity, this is indicated accordingly. Red arrows indicate inhibitory effects, and green arrows indicate stimulatory effects. Cer ceramide, CerS ceramide synthase, ER endoplasmic reticulum, FFA free fatty acid, HGP hepatic glucose production, LDL low density lipoprotein, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis, NO nitric oxide, PVH paraventricular hypothalamus, VMH ventromedial hypothalamus
Ceramides in the adipose tissue
White adipose tissue (WAT) is a multifactorial organ that can store large amounts of TAGs and communicate the status of endogenous fat storage to other tissues by endocrine signaling to adapt nutrient intake, storage, and utilization [229]. These processes are often disturbed in obese individuals triggering dyslipidemia and metabolic dysfunction [229]. While expanding visceral WAT depots are associated with risk for metabolic disease, anatomically distinct depots of subcutaneous WAT may elicit more protective effects on energy homeostasis [229].
Human correlative studies have demonstrated an association between increased ceramide content in distinct adipose tissue depots and obesity-related pathologies [23, 230,231,232,233,234]. For example, C16:0 ceramides were elevated in subcutaneous- but not in visceral WAT in a small cohort of obese patients with type 2 diabetes as compared to obese non-diabetics [23]. In a small group of obese non-diabetic women with hepatic steatosis, C24:1 ceramides showed increased levels in the inflamed subcutaneous WAT [231]. In another group of obese women, higher total ceramide levels were measured in visceral- compared to subcutaneous WAT, where C16:0 and C18:0 ceramides related to systemic metabolic defects [233]. Moreover, in a study involving subjects across different BMIs, an increase in most ceramides was recorded in both the subcutaneous- and visceral epicardial WAT depot in obese individuals, with a close association between C16:0 ceramides in subcutaneous WAT and high HOMA-IR [232]. A challenging task will be to understand the variability of obesity-related changes in ceramide content within the different WAT depots between groups of patients.
Recently, an in-depth lipidomic profile of human WAT (AdipoAtlas) confirmed that C16:0 ceramides with the usual sphingoid base sphingosine (d18:1) are the most abundant species also in the adipose tissue of humans [234]. In addition, the authors found that WAT exhibits high relative amounts of potentially lipotoxic deoxy-ceramides (> 10% of all ceramide subclasses), a yet poorly studied ceramide molecular species produced from alanine instead of serine [234]. The obese WAT showed a marked upregulation of ceramides with the unusual sphingoid base sphingadienine (d18:2) with a variety of amide-linked acyl chains (C14:0-C24:0), illustrating the need for analysis of ceramides also with alternative sphingoid bases in the context of obesity-related WAT dysfunction [234].
In genome-wide association studies, the SPT suppressor ORMDL3 was identified as an obesity-related gene, and its expression in human subcutaneous WAT inversely correlates with BMI [235, 236]. Conversely, Ormdl3-deficient mice show elevated ceramide levels in WAT, increased body weight gain upon high-fat diet feeding, and insulin resistance, which was attributed to decreased thermogenesis and impaired WAT browning [235]. Myriocin treatment reversed the detrimental phenotypes in these mice, indicating that the inhibition of ceramide synthesis by ORMDL3 is a critical mechanism for maintaining adipose tissue function and systemic energy homeostasis [235]. Furthermore, by adipocyte-specific deletion of Sptlc2 in obese mice, Chaurasia and colleagues found that the inhibition of ceramide synthesis decreased C24:0 and C24:1 ceramides in epididymal- and C16:0–C24:0 and C24:1 ceramides in subcutaneous WAT, which improved adipose tissue function preferentially in subcutaneous depots [23]. More specifically, adipocyte-specific Sptlc2 deficiency stimulated M2 macrophage polarization, increased thermogenic gene expression, and abolished inflammation [23]. These animals showed improved energy expenditure, insulin sensitivity, glucose tolerance, and decreased hepatic steatosis, indicating that the adipocyte-autonomous effects of ceramides affect systemic metabolic function [23]. Similarly, adipocyte-specific deletion of Degs1 in obese mice improved systemic insulin sensitivity and glucose tolerance but without effects on adiposity and energy expenditure [25]. Beneficial effects were also observed in high-fat diet-fed mice with inducible adipocyte-specific overexpression of CDase, which reduced total ceramide levels in different visceral and subcutaneous depots (C16:0 and C18:0 ceramides showed the most consistent and robust decrease (± 50%)) that markedly improved systemic and adipose tissue-specific insulin sensitivity [44]. Adipocyte-specific CDase overexpression in obesity also reduced C16:0 and C18:0 ceramides in the liver, associated with improved hepatic insulin sensitivity and protection from diet-induced hepatic steatosis [44]. In contrast to most studies showing that ceramide-lowering interventions in adipose tissue alleviate metabolic dysfunction in obesity, it was reported that reducing ceramide content by the deletion of Sptlc1 or -2 impairs adipose tissue remodeling and causes lipodystrophy, which may have occurred due to impairments in adipocyte differentiation [237, 238]. These findings reemphasize the need to identify and selectively modulate the specific lipotoxic ceramide species in adipose tissue to avoid the adverse effects associated with reducing other ceramides crucial for maintaining adipocyte function and survival.
C16:0 ceramides were investigated in more detail concerning their roles in regulating adipose tissue function in obesity. An increase in the content of C16:0 ceramides in both visceral and subcutaneous WAT can be observed in mice and humans and may be attributed to increased CerS6-dependent ceramide synthesis in these tissues [27]. In a cohort of 439 obese versus lean subjects, significant correlations were found between WAT CERS6 mRNA expression and BMI, systemic insulin resistance, adipocyte size, and circulating leptin and HbA1c levels [27]. Conversely, the specific blockage of C16:0 ceramide production through conventional knockout of CerS6 prevented the diet-induced elevations in C16:0 ceramides in WAT, which led to decreased body fat content, reduced adipocyte size, and reduced macrophage WAT infiltration [27]. These findings have indicated that the accumulation of CerS6-derived C16:0 ceramide in WAT is involved in the obesity-related impairment of WAT function; however, an adipocyte-specific model of CerS6 deficiency has not yet been described, which is necessary to conclude adipocyte-autonomous effects of CerS6-derived C16:0 ceramides in vivo.
Distinct from WAT are depots of BAT with high mitochondrial density that dissipate energy to produce heat. In BAT of obese rodents, de novo ceramide synthesis (particularly of C16:0 and C18:0 ceramides) is increased [24, 27]. Conversely, decreased ceramide synthesis was found in primary brown adipocytes following β-adrenergic stimulation due to reduced expression of Sptlc2 and CerS6 [24]. In rodents, β-adrenergic agonists are effective thermogenic, anti-obesity, and insulin-sensitizing agents that exert their effects primarily through actions in WAT, BAT, and muscle. Thus, it is postulated that the metabolically beneficial effects of β-adrenergic stimulation are partly due to the blockage of ceramide build-up in these tissues. Accordingly, deletion of Sptlc2 in UCP1-positive brown adipocytes protects mice from diet-induced obesity by increasing BAT function and systemic energy expenditure [24]. In turn, thermogenic regulation is impaired when ceramide degradation is inhibited through BAT-specific deletion of acid CDase (Asah1), exacerbating obesity, hepatic steatosis, and insulin resistance [24]. Interestingly, these phenotypes were related to changes in mitochondrial structure and function, respectively [24]. Whereas the inhibition of ceramide synthesis increased mitochondrial density, size, and respiration in BAT of obese mice, blocking ceramide degradation in lean animals decreased mitochondrial density and impaired mitochondrial respiration [24]. Thus, ceramides may be involved in the deregulation of mitochondrial morphology and respiratory function in BAT with adverse metabolic consequences. Specifically, increased formation of CerS6-derived C16:0 ceramides in primary brown adipocytes is sufficient to disturb mitochondrial morphology and function [24], and the deletion of CerS6 in UCP1-positive cells increases mitochondrial β-oxidation capacity in BAT to improve energy expenditure and glucose tolerance in diet-induced obese mice [27]. Together, these studies support the notion that CerS6-derived C16:0 ceramides promote metabolic dysfunction at least in part through adverse actions on mitochondrial function in brown adipocytes. C16:0 ceramides may also impair brown adipogenesis, thus contributing to impairments of the aged BAT [239]. Chaurasia and colleagues made additional suggestions that ceramides in BAT slow lipolysis by inhibiting HSL phosphorylation, inhibit insulin-stimulated AKT phosphorylation, and reduce FFA uptake [24].
Ceramides in the liver
Excessive hepatic lipid storage in obesity can lead to non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH). These conditions are characterized by a particular increase in the liver TAG content, often associated with hepatic and systemic insulin resistance (NAFLD) alongside inflammation that can promote hepatic cirrhosis and fibrosis (NASH) [240]. TAG concentrations in the liver exhibit a strong predictive value for insulin resistance [241], which is believed to arise from the secondary accumulation of deleterious TAG metabolites, including ceramides. Hepatic ceramide levels increase in conditions of dyslipidemia and insulin resistance, e.g., in the liver of lard oil- or dexamethasone-infused rats, high-fat diet-fed mice, and genetically obese rodent models [12, 81, 192]. This is attributable to increases in specific ceramide species (most consistently C16:0–C20:0), which in rodents positively correlate with the degree of steatosis, insulin resistance, hepatic oxidative stress, and inflammation, while other ceramide species remain unaltered or even decrease under these conditions [26, 27, 33, 217, 242,243,244,245,246,247,248,249] (Table 1). Consistent with the data obtained in rodents, specific ceramide species are elevated in the liver of humans with NAFLD. Luukkonen and colleagues observed that hepatic C16:0 and C18:0 ceramides derived from de novo synthesis in obese patients with NAFLD strongly correlate with insulin resistance as measured by HOMA-IR [250]. Intriguingly, patients with NAFLD induced by a mutation in the gene encoding patatin-like phospholipase domain-containing protein 3 (PNPLA3), who are relatively protected against insulin resistance, did not show the same increase in hepatic ceramide levels, linking ceramide accumulation in NAFLD to the development of metabolic deficits [250]. Similarly, an increase in total hepatic ceramide content and that of specific dihydroceramide species (C16:0, C22:0, C24:1) was found in patients with NASH compared to those having fatty liver disease without hepatic inflammation [251].
Evidence for a causal role of ceramides in the liver to systemic metabolic dysfunction is given from obese animal models in which disruption of ceramide synthesis consistently prevented or reversed hepatic ceramide accumulation and improved insulin sensitivity, glucose tolerance, and hepatic steatosis [12, 29, 33, 217, 244,245,246, 252, 253]. Liver-specific effects of ceramides were demonstrated through disruption of ceramide synthesis in obese mice (via hepatocyte-restricted deletion of Degs1) or stimulation of ceramide degradation (via hepatocyte-restricted stimulation of CDase expression or activity), which sufficiently attenuated hepatic steatosis and improved systemic glucose metabolism [25, 44, 192, 203, 205]. These studies have indicated that the liver presents a key site for the adverse effects of ceramide accumulation in obesity and that interventions to reduce hepatic ceramides are promising to alleviate obesity-related metabolic defects. Specifically, of the many ceramide species, CerS6-derived C16:0 ceramides elicit lipotoxic reactions in the liver [26, 27, 93, 243] (Table 1). Both mRNA and protein expression of CerS6 is increased in the liver of diet-induced obese mice concomitant with increased hepatic C16:0 ceramide content [26]. Conversely, improved glucose metabolism in high-fat diet-fed or genetically obese mice can be achieved by conventional [27], whole-body inducible [26, 243], and liver-specific disruption of CerS6-dependent C16:0 ceramide synthesis. Beneficial effects of reducing C16:0 ceramides were also observed in mice fed a methionine-choline deficient diet that promotes NASH independent of obesity [254]. In these animals, treatment with the GLP-1 receptor agonist liraglutide prevented the diet-induced accumulation of C16:0 and C24:0 ceramides in the liver and alleviated hepatic inflammation and fibrosis [254]. These findings have demonstrated the multifaceted effects on liver physiology induced by C16:0 ceramide accumulation. Other studies have indicated that specific ceramides (C16:0 and C18:0) accumulate during the progression from the steatotic liver to the NASH liver in mice fed a fat- and cholesterol-rich diet [255]. In turn, diminishing hepatic ceramide synthesis by Myriocin treatment can prevent steatosis and fibrosis by ameliorating hepatic inflammation, autophagy, and apoptosis in high-fat diet-induced obese rats [256, 257].
Substantial differences exist between CerS5- and CerS6-derived C16:0 ceramides in the liver, as only reducing the latter in mice prevents the development of diet-induced insulin resistance and hepatic steatosis [26]. We found that the deletion of CerS6 in mice results in reduced C16:0 ceramide levels in mitochondria and MAM [26]. This protected the animals against diet-induced mitochondrial fragmentation in hepatocytes and improved mitochondrial respiratory function, highlighting the significance of the subcellular site of ceramide accumulation [26]. Furthermore, liver-specific targeting of CerS6 to reduce hepatic C16:0 ceramide synthesis in obesity can even reverse the detrimental effects of obesity on mitochondrial morphology and glucose metabolism, highlighting the therapeutic potential of CerS6 inhibition to treat obesity-associated hepatic and systemic defects [26]. CerS6-derived C16:0 ceramides in the liver also promote ER stress in the modulation of lipogenesis and hepatic lipid loading upon fatty acid excess by stimulating SREBP1 processing [95]. The ceramide-dependent regulation of CD36-dependent fatty acid uptake in hepatocytes may be relevant in this process [25, 27, 44]. Collectively, these observations indicate that C16:0 ceramides, as generated by CerS6 in hepatocytes, elicit diverse effects on liver metabolism in the obesity-associated deterioration of liver-specific and systemic metabolic homeostasis.
While the roles of hepatic ceramides in promoting metabolic disease have been a significant focus, some evidence points toward beneficial effects of specific ceramide species in this context. In mouse models of type 1 diabetes and diet-induced obesity, C24:1 ceramides were reduced in the liver and plasma [258]. In turn, restoring hepatic C24:1 ceramides by dietary supplementation of nervonic acid, a C24:1 ω-9 fatty acid, reduced body weight gain and improved glucose tolerance and insulin sensitivity [259]. Similarly, elevated C18:1 ceramides in mice fed a palmitate-enriched diet by deleting alkaline CDase 3 (Acer3) alleviated early inflammation and fibrosis, possibly by suppressing hepatocellular oxidative stress in the NASH liver [260]. These results underscore the distinct and partly opposing roles of different ceramide species and point out the need to consider differential changes in the array of hepatic ceramides concerning metabolic disease development and progression.
Ceramides in the skeletal muscle
Skeletal muscle is a highly active metabolic organ and a key site for glucose disposal during hyperglycemia depending on insulin action in myocytes. In obesity, skeletal muscle accumulates lipids caused by an imbalance of nutrient supply and utilization, with lipotoxic effects on insulin sensitivity. This phenomenon used to be under controversial debate given the “athlete’s paradox” that arose from observations in endurance-trained athletes, which exhibit enhanced muscle insulin sensitivity but high amounts of intramyocellular glycerolipids (particularly TAGs) [261]. This phenomenon indicates that the classic neutral lipids are unlikely to be the primary cause of insulin resistance in muscle, which has switched the focus to other lipid metabolites. Studies have recently demonstrated a more consistent link between skeletal muscle ceramide content and impaired insulin sensitivity in rodent models and humans [262].
In skeletal muscle, C18:0 ceramides account for most ceramide content, which in mice almost entirely depends on muscle-specific CerS1-mediated ceramide formation [28]. Endogenous ceramide production in skeletal muscle is increased in mouse models of diet-induced obesity, which show increased CerS1 expression and elevated muscle C18:0 ceramide content [28, 263]. This can be observed in mice as early as three weeks of high-fat diet feeding, accompanied by reduced glucose tolerance [264]. The Watt laboratory has identified an additional mechanism of ceramide accrual in muscle in type 2 diabetic patients, involving LDL-mediated transport of ceramides and uptake by myotubes, which is sufficient to induce systemic insulin resistance by mediating decreased insulin action in muscle [265]. While skeletal muscle C18:0 ceramides are consistently increased in obese rodent models (Table 2), other ceramide species (C14:0, C24:0, and C24:1) decrease after chronic HFD feeding in rats, pointing towards the differential roles of selected ceramide species in muscle [266]. Similarly, most lipid profiling studies on human muscle biopsies revealed increased ceramide content in obesity and diabetes [10, 267,268,269,270], with a C18:0 ceramide signature for insulin resistance [271, 272]. In particular, increased C18:0 ceramide content was found in the muscle of obese and insulin-resistant individuals compared to obese and insulin-sensitive or lean counterparts [271]. Similarly, C18:0 ceramides were increased in skeletal muscle of obese subjects with type 2 diabetes compared to obese non-diabetics and decreased in endurance-trained athletes, especially after acute exercise [272]. Correlation analysis further revealed a strong association between skeletal muscle C18:0 ceramides and BMI and an inverse relationship with insulin sensitivity [272]. In turn, weight loss interventions in humans, such as acute [273] and chronic exercise training [267, 274,275,276], or bariatric surgery [277], consistently decrease muscle ceramide content in conjunction with improved insulin sensitivity and, in some cases, increased skeletal muscle β-oxidation. However, no such correlations were found in a minor subset of lipid profiling studies, which has sparked a controversial debate on the extent to which muscle ceramides were involved in regulating insulin sensitivity at all [278, 279]. However, these conflicting results reemphasize that it may not be the total content of muscle ceramides that matters but changes in specific ceramide species in discrete subcellular pools. In support of this notion, a correlation study on human muscle biopsies suggested that the accumulation of C18:0 ceramides, specifically in a mitochondrial/ER subsarcolemmal fraction, underlies decreased insulin sensitivity [109].
Data obtained from in vitro experiments and murine models confirm that ceramides in myocytes play a crucial role in the deterioration of systemic glucose metabolism. Thus, inhibition of ceramide synthesis in cultured myotubes prevents palmitate-induced changes in mitochondrial morphology and function and restores insulin sensitivity [83, 118]. Similarly, reducing ceramide content in muscle of obese rodents, e.g., by blocking general ceramide synthesis using Myriocin treatment, consistently improves energy expenditure and glucose homeostasis [12, 30, 118, 263, 266] (Table 2). Knockout experiments in mice have further confirmed the exceptionally critical role of CerS1-derived C18:0 ceramides in skeletal muscle in obesity [28]. Ablation of muscle C18:0 ceramide synthesis by myocyte-specific knockout of CerS1 significantly improved insulin sensitivity in high-fat diet-fed mice despite unchanged adiposity [28]. In contrast, the myocyte-specific deletion of CerS5 and CerS6 did not affect insulin sensitivity and glucose metabolism in obesity, highlighting the tissue-specific regulation and roles of distinct CerSs and their specific ceramide products [28]. Surprisingly, deleting CerS1 in skeletal muscle to reduce C18:0 ceramide synthesis in obesity did not appreciably affect muscle-specific insulin signaling or glucose uptake [28]. Still, it improved insulin’s ability to suppress hepatic glucose production and systemic glucose tolerance through increased circulating concentrations of muscle-derived FGF21 [28]. FGF21 is a proteotypic effector of an integrated stress response, particularly to mitochondrial damage [280]. This finding might indicate that CerS1 regulates mitochondrial integrity in vivo, but no overt structural or functional changes of mitochondria were observed in skeletal muscle due to myocyte-specific CerS1 deficiency [28]. Interestingly, pharmacologic inhibition of CerS1 in diet-induced obese mice with a recently developed specific inhibitor (P053) demonstrated a role of CerS1 inhibition on muscle fatty acid oxidation [34]. P053 treatment in high-fat diet-fed mice decreased muscle C18:0 ceramide content by half, associated with increased mitochondrial β-oxidation and significantly reduced TAG levels in muscle [34]. However, compared to the more comprehensive genetic inactivation of CerS1, partial pharmacological inhibition of CerS1 did not alter systemic insulin sensitivity or glucose tolerance [34]. Still, it is predicted that CerS1-derived C18:0 ceramides cause metabolic deterioration in obesity and that CerS1 inhibition provides a promising strategy for treating obesity-related metabolic abnormalities.
Ceramides in the pancreas
Pancreatic β-cells located in the islets of Langerhans are integral to the systemic control of glucose homeostasis through their unique ability to produce and secrete insulin. Evidence suggests that high concentrations of fatty acids and glucose in obesity trigger glucolipotoxic responses that gradually impair the ability of β-cell to provide insulin due to dedifferentiation or altered mass, which is a hallmark of late-stage type 2 diabetes mellitus [281, 282]. Accordingly, mitigation of plasma FFA levels prevents β-cell dysfunction in obese rodents and reduces hyperinsulinemia and hyperglycemia [283]. Pancreatic lipid concentrations, particularly of TAGs, are increased in subjects with type 2 diabetes and decreased after bariatric surgery [284]; however, as also suggested for other cells, TAGs are unlikely to be cytotoxic to pancreatic cells. Thus, the ability of β-cells to route fatty acids to TAG synthesis protects against cellular dysfunction and apoptosis [285]. On the other hand, increased FFA influx into β-cells fuels de novo ceramide synthesis, which can impair pancreatic function [163, 286]. This has been identified in the seminal work of the Unger laboratory, where the treatment with either fatty acids (2:1 oleate/palmitate) or C2 ceramide increased apoptotic DNA fragmentation in islets isolated from prediabetic rats [161]. This response was abolished upon CerS inhibition by fumonisin B1, indicating a critical role of de novo ceramide formation in this process [161]. Accordingly, the lipotoxic effects of fatty acids on β-cells were negated through inhibition of SPT using L-cycloserine in vivo, which ameliorated excessive pancreatic apoptosis and hyperglycemia [32]. It has been proposed that ceramides also account for the profound mitochondrial alterations and ER stress observed in β-cells of the Zucker diabetic fatty rat model, thus promoting pancreatic failure in the pathophysiology of diabetes [287]. Two independent studies demonstrated an additional function of ceramides in cultured pancreatic cells, suggesting a role in modulating glucose-stimulated insulin production [288, 289].
Furthermore, in cultured cells, it was found that chronic exposure of β-cells to supraphysiological levels of glucose and fatty acids promotes the cytotoxic production of selected ceramide species [290, 291]. In INS-1 β-cells, stimulation with glucose and fatty acids induced expression of CerS4 and the formation of specific ceramide species (C18:0, C22:0, and C24:1) concomitant with increased cell death [290]. In turn, inhibition of global ceramide synthesis or selective knockdown of CerS4 in these cells partially prevented palmitate-induced apoptosis [290]. In another study using a mouse insulinoma cell line that exhibits β-cell characteristics, treatment with palmitate increased the expression of CerS5 and CerS6 [291]. Here, the adverse effects of palmitate on β-cells were attributed to both newly generated and salvaged ceramides (C14:0, C16:0, and C24:0), suggesting that these species may be of particular importance in regulating β-cell fate and function also in vivo [291]. However, detailed sphingolipidomic analyses of pancreatic islets in obesity and reports on complementary animal models with pancreas-specific CerS manipulation are lacking. This includes studies investigating the role of ceramides in alternative pancreatic cells, such as α-cells, which are involved in the regulation of systemic glucose metabolism by secreting the peptide hormone glucagon to promote hepatic glucose production, and may also be susceptible to ceramide-induced lipotoxicity in the development of metabolic disease.
Ceramides in the cardiovascular system
Obesity predisposes to cardiovascular complications such as coronary artery disease, cardiomyopathy, and heart failure, which are primary reasons for the morbidity associated with obesity [292]. Excessive lipid deposition in the vascular endothelium and myocardium in obesity disrupts heart and blood vessel function promoting the development of cardiovascular defects [292]. Emerging studies indicate that plasma concentrations of individual ceramide species bear important prognostic value for cardiometabolic impairments, including atherosclerosis, diabetes, heart failure, and death [293]. Remarkably, specific circulating (dihydro)ceramides in humans were able to predict type 2 diabetes even up to 9 years before disease onset [294] and may also be used for assessment of heart failure and atherosclerosis risk in the general population for primary prevention purposes [295, 296]. More specifically, in the majority of studies, high concentrations of ceramides with C16:0, C18:0, and C24:1 acyl chains and low levels of C24:0 ceramides were associated with poor cardiovascular outcomes and increased mortality [297]. On this basis, diagnostic tests to identify subjects at risk of cardiovascular complications by determining particular ceramide ratios and scores are being established [297]. Interestingly, the biomarkers predicting fatal cardiovascular outcomes are proposed to be driven in part by ceramide biosynthesis in hepatocytes [298], adipocytes [299], and intestinal epithelial cells [222].
Ceramides not only can monitor and predict cardiovascular impairment but themselves promote lipotoxic cardiometabolic disease [165]. Accordingly, while ceramides in heart and vasculature are substantially elevated in rodent models of cardiac lipotoxicity and vascular dysfunction, global inhibition of ceramide synthesis improves cardiovascular integrity in obesity [31, 300,301,302]. A critical role of ceramides appears to be their ability to control blood vessel reactivity through actions in the vascular endothelium [165]. Through actions in endothelial cells lining the vessel intima, ceramides affect vascular tone and contribute to arterial dysfunction. As such, in mouse and bovine coronary arteries ex vivo, treatment with C2 ceramide impaired the controlled reduction of vascular tension (vasodilation) [303, 304] and exacerbated blood vessel narrowing (vasoconstriction) in isolated canine cerebral arterial rings [305]. Here, only the treatment with C16:0 ceramides, but not C24:0 and C24:1 ceramides, triggered the constriction of isolated cerebral vascular smooth muscle, indicating ceramide species-specific effects in this process [305]. In addition, increased sphingomyelin hydrolysis has been involved in acute vascular oxygen sensing in the vasoconstrictor response induced by two opposite stimuli, such as hypoxia (in pulmonary and chorioallantoic arteries) and normoxia (in ductus arteriosus) [306]. C6 ceramide treatment in cultured human endothelial cells further indicated that ceramides promote oxidative stress by reducing nitric oxide (NO) generation, which is a critical molecule in maintaining basal vascular tone, leading to ROS formation at the expense of NO synthesis [307]. Ceramide-mediated reductions in NO levels are largely due to PP2A-dependent effects on endothelial NO synthase (eNOS) activity, as demonstrated in cultured endothelial cells subjected to high palmitate concentrations [31, 308]. This response can be restored by inhibiting ceramide synthesis using Myriocin or genetic modification of ceramide biosynthetic genes in models of obesity and hyperlipidemia to improve eNOS activity, NO production, and endothelial cell-dependent vasodilation [31, 308, 309].
Furthermore, ceramide accumulation through increased de novo ceramide production may dictate endothelial cell fate and injury [197]. Endothelial cell apoptosis in response to hyperglycemia has been related to the intercellular transfer of high concentrations of C16:0 ceramides in large extracellular vesicles derived from nSMase2-dependent sphingomyelin hydrolysis, thereby causing endothelial dysfunction in obesity and diabetes [310]. By correlating C16:0 ceramide levels in thoracic adipose tissue and circulation with the deregulation of the vascular redox state and inflammation in human atherosclerotic patients, together with complementary experiments on human tissue ex vivo and primary cultured cells in vitro, it has been suggested that adipose-tissue-derived C16:0 ceramides increase the risk of cardiovascular death by acting on endothelial cells to reduce vasodilation, induce inflammation, and promote oxidative stress via eNOS uncoupling [299]. Notably, eNOS uncoupling is increased in patients with endothelial dysfunction resulting from metabolic diseases such as type 2 diabetes mellitus [311], suggestive of a critical role of ceramides in this process.
Endothelial dysfunction in the tunica intima in obese subjects also promotes the development of atherosclerotic lesions in coronary arteries. Here, lipid accumulation in the endothelium and extracellular matrix and inflammatory cell infiltration into subjacent tissue contributes to the onset of atherosclerotic plaque formation. It was recently reported that patients with coronary artery disease show a two-fold increase in TAG content of the right atrial appendage but no alterations in DAGs, associated with a reduction in adipose triglyceride lipase (ATGL) expression, a rate-limiting enzyme in TAG hydrolysis [312]. Additionally, ceramides accumulate in atherosclerotic plaques, implicated in lipoprotein aggregation (in particular LDL) [313]. In human patients, ceramides were enriched in symptomatic versus asymptomatic atherosclerotic carotid plaques, correlating with plaque content of LDL and inflammatory markers [314]. This study also assigned a causal role to ceramides by showing that they promote an inflammatory response in cultured human coronary artery smooth muscle cells, suggesting that ceramides may attract inflammatory cells to the site of atherosclerotic plaque formation [314]. Conversely, inhibition of de novo ceramide synthesis decreased vascular ceramide content and prevented vascular dysfunction and hypertension in high-fat diet-fed mice [31]. Similar beneficial effects were observed upon pharmacologic treatment with Myriocin in the hyperlipidemic and atherosclerosis-prone apolipoprotein E (ApoE)-deficient mouse model, which prevented the development of atherosclerotic plaques and enabled the regression of established lesions [315]. Interestingly, circulating ceramides derived from the intestine promote the development of atherosclerosis, and decreasing plasma ceramides through suppression of the intestinal FXR/Smpd3 axis reduced lesion areas in the aortas of ApoE-deficient mice [222]. In turn, replenishment of C16:0 ceramides could partially reverse these improvements, suggesting a specific role of C16:0 ceramides in atherosclerosis [222]. Collectively, it is emphasized that lowering plasma ceramide levels may be an effective strategy to improve vascular health in obesity. Still, causal relationships between specific ceramide molecular species and atherosclerosis need to be worked out.
Besides the critical role in the vasculature, ceramides are necessary for maintaining cardiac integrity, as exemplified by the diminished heart function in mice with heart-specific Sptlc2 deficiency [316]. Similar to ceramide depletion, also the accumulation of ceramides in the heart is associated with cardiotoxicity, and ceramide content increases during obesity-related progressive cardiac remodeling and dysfunction [317, 318]. It has been demonstrated that diets rich in myristate promote early development of cardiac hypertrophy, left ventricular systolic and diastolic dysfunction, and autophagy due to increased CerS5-dependent C14:0 ceramide synthesis [319]. Accordingly, in isolated primary cardiomyocytes, myristate but not palmitate could induce CerS5-dependent hypertrophy and autophagic flux, indicating that CerS5-derived C14:0 ceramides may be involved in cardiomyocyte autophagy and lipotoxic diabetic cardiomyopathy [319]. In a follow-up study, a longer exposure to the diet increased myocardial C18:0 and C18:1 ceramides but also CerS2-derived C22:0 and C24:0 ceramide species [320]. Interestingly, while the overexpression of either CerS2 or CerS5 triggered cerdiomyocyte apoptosis, only the overexpression of CerS2 induced mitochondrial dysfunction and mitophagy in cardiomyocytes; however, it did not affect hypertrophy, suggesting that CerS2 and CerS5 have distinct roles in this process [320].
Myriocin treatment and the selective reduction of specific ceramides (C16:0, C24:0, and C24:1) in the heart of mice with ischemic cardiomyopathy reduced ventricular remodeling, fibrosis, and macrophage content following myocardial infarction [318]. In another study, Myriocin treatment significantly blunted the increase of myocardial ceramides in the lipid-overloaded heart of a mouse model of dilated cardiomyopathy, i.e., in mice expressing glycosylphosphatidylinositol (GPI)-anchored human lipoprotein lipase (LpLGPI) [80]. The reduction of ceramide de novo synthesis in these animals improved myocardial glucose oxidation rates, cardiac efficiency, and survival and reduced the expression of heart failure markers [80, 321]. Similar beneficial effects were obtained after Myriocin treatment in diet-induced obese mice, which decreased myocardial ceramide content and improved glycolysis and glucose oxidation in isolated aerobic perfused working hearts in the presence of insulin [302]. From a mechanistic point of view, myocardial ceramides trigger ER stress and apoptosis, decrease mitochondrial function, and promote insulin resistance, thereby contributing to the pathophysiology of cardiomyopathy [322,323,324]. Collectively, it is predicted that targeting the production of specific ceramide species may have profound beneficial cardiovascular effects to improve obesity-associated cardiometabolic complications.
Ceramides in hypothalamic neurons
In the central nervous system, a dynamic network of neurons located in spatially distinct areas of the hypothalamus responds to hormonal and nutritional cues in order to compute the organism’s energy state and adapt food intake and metabolic rate [325]. Different hypothalamic nuclei, including the arcuate nucleus (ARC), the ventromedial nucleus (VMH), and the lateral hypothalamic area (LHA), have profound roles in regulating food intake, glucose homeostasis, and metabolism [326]. Acute high-fat diet feeding and chronic over-nutrition are associated with a rise in hypothalamic lipid concentrations that promotes a decline in sensitivity toward hormonal input within the hypothalamic melanocortin circuitry [327, 328]. Interestingly, central actions of both the adipokine leptin and the gastric hormone ghrelin involve changes in hypothalamic ceramide content to pursue their respective effects on food intake, indicating critical regulatory functions of ceramides in this process [329, 330].
Given the highly heterogeneous composition of neurons in the hypothalamus, it is likely that significant differences in ceramide species occur across different neuronal populations, which has not been resolved thus far. Still, evidence now clearly indicates that ceramides mediate many of the adverse effects of fatty acids on neuronal integrity, contributing to metabolic impairment when they accumulate in the hypothalamus, as is the case in hyperlipidemic and high-fat diet-fed animal models [331]. Studies revealed increases in selected ceramide species in the hypothalamus of high-fat diet-induced obese mice (C18:0, C22:0, C24:0), diabetic and dyslipidemic rats (C16:0, C18:0, C20:0), and in the lipid-overloaded hypothalamus of obese Zucker rats (C16:0, C18:0) [135, 332, 333]. Intriguingly, hypothalamic accumulation of ceramides appears to be sexually dimorphic, as elevated levels of ceramides were only found in the hypothalamus of males compared to female mice following consumption of high-fat diets [334]. Here, ceramide levels correlated with reduced PGC-1α and estrogen receptor α (ERα) to promote hypothalamic inflammation and myocardial dysfunction in a sex-specific manner [334]. Sex dimorphisms are primarily due to hormonal differences between sexes, e.g., ovarian steroids that deeply affect metabolic networks in females [334]. Accordingly, estradiol (E2) controls ceramide content in the hypothalamus of female rats [335]. E2 is implicated in sexual maturation and regulates food intake through effects on proopiomelanocortin (POMC)-expressing neurons in the ARC as well as BAT thermogenesis through impact on the VMH [336]. Ovarian insufficiency, in turn, is associated with ceramide accumulation in the mediobasal hypothalamus of rats and hyperphagia, reduced energy expenditure, and increased weight gain [335]. Conversely, central E2 treatment reduces hypothalamic ceramide content, possibly via AMPK, and ameliorates ceramide-induced lipotoxicity and ER stress by affecting the sympathetic nervous system and BAT thermogenesis [335]. Ceramide accumulation in the hypothalamus was also observed in mice with deficiency for the lipoprotein lipase (LPL) in astrocytes, which represent important sites of brain lipid sensing [337]. Astrocytic ceramide accrual increased hypothalamic immunoreactivity of the appetite-regulating agouti-related peptide (AgRP) and ER stress marker gene expression in conjunction with elevations in food intake, body weight gain, adiposity, and glucose intolerance [337]. Furthermore, transcript levels of sphingolipid-metabolic genes, including that of specific CerSs, are increased in particular neurons of diet-induced obese mice, as was recently suggested from transcriptomic analysis in POMC neurons [338].
Ceramides derived from endogenous ceramide synthesis elicit direct effects on hypothalamic neurons. In cultured hypothalamic GT1-7 neuronal cells, palmitate-dependent increases in ceramide content decreased insulin sensitivity [339]. Myriocin treatment or Sptlc2 knockdown in these cells abolished the inhibition of insulin sensitivity, indicating that ceramides produced through de novo synthesis have critical roles in the manifestation of neuronal insulin resistance during fatty acid excess [339]. Accordingly, ICV infusion of C2 ceramides in obese Zucker rats impaired and Myriocin infusion improved hypothalamic insulin sensitivity and systemic glucose tolerance, which was attributed to increased glucose-stimulated insulin secretion and β-cell mass [339]. TLR4 signaling was found essential for lipid-induced hypothalamic ceramide accumulation, and TLR4 deficiency in mice prevents hypothalamic ceramide accrual in response to lard oil infusion, protecting the animals from fatty acid-induced insulin resistance and systemic glucose intolerance [192, 340]. Consistently, inhibition of IKK-β in obese animals can lower hypothalamic ceramide concentrations, leading to similar beneficial metabolic outcomes, demonstrating the functional relevance of inflammatory signals in regulating hypothalamic ceramide turnover and metabolic homeostasis.
Campana et al. found a positive impact of PKC inhibition on fatty acid- and ceramide-induced insulin resistance in cultured hypothalamic neurons [339]. Here, pharmacological inhibition of PKCs or expression of a dominant-negative version of PKCζ counteracted the inhibition of AKT phosphorylation induced by either C2 ceramide or palmitate treatment in GT1-7 cells [339]. Ceramide-mediated PKCζ regulation may thus be central to modulation of insulin sensitivity also in hypothalamic neurons, but this has not been confirmed in vivo. The detrimental effects of ceramide accrual in the hypothalamus are also partly mediated through ER stress and associated inflammation. In mHypoE-N42 cells, inhibition of de novo ceramide formation through L-cycloserine treatment reduced palmitate-induced inflammation [341]. Other fatty acids, namely oleic- and eicosatetraenoic acid, showed anti-inflammatory effects by decreasing palmitate-induced ceramide build-up [341].
Contreras et al. found that hypothalamic ceramides promote ER stress in the VMH, thereby reducing sympathetic tone that impairs BAT metabolic function in the control of systemic energy metabolism [135]. In this study, ICV treatment of rats with C6 ceramide increased C16:0 ceramide content in the mediobasal hypothalamus, which was associated with increased expression of inflammatory markers and elevated ER stress [135]. Central administration of C6 ceramide reduced sympathetic nerve activity, diminished the thermogenic capacity of BAT, and impaired systemic insulin sensitivity [135]. Similarly, increasing ceramide synthesis by overexpression of SPTLC1/2 in the VMH increased ER stress in hyperthyroid rats [210]. Downregulation of SPTLC1 in VMH, in turn, ameliorated ER stress and improved metabolic health in ovariectomized rats [335]. Decreases in hypothalamic ceramide content induced by T3 treatment were associated with reduced hypothalamic ER stress, in conjunction with improved BAT mitochondrial activity, thermogenesis, and metabolic homeostasis [210]. Together, these studies indicate that ceramide accumulation in the hypothalamus, as present in obesity, promotes hypothalamic ER stress, particularly in the VMH, leading to systemic metabolic impairments.
In addition, a role of ceramides in the PVH concerning sexual maturation in obesity has been identified [342]. In contrast to undernutrition that delays puberty, childhood obesity often accelerates puberty onset, linked to a higher disease burden later in life [343]. It was recently found that de novo synthesis of nearly all ceramide species is increased in the PVH of early-onset obese female rats, leading to ceramide accumulation and precocious puberty [342]. Central administration of C6 ceramides induced pubertal precocity, while it was delayed in lean female rats after inhibiting ceramide synthesis using Myriocin [342]. In particular, the PVH has been proposed as a critical hypothalamic region for transmitting sympathetic neural information to the ovary to control ovarian maturation and function [342, 344]. Accordingly, PVH de novo synthesized ceramides triggered an increase in ovarian sympathetic tone in early-overfed rats through interplay with kisspeptin in a non-canonical pathway of the central control of puberty [342].
Collectively, there is emerging evidence for a specific role of hypothalamic ceramides in obesity-related deregulations. However, the particular neuronal populations and potential other cell types in the hypothalamus in which specific ceramides affect metabolic homeostasis have not yet been clearly defined.
Is it possible to modulate ceramide metabolism for the treatment of obesity-related diseases?
As highlighted in this article, plasma and tissue ceramide levels increase during obesity development associated with the onset and progression of metabolic diseases in both animal models and humans. An obvious question arising from these findings is whether inhibiting ceramide synthesis or stimulating ceramide degradation would provide strategies to efficiently improve metabolic health and treat obesity-related disorders in human patients. Results from rodent studies support this idea, showing that the disruption of ceramide synthesis protects from insulin resistance and other metabolic complications of obesity and can reverse these pathologies when achieved in an inducible manner [12, 29, 30, 33]. The fact that conditional, tissue-specific reduction in ceramide content in obese rodent models is sufficient to improve metabolic homeostasis emphasizes the possibility of developing tissue-restrictive drugs for selective inhibition of ceramide production. Thus, peripherally acting pharmacotherapeutics may circumvent adverse reactions associated with ceramide-lowering interventions such as neurodegenerative processes in the brain [345]. However, inhibitors to modulate components of the ceramide metabolic pathway in humans with tolerable side effects are not yet available. Therefore, to the authors’ knowledge, there is currently no publicly available information about clinical experience with specific inhibitors of ceramide biosynthetic enzymes in obese and diabetic patients.
Strategies to improve metabolic health, such as the treatment with insulin-sensitizing agents (metformin and pioglitazone), acute exercise, or weight loss, reduce ceramide levels in tissues and circulation [275, 346, 347]. In addition, it was found that people consuming “healthier diets,” such as Nordic or Mediterranean diets, exhibit lower circulating ceramide levels and a lower risk for cardiovascular disease and diabetes as compared with those consuming more typical foods [348, 349]. Plasma ceramide levels are also reduced following the application of statin-based pharmacotherapies in patients with metabolic syndrome and coronary artery disease, used to treat hypercholesterolemia for primary and secondary prevention of cardiovascular disease [350, 351]. Gastric bypass surgery in obese subjects also lowers circulating ceramide levels [352], and this intervention is applied for the prevention and remission of diabetes, hypertension, and dyslipidemia [353]. Collectively, although the studies only show correlations between ceramides and metabolic integrity, it is interesting to speculate that these interventions cause beneficial metabolic outcomes by affecting ceramide content. In addition, they show that ceramide levels in humans are indeed modifiable, highlighting the potential of ceramide-lowering interventions in future clinical settings. It is also interesting to note that extracts from the endoparasitic fungus Cordyceps sinclairii, from which the SPT inhibitor Myriocin was initially isolated, are commonly applied to patients in traditional Chinese medicine to treat an array of health impairments, including diabetes [354]. However, long-term treatment with Myriocin itself can exert adverse health effects, e.g., hepatoxicity, as shown in male Wistar rats [355]. The need to identify novel and more specific inhibitors of ceramide synthesis is thus clearly emphasized.
It should be noted that the complete inhibition of global ceramide formation poses considerable risk of adverse side effects due to the multifaceted cellular functions of ceramides and their sphingolipid derivatives. This is also exemplified by the embryonic lethality in mice with homozygous deletion of Sptlc1 and Sptlc2 [356], the development of liver cancer upon hepatocyte-specific knockout of Sptlc2, and the development of inflammatory bowel disease and early lethality when Sptlc2 is deleted in an inducible and intestine-specific manner [357, 358]. Along the same lines, mice with homozygous deletion of Degs1 reveal an incompletely penetrant lethality, and the surviving animals show growth retardation with several health complications [12]. Similarly, DEGS1 missense mutations in humans cause severe neurological disorders [359,360,361]. Nevertheless, the development of pharmacological approaches to partially inhibit DES1 in obesity to treat cardiometabolic diseases is being pushed forward [362]. A similar suggestion has been made for CERS6 to avoid the broad spectrum of possible side effects [35]. Indeed, the CerS enzymes could offer attractive drug targets for obesity and diabetes therapy [363]. This suggestion is mainly based on the observation that human CERSs and the regulation of the corresponding ceramide products during obesity development are conserved from mice to humans and that the inhibition of specific CerSs is sufficient for alleviating obesity-associated metabolic dysfunction in related murine studies [363]. Specifically, the disruption of C16:0 ceramide synthesis either by the inducible deletion of CerS6 or treatment with CerS6-specific ASOs can improve systemic glucose metabolism in obese and insulin-resistant mice [26, 243]. Similarly, pharmacological inhibition of CerS1 in obese mice using P053 treatment to reduce C18:0 ceramide content predominantly in skeletal muscle efficiently improves lipid metabolism [34], and no adverse effects as a consequence of either intervention have been reported to date.
When CerS enzymes are selected as targets for pharmacological intervention, it is important to consider the different physiological roles of their ceramide products and the consequences of reducing them, which may result in a compensatory increase in other cytotoxic ceramide molecular species. In this context, it is also important to reiterate that even ceramides with a specific acyl chain length can have different physiological functions and pathological effects depending on their intracellular localization, which is partly determined by the specific synthesizing CerS [26]. This specificity could provide a highly advantageous opportunity to target only restricted pools of ceramide species that exert adverse effects on metabolic homeostasis while leaving other populations unaffected and available to maintain critical cellular processes. The development of such specific inhibitors of ceramide synthesis could pave the way for sophisticated novel therapeutic strategies to combat the epidemic of obesity and its comorbidities.
Conclusion and future perspectives
Ceramides are bioactive lipids that exert a plethora of metabolic functions through their unique biophysical properties in membranes and their abilities to control intracellular signaling pathways in part through binding to regulatory proteins. As discussed herein, the biological roles and pathological effects of ceramides highly depend on the molecular composition of the ceramide species, partly defined by the length of the acyl chain. Recent advances in high-resolution mass spectrometry-based lipidomics have laid the foundations for dissecting the complexity of the acyl chain ceramide distribution in membranes and its dynamic regulation upon varying physiological conditions. However, we still face limitations in the technical abilities to study ceramides in small cell populations and at the single-cell level, which will be crucial to understand the cell-type-specific regulation of ceramides in greater detail in vivo. The need for a cell to exhibit this striking diversity is not fully understood, as are the stimuli that control membrane ceramide plasticity. Still, it underscores the need for multilayered lipid-regulated mechanisms to fine-tune biological processes through appropriate alterations in membrane dynamics and membrane-emanating signaling cascades in response to a wide array of stimuli. In this light, ceramides may act as critical metabolic messengers to control lipid and glucose homeostasis, mitochondrial plasticity, and inflammatory signaling in conditions of fatty acid excess. We have discussed studies indicating that several metabolism-regulatory pathways (e.g., adiponectin receptor signaling) mediate their cellular actions by modulating ceramide turnover and that deregulation within these processes impacts metabolic health depending on specific ceramide species. In accordance, it has been consistently demonstrated that ceramide metabolism is altered in obesity and that the accumulation of selected ceramide molecular species can have detrimental pathological consequences in a tissue-specific manner.
Whereas initially, only total amounts of ceramides in cell and tissue homogenates were reported, and interventions were aimed at inhibiting general ceramide synthesis, studies over the past decade have demonstrated that quantifying total ceramide levels is insufficient to identify alterations in ceramide metabolism; this is due to the distinction of specific acyl chain length molecular species that can be regulated independently of each other and exert defined metabolic and organ-specific roles. In particular, CerS6-derived C16:0 ceramides in the liver and BAT and CerS1-derived C18:0 ceramides in skeletal muscle evolved as critical regulators of metabolic integrity and dysfunction in obesity. Defining the roles of the relevant ceramide species also in other tissues, such as the pancreas, heart, vasculature, and hypothalamus, will provide additional insights into the tissue-specific functional versatility of ceramides, allowing us to unravel further the immense complexity of lipid-based mechanism in maintaining or disturbing metabolic homeostasis in obesity. Characterizing cell-type-specific CerS knockout mouse models and developing CerS-selective inhibitors to modulate the synthesis of specific ceramide molecular species will be important in the future. Nevertheless, recent observations indicate that—depending on the research objective—simply distinguishing between levels of particular ceramides is no longer sufficient, given that the same molecular species can be regulated differently in different cellular compartments. The sub-compartmentalization of these ceramides can have specific biological roles with independent pathological consequences when they accrue, further supporting the need for analyses of ceramides within their relevant subcellular locations. In addition, although the physicochemical properties of ceramides have been subject to extensive research in vitro and studies on the cellular actions and pathological properties of ceramides have emerged, definite causal relationships often remain to be established. By linking both research areas in the future, it is expected that new avenues can be explored to dissect the biological functions of particular acyl chain ceramide species in obesity.
This article addresses the impact of ceramides with specific acyl chain lengths in obesity. However, it has to be kept in mind that further layers of ceramide complexity confer additional functional specificity in physiology and disease. This involves (a) varying sphingoid bases, (b) acyl chain unsaturation, and (c) ceramide headgroup modification (e.g., phosphorylation and glycosylation). Moreover, it should by no means be ruled out that other lipid species make a decisive contribution to metabolic regulation and promote lipotoxicity in the deterioration of metabolic homeostasis, such as already proposed for sn-1,2-DAGs [364]. Nevertheless, as hopefully appreciated by the reader, the research on ceramides in obesity and their specific roles in metabolic disease pathogenesis has begun to evolve. Further research is undoubtedly required to unravel the precise mechanisms by which specific bioactive ceramide species are regulated in obesity, and to better understand how they modulate metabolic processes in the tissue-specific (de)regulation of metabolic homeostasis.
In particular, as the obesity epidemic continues to spread and cases of obesity-related metabolic diseases inexorably increase, new targets must be found for therapeutic intervention. Restraining acyl chain length-specific ceramides in production or action could be a novel approach in this context. The identification of the specific ceramide target proteins in distinct cellular compartments and the prevention of ceramide-(binding)-induced deregulations in downstream signaling pathways might bring us closer to achieving this goal. Furthermore, pharmacological inhibition of specific CerS enzymes in obesity—promising candidates are CerS1 and CerS6—holds the potential to treat obesity-related metabolic disease while circumventing the adverse consequences associated with inhibiting global ceramide production.
Box 1
Box 2
Availability of data and materials
No data sets were generated for this review.
References
Collaborators GBDO et al (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27
Ebbeling CB, Pawlak DB, Ludwig DS (2002) Childhood obesity: public-health crisis, common sense cure. Lancet 360(9331):473–482
WHO. Controlling the global obesity epidemic. https://www.who.int/activities/controlling-the-global-obesity-epidemic
Bluher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15(5):288–298
Muller, T.D., et al., Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov, 2021.
Bluher M (2013) Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab 27(2):163–177
Unger RH et al (2010) Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801(3):209–214
Chavez JA, Summers SA (2010) Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta 1801(3):252–265
Turinsky J, O’Sullivan DM, Bayly BP (1990) 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 265(28):16880–16885
Adams JM 2nd et al (2004) Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53(1):25–31
Haus JM et al (2009) Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58(2):337–343
Holland WL et al (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5(3):167–179
Ying L, Tippetts TS, Chaurasia B (2019) Ceramide dependent lipotoxicity in metabolic diseases. Nutrition Healthy Aging 5:1–12
Hannun YA, Obeid LM (2011) Many ceramides. J Biol Chem 286(32):27855–27862
Grosch S, Schiffmann S, Geisslinger G (2012) Chain length-specific properties of ceramides. Prog Lipid Res 51(1):50–62
Turpin-Nolan SM, Bruning JC (2020) The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol 16(4):224–233
Futerman AH, Hannun YA (2004) The complex life of simple sphingolipids. EMBO Rep 5(8):777–782
Leipelt M, Merrill AH (2004) Sphingolipid Biosynthesis. In: Lennarz WJ, Lane MD (eds) Encyclopedia of biological chemistry. Elsevier, New York, pp 76–81
Gault CR, Obeid LM, Hannun YA (2010) An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol 688:1–23
Lone MA et al (2020) Subunit composition of the mammalian serine-palmitoyltransferase defines the spectrum of straight and methyl-branched long-chain bases. Proc Natl Acad Sci USA 117(27):15591–15598
Levy M, Futerman AH (2010) Mammalian ceramide synthases. IUBMB Life 62(5):347–356
Siddique MM et al (2015) Dihydroceramides: from bit players to lead actors. J Biol Chem 290(25):15371–15379
Chaurasia B et al (2016) Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab 24(6):820–834
Chaurasia B et al (2021) Ceramides are necessary and sufficient for diet-induced impairment of thermogenic adipocytes. Mol Metab 45:101145
Chaurasia B et al (2019) Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365(6451):386–392
Hammerschmidt P et al (2019) CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177(6):1536-1552 e23
Turpin SM et al (2014) Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20(4):678–686
Turpin-Nolan SM et al (2019) CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep 26(1):1-10 e7
Yang G et al (2009) Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab 297(1):E211–E224
Ussher JR et al (2010) Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59(10):2453–2464
Zhang QJ et al (2012) Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61(7):1848–1859
Shimabukuro M et al (1998) Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem 273(49):32487–32490
Bikman BT et al (2012) Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J Biol Chem 287(21):17426–17437
Turner N et al (2018) A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat Commun 9(1):3165
Raichur S et al (2019) The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab 21:36–50
Senkal CE et al (2017) Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab 25(3):686–697
Hanada K (2017) Ceramide transport from the endoplasmic reticulum to the trans golgi region at organelle membrane contact sites. Adv Exp Med Biol 997:69–81
Kumagai K, Hanada K (2019) Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites. FEBS Lett 593(17):2366–2377
Kumagai K et al (2005) CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem 280(8):6488–6495
Goto A, Mizuike A, Hanada K (2020) Sphingolipid metabolism at the ER-golgi contact zone and its impact on membrane trafficking. Contact 3:2515256420959514
Kitatani K, Idkowiak-Baldys J, Hannun YA (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20(6):1010–1018
Coant N et al (2017) Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 63:122–131
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9(2):139–150
Xia JY et al (2015) Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab 22(2):266–278
Holland WL et al (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17(1):55–63
Hannun YA, Obeid LM (2018) Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19(3):175–191
Li S et al (2021) Structural insights into the assembly and substrate selectivity of human SPT-ORMDL3 complex. Nat Struct Mol Biol 28(3):249–257
Breslow DK et al (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463(7284):1048–1053
Han G et al (2019) The ORMs interact with transmembrane domain 1 of Lcb1 and regulate serine palmitoyltransferase oligomerization, activity and localization. Biochim Biophys Acta Mol Cell Biol Lipids 1864(3):245–259
Davis DL et al (2019) The ORMDL/Orm-serine palmitoyltransferase (SPT) complex is directly regulated by ceramide: reconstitution of SPT regulation in isolated membranes. J Biol Chem 294(13):5146–5156
Green CD et al (2021) CRISPR/Cas9 deletion of ORMDLs reveals complexity in sphingolipid metabolism. J Lipid Res 62:100082
Chaurasia B, Summers SA (2021) Ceramides in metabolism: key lipotoxic players. Annu Rev Physiol 83:303–330
Ogretmen B et al (2002) Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem 277(15):12960–12969
Summers SA, Chaurasia B, Holland WL (2019) Metabolic messengers: ceramides. Nat Metab 1(11):1051–1058
Alonso A, Goni FM (2018) The physical properties of ceramides in membranes. Annu Rev Biophys 47:633–654
Bockelmann S et al (2018) A search for ceramide binding proteins using bifunctional lipid analogs yields CERT-related protein StarD7. J Lipid Res 59(3):515–530
Chavez JA, Summers SA (2012) A ceramide-centric view of insulin resistance. Cell Metab 15(5):585–594
Roszczyc-Owsiejczuk K, Zabielski P (2021) Sphingolipids as a culprit of mitochondrial dysfunction in insulin resistance and type 2 diabetes. Front Endocrinol (Lausanne) 12:635175
Jain A, Dadsena S, Holthuis JCM (2020) A switchable ceramide transfer protein for dissecting the mechanism of ceramide-induced mitochondrial apoptosis. FEBS Lett 594(22):3739–3750
Weijers RN (2012) Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr Diabetes Rev 8(5):390–400
Pilon M (2016) Revisiting the membrane-centric view of diabetes. Lipids Health Dis 15(1):167
Pascher I (1976) Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim Biophys Acta 455(2):433–451
Bollinger CR, Teichgraber V, Gulbins E (2005) Ceramide-enriched membrane domains. Biochim Biophys Acta 1746(3):284–294
de la Arada I et al (2020) Exploring polar headgroup interactions between sphingomyelin and ceramide with infrared spectroscopy. Sci Rep 10(1):17606
Megha, London E (2004) Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem 279(11):9997–10004
Garcia-Arribas AB, Alonso A, Goni FM (2016) Cholesterol interactions with ceramide and sphingomyelin. Chem Phys Lipids 199:26–34
Pinto SN et al (2011) Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim Biophys Acta 1808(11):2753–2760
Silva LC et al (2012) Ablation of ceramide synthase 2 strongly affects biophysical properties of membranes. J Lipid Res 53(3):430–436
Imgrund S et al (2009) Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem 284(48):33549–33560
Pewzner-Jung Y et al (2010) A critical role for ceramide synthase 2 in liver homeostasis: II. Insights into molecular changes leading to hepatopathy. J Biol Chem 285(14):10911–10923
Pewzner-Jung Y et al (2010) A critical role for ceramide synthase 2 in liver homeostasis: I. Alterations in lipid metabolic pathways. J Biol Chem 285(14):10902–10910
Pinto SN et al (2014) Changes in membrane biophysical properties induced by sphingomyelinase depend on the sphingolipid N-acyl chain. J Lipid Res 55(1):53–61
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9(2):112–124
Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327(5961):46–50
Contreras FX et al (2012) Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 481(7382):525–529
Haberkant P et al (2016) Bifunctional sphingosine for cell-based analysis of protein-sphingolipid interactions. ACS Chem Biol 11(1):222–230
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414(6865):799–806
Summers SA et al (1998) Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18(9):5457–5464
Schmitz-Peiffer C, Craig DL, Biden TJ (1999) Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274(34):24202–24210
Park TS et al (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49(10):2101–2112
Longato L et al (2012) High fat diet induced hepatic steatosis and insulin resistance: role of dysregulated ceramide metabolism. Hepatol Res 42(4):412–427
Chavez JA et al (2005) Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280(20):20148–20153
Watson ML, Coghlan M, Hundal HS (2009) Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem J 417(3):791–801
Fox TE et al (2007) Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282(17):12450–12457
Hajduch E et al (2008) Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J 410(2):369–379
Blouin CM et al (2010) Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59(3):600–610
Mukhopadhyay A et al (2009) Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J 23(3):751–763
Stratford S et al (2004) Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 279(35):36608–36615
JeBailey L et al (2007) Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 56(2):394–403
Hage Hassan R et al (2016) Sustained action of ceramide on the insulin signaling pathway in muscle cells: implication of the double-stranded RNA-activated protein kinase. J Biol Chem 291(6):3019–3029
Park JW et al (2013) Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology 57(2):525–532
Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375(9733):2267–2277
Raichur S et al (2014) CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 20(4):687–695
James DE, Stockli J, Birnbaum MJ (2021) The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol 22(11):751–771
Kim YR et al (2019) Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp Mol Med 51(11):129
Brown MS, Goldstein JL (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7(2):95–96
Vatner DF et al (2015) Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci U S A 112(4):1143–1148
Suomalainen A, Battersby BJ (2018) Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol 19(2):77–92
Silva JP et al (2000) Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet 26(3):336–340
Szendroedi J, Phielix E, Roden M (2011) The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 8(2):92–103
Mantena SK et al (2009) High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 417(1):183–193
Novgorodov SA et al (2011) Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem 286(28):25352–25362
El Bawab S et al (2000) Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem 275(28):21508–21513
Wu BX et al (2010) Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem 285(23):17993–18002
Yu J et al (2007) JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem 282(35):25940–25949
Bionda C et al (2004) Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382(Pt 2):527–533
Novgorodov SA et al (2011) Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J Biol Chem 286(6):4644–4658
Aaltonen MJ et al (2022) Serine palmitoyltransferase assembles at ER-mitochondria contact sites. Life Sci Alliance 5(2):e202101278
Perreault L et al (2018) Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight. https://doi.org/10.1172/jci.insight.96805
Kogot-Levin A, Saada A (2014) Ceramide and the mitochondrial respiratory chain. Biochimie 100:88–94
Garcia-Ruiz C et al (1997) Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272(17):11369–11377
Gudz TI, Tserng KY, Hoppel CL (1997) Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem 272(39):24154–24158
Di Paola M, Cocco T, Lorusso M (2000) Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry 39(22):6660–6668
Zigdon H et al (2013) Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J Biol Chem 288(7):4947–4956
Mishra P, Chan DC (2016) Metabolic regulation of mitochondrial dynamics. J Cell Biol 212(4):379–387
Wai T, Langer T (2016) Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab 27(2):105–117
Song JE et al (2021) Mitochondrial fission governed by Drp1 regulates exogenous fatty acid usage and storage in hela cells. Metabolites 11(5):322
Smith ME et al (2013) Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem J 456(3):427–439
Parra V et al (2008) Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res 77(2):387–397
Veluthakal R et al (2005) Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis 10(4):841–850
Hoglinger D et al (2017) Trifunctional lipid probes for comprehensive studies of single lipid species in living cells. Proc Natl Acad Sci USA 114(7):1566–1571
Huang H et al (2011) piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell 20(3):376–387
Erion DM, Shulman GI (2010) Diacylglycerol-mediated insulin resistance. Nat Med 16(4):400–402
Saita S et al (2018) PARL partitions the lipid transfer protein STARD7 between the cytosol and mitochondria. EMBO J. https://doi.org/10.15252/embj.201797909
Kong JN et al (2018) Novel function of ceramide for regulation of mitochondrial ATP release in astrocytes. J Lipid Res 59(3):488–506
Hayashi T, Fujimoto M (2010) Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol 77(4):517–528
Rieusset J (2018) The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis 9(3):388
Zelnik ID et al (2020) The role of ceramide in regulating endoplasmic reticulum function. Biochim Biophys Acta Mol Cell Biol Lipids 1865(1):158489
Rodriguez-Gallardo S et al (2020) Ceramide chain length-dependent protein sorting into selective endoplasmic reticulum exit sites. Sci Adv. https://doi.org/10.1126/sciadv.aba8237
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21(8):421–438
Ozcan L, Tabas I (2012) Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med 63:317–328
Fu S et al (2011) Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473(7348):528–531
Park WJ, Park JW (2020) The role of sphingolipids in endoplasmic reticulum stress. FEBS Lett 594(22):3632–3651
Correnti J et al (2020) Liver-specific ceramide reduction alleviates steatosis and insulin resistance in alcohol-fed mice. J Lipid Res 61(7):983–994
Contreras C et al (2014) Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep 9(1):366–377
Han S et al (2010) Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc Natl Acad Sci USA 107(13):5851–5856
Tam AB et al (2018) The UPR activator ATF6 responds to proteotoxic and lipotoxic stress by distinct mechanisms. Dev Cell 46(3):327-343 e7
Liu F et al (2016) Ceramide activates lysosomal cathepsin B and cathepsin D to attenuate autophagy and induces ER stress to suppress myeloid-derived suppressor cells. Oncotarget 7(51):83907–83925
Park MA et al (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 7(10):1648–1662
Martinez L et al (2015) Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget 6(39):41479–41496
Choi S et al (2018) Myristate-induced endoplasmic reticulum stress requires ceramide synthases 5/6 and generation of C14-ceramide in intestinal epithelial cells. FASEB J 32(10):5724–5736
Liu LK et al (2017) An inducible ER-Golgi tether facilitates ceramide transport to alleviate lipotoxicity. J Cell Biol 216(1):131–147
Gjoni E et al (2014) Glucolipotoxicity impairs ceramide flow from the endoplasmic reticulum to the Golgi apparatus in INS-1 beta-cells. PLoS ONE 9(10):e110875
Bandet CL et al (2018) Ceramide transporter CERT is involved in muscle insulin signaling defects under lipotoxic conditions. Diabetes 67(7):1258–1271
Senkal CE et al (2010) Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J 24(1):296–308
Senkal CE et al (2011) Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. J Biol Chem 286(49):42446–42458
Vandanmagsar B et al (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17(2):179–188
Ali M et al (2013) Altering the sphingolipid acyl chain composition prevents LPS/GLN-mediated hepatic failure in mice by disrupting TNFR1 internalization. Cell Death Dis 4:e929
Ververs FA et al (2018) Immunometabolic activation of invariant natural killer T cells. Front Immunol 9:1192
Zhou D et al (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306(5702):1786–1789
Lynch L et al (2012) Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37(3):574–587
Ji Y et al (2012) Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem 287(17):13561–13571
Wu L et al (2012) Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA 109(19):E1143–E1152
Albeituni S, Stiban J (2019) Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv Exp Med Biol 1161:169–191
Sims K et al (2010) Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem 285(49):38568–38579
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469(7330):323–335
Camell CD et al (2015) Macrophage-specific de novo synthesis of ceramide is dispensable for inflammasome-driven inflammation and insulin resistance in obesity. J Biol Chem 290(49):29402–29413
Kolesnick RN, Kronke M (1998) Regulation of ceramide production and apoptosis. Annu Rev Physiol 60:643–665
Pettus BJ, Chalfant CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585(2–3):114–125
Mullen TD, Obeid LM (2012) Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem 12(4):340–363
Shimabukuro M et al (1998) Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95(5):2498–2502
Turpin SM et al (2006) Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 291(6):E1341–E1350
Boslem E, Meikle PJ, Biden TJ (2012) Roles of ceramide and sphingolipids in pancreatic beta-cell function and dysfunction. Islets 4(3):177–187
Pagadala M et al (2012) Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab 23(8):365–371
Choi RH et al (2021) Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 18(10):701–711
Cremesti A et al (2001) Ceramide enables fas to cap and kill. J Biol Chem 276(26):23954–23961
Verheij M et al (1996) Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380(6569):75–79
Zhang Y et al (1997) Kinase suppressor of Ras is ceramide-activated protein kinase. Cell 89(1):63–72
Heinrich M et al (2000) Ceramide as an activator lipid of cathepsin D. Adv Exp Med Biol 477:305–315
Hernandez-Corbacho MJ et al (2015) Tumor necrosis factor-alpha (TNFalpha)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death. J Biol Chem 290(42):25356–25373
Kajimoto T et al (2004) Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem 279(13):12668–12676
Jain A et al (2017) Diverting CERT-mediated ceramide transport to mitochondria triggers Bax-dependent apoptosis. J Cell Sci 130(2):360–371
Chipuk JE et al (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148(5):988–1000
Kashkar H et al (2005) Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem 280(21):20804–20813
Lee H et al (2011) Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE 6(6):e19783
Schull S et al (2015) Cytochrome c oxidase deficiency accelerates mitochondrial apoptosis by activating ceramide synthase 6. Cell Death Dis 6:e1691
Dadsena S et al (2019) Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun 10(1):1832
Siskind LJ, Kolesnick RN, Colombini M (2002) Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem 277(30):26796–26803
Siskind LJ, Colombini M (2000) The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem 275(49):38640–38644
Stiban J, Perera M (2015) Very long chain ceramides interfere with C16-ceramide-induced channel formation: a plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta 1848(2):561–567
Sentelle RD et al (2012) Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol 8(10):831–838
Nganga R et al (2019) Receptor-interacting Ser/Thr kinase 1 (RIPK1) and myosin IIA-dependent ceramidosomes form membrane pores that mediate blebbing and necroptosis. J Biol Chem 294(2):502–519
Larsen PJ, Tennagels N (2014) On ceramides, other sphingolipids and impaired glucose homeostasis. Mol Metab 3(3):252–260
Norris GH, Blesso CN (2017) Dietary and endogenous sphingolipid metabolism in chronic inflammation. Nutrients 9(11):1180
Johnson EL et al (2020) Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat Commun 11(1):2471
Rosqvist F et al (2019) Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J Clin Endocrinol Metab 104(12):6207–6219
Luukkonen PK et al (2018) Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 41(8):1732–1739
Vijay A et al (2021) Dietary interventions reduce traditional and novel cardiovascular risk markers by altering the gut microbiome and their metabolites. Front Cardiovasc Med 8:691564
Grabner GF et al (2021) Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab 3(11):1445–1465
Maceyka M, Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510(7503):58–67
Lancaster GI et al (2018) Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab 27(5):1096-1110 e5
Holland WL et al (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121(5):1858–1870
Davis CN et al (2006) IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem 98(5):1379–1389
Brenner B et al (1997) Fas- or ceramide-induced apoptosis is mediated by a Rac1-regulated activation of Jun N-terminal kinase/p38 kinases and GADD153. J Biol Chem 272(35):22173–22181
Dumitru CA, Gulbins E (2006) TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene 25(41):5612–5625
Gulbins E et al (1995) FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity 2(4):341–351
Xu J et al (1998) Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem 273(26):16521–16526
Wiegmann K et al (1994) Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell 78(6):1005–1015
Straub LG, Scherer PE (2019) Metabolic messengers: adiponectin. Nat Metab 1(3):334–339
Yamauchi T et al (2003) Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278(4):2461–2468
Vasiliauskaite-Brooks I et al (2017) Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544(7648):120–123
Okada-Iwabu M et al (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503(7477):493–499
Holland WL et al (2017) Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab 6(3):267–275
Choi SR et al (2018) Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 85:348–360
Holland WL et al (2013) An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 17(5):790–797
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19(2):121–135
Ruderman NB et al (2013) AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123(7):2764–2772
Erickson KA et al (2012) AICAR inhibits ceramide biosynthesis in skeletal muscle. Diabetol Metab Syndr 4(1):45
Blazquez C et al (2001) The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett 489(2–3):149–153
Martinez-Sanchez N et al (2017) Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab 26(1):212-229 e12
Pertwee RG et al (2010) International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62(4):588–631
Silvestri C, Di Marzo V (2013) The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17(4):475–490
Ravinet Trillou C et al (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28(4):640–648
Scheen AJ et al (2006) Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368(9548):1660–1672
Despres JP et al (2005) Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353(20):2121–2134
Guzman M, Galve-Roperh I, Sanchez C (2001) Ceramide: a new second messenger of cannabinoid action. Trends Pharmacol Sci 22(1):19–22
Cinar R et al (2014) Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 59(1):143–153
Gonzalez FJ et al (2017) Intestinal farnesoid X receptor signaling modulates metabolic disease. Dig Dis 35(3):178–184
Jiang C et al (2015) Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 6:10166
Xie C et al (2017) An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66(3):613–626
Jiang C et al (2015) Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 125(1):386–402
Wu Q et al (2021) Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J Clin Invest. https://doi.org/10.1172/JCI142865
Parseus A et al (2017) Microbiota-induced obesity requires farnesoid X receptor. Gut 66(3):429–437
Xie C et al (2017) Activation of intestinal hypoxia-inducible factor 2alpha during obesity contributes to hepatic steatosis. Nat Med 23(11):1298–1308
Zhang X et al (2019) Adipocyte hypoxia-inducible factor 2alpha suppresses atherosclerosis by promoting adipose ceramide catabolism. Cell Metab 30(5):937-951 e5
Luo Y et al (2021) Intestinal MYC modulates obesity-related metabolic dysfunction. Nat Metab 3(7):923–939
Aronova S et al (2008) Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab 7(2):148–158
Muir A et al (2014) TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. Elife. https://doi.org/10.7554/eLife.03779
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156(1–2):20–44
Blachnio-Zabielska AU et al (2012) Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obesity (Silver Spring) 20(12):2341–2347
Kolak M et al (2007) Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56(8):1960–1968
Blachnio-Zabielska AU et al (2012) Increased bioactive lipids content in human subcutaneous and epicardial fat tissue correlates with insulin resistance. Lipids 47(12):1131–1141
Choromanska B et al (2019) Metabolic syndrome is associated with ceramide accumulation in visceral adipose tissue of women with morbid obesity. Obesity (Silver Spring) 27(3):444–453
Lange M et al (2021) AdipoAtlas: a reference lipidome for human white adipose tissue. Cell Rep Med 2(10):100407
Song Y et al (2021) Ablation of ORMDL3 impairs adipose tissue thermogenesis and insulin sensitivity by increasing ceramide generation. Mol Metab 56:101423
Pan DZ et al (2018) Integration of human adipocyte chromosomal interactions with adipose gene expression prioritizes obesity-related genes from GWAS. Nat Commun 9(1):1512
Alexaki A et al (2017) De novo sphingolipid biosynthesis is required for adipocyte survival and metabolic homeostasis. J Biol Chem 292(9):3929-3939
Lee SY et al (2017) Adipocyte-specific deficiency of de novo sphingolipid biosynthesis leads to lipodystrophy and insulin resistance. Diabetes 66(10):2596–2609
Gohlke S et al (2019) Identification of functional lipid metabolism biomarkers of brown adipose tissue aging. Mol Metab 24:1–17
Byrne CD, Targher G (2015) NAFLD: a multisystem disease. J Hepatol 62(1 Suppl):S47-64
Korenblat KM et al (2008) Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134(5):1369–1375
Yetukuri L et al (2007) Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol 1:12
Raichur S et al (2019) The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab. https://doi.org/10.1016/j.molmet.2018.12.008
Kasumov T et al (2015) Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS ONE 10(5):e0126910
Zabielski P et al (2018) The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci Rep 8(1):7249
Zabielski P et al (2019) The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J Cell Physiol 234(2):1851–1861
Chocian G et al (2010) High fat diet induces ceramide and sphingomyelin formation in rat’s liver nuclei. Mol Cell Biochem 340(1–2):125–131
Montgomery MK et al (2016) Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: a beneficial role for very long-chain sphingolipid species. Biochim Biophys Acta 1861(11):1828–1839
Montgomery MK et al (2013) Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56(5):1129–1139
Luukkonen PK et al (2016) Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 64(5):1167–1175
Apostolopoulou M et al (2018) Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 41(6):1235–1243
Kurek K et al (2014) Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int 34(7):1074–1083
Fucho R et al (2014) ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J Hepatol 61(5):1126–1134
Somm E et al (2021) The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res 227:75–88
Sanyal AJ, Pacana T (2015) A lipidomic readout of disease progression in a diet-induced mouse model of nonalcoholic fatty liver disease. Trans Am Clin Climatol Assoc 126:271–288
Jiang M et al (2019) Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 10:665
Yang RX et al (2019) Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids Health Dis 18(1):179
Fox TE et al (2011) Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res 52(3):509–517
Keppley LJW et al (2020) Nervonic acid limits weight gain in a mouse model of diet-induced obesity. FASEB J 34(11):15314–15326
Wang K et al (2020) Targeting alkaline ceramidase 3 alleviates the severity of nonalcoholic steatohepatitis by reducing oxidative stress. Cell Death Dis 11(1):28
Goodpaster BH et al (2001) Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86(12):5755–5761
Bandet CL et al (2019) Sphingolipid metabolism: new insight into ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci 20(3):479
Frangioudakis G et al (2010) Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology 151(9):4187–4196
Turner N et al (2013) Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56(7):1638–1648
Boon J et al (2013) Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62(2):401–410
Blachnio-Zabielska AU et al (2016) The crucial role of C18-Cer in fat-induced skeletal muscle insulin resistance. Cell Physiol Biochem 40(5):1207–1220
Amati F et al (2011) Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60(10):2588–2597
Coen PM et al (2013) Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 21(11):2362–2371
de la Maza MP et al (2015) Skeletal muscle ceramide species in men with abdominal obesity. J Nutr Health Aging 19(4):389–396
Straczkowski M et al (2007) Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50(11):2366–2373
Tonks KT et al (2016) Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity (Silver Spring) 24(4):908–916
Bergman BC et al (2016) Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia 59(4):785–798
Schenk S, Horowitz JF (2007) Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117(6):1690–1698
Dube JJ et al (2008) Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 294(5):E882–E888
Dube JJ et al (2011) Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54(5):1147–1156
Bruce CR et al (2006) Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291(1):E99–E107
Coen PM et al (2015) Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64(11):3737–3750
Petersen MC, Jurczak MJ (2016) CrossTalk opposing view: Intramyocellular ceramide accumulation does not modulate insulin resistance. J Physiol 594(12):3171–3174
Summers SA, Goodpaster BH (2016) CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol 594(12):3167–3170
Forsstrom S et al (2019) Fibroblast growth factor 21 drives dynamics of local and systemic stress responses in mitochondrial myopathy with mtDNA deletions. Cell Metab 30(6):1040-1054 e7
Talchai C et al (2012) Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150(6):1223–1234
Butler AE et al (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52(1):102–110
Unger RH (1995) Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic Clin Implic Diabetes 44(8):863–870
Gaborit B et al (2015) Ectopic fat storage in the pancreas using 1H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes (Lond) 39(3):480–487
Listenberger LL et al (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100(6):3077–3082
Pearson GL et al (2016) A comprehensive lipidomic screen of pancreatic beta-cells using mass spectroscopy defines novel features of glucose-stimulated turnover of neutral lipids, sphingolipids and plasmalogens. Mol Metab 5(6):404–414
Unger RH, Orci L (2002) Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta 1585(2–3):202–212
Sjoholm A (1995) Ceramide inhibits pancreatic beta-cell insulin production and mitogenesis and mimics the actions of interleukin-1 beta. FEBS Lett 367(3):283–286
Kelpe CL et al (2003) Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 278(32):30015–30021
Veret J et al (2011) Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 beta-cells. Biochem J 438(1):177–189
Manukyan L et al (2015) Palmitate-induced impairments of beta-cell function are linked with generation of specific ceramide species via acylation of sphingosine. Endocrinology 156(3):802–812
Bastien M et al (2014) Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56(4):369–381
Summers SA (2018) Could ceramides become the new cholesterol? Cell Metab 27(2):276–280
Wigger L et al (2017) Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep 18(9):2269–2279
Vasile VC et al (2021) Ceramide scores predict cardiovascular risk in the community. Arterioscler Thromb Vasc Biol 41(4):1558–1569
Wittenbecher C et al (2021) Lipid profiles and heart failure risk: results from two prospective studies. Circ Res 128(3):309–320
Hilvo M et al (2020) Ceramides and ceramide scores: clinical applications for cardiometabolic risk stratification. Front Endocrinol (Lausanne) 11:570628
Schmidt S et al (2021) Silencing of ceramide synthase 2 in hepatocytes modulates plasma ceramide biomarkers predictive of cardiovascular death. Mol Ther. https://doi.org/10.1016/j.ymthe.2021.08.021
Akawi N et al (2021) Fat-secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. J Am Coll Cardiol 77(20):2494–2513
Chiu HC et al (2001) A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107(7):813–822
Zhou YT et al (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97(4):1784–1789
Ussher JR et al (2012) Inhibition of serine palmitoyl transferase I reduces cardiac ceramide levels and increases glycolysis rates following diet-induced insulin resistance. PLoS ONE 7(5):e37703
Zhang DX, Zou AP, Li PL (2001) Ceramide reduces endothelium-dependent vasodilation by increasing superoxide production in small bovine coronary arteries. Circ Res 88(8):824–831
Zhang DX, Zou AP, Li PL (2003) Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol 284(2):H605–H612
Zheng T et al (2000) Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca(2+)](i) in canine cerebral vascular muscle. Am J Physiol Heart Circ Physiol 278(5):H1421–H1428
Moreno L et al (2014) Ceramide mediates acute oxygen sensing in vascular tissues. Antioxid Redox Signal 20(1):1–14
Li H et al (2002) Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 106(17):2250–2256
Bharath LP et al (2015) Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes 64(11):3914–3926
Chun L et al (2011) Inhibition of ceramide synthesis reverses endothelial dysfunction and atherosclerosis in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract 93(1):77–85
Zietzer A et al (2021) Activation of neutral sphingomyelinase 2 through hyperglycemia contributes to endothelial apoptosis via vesicle-bound intercellular transfer of ceramides. Cell Mol Life Sci 79(1):48
Heitzer T et al (2000) Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43(11):1435–1438
Knapp M et al (2020) The gene and protein expression of the main components of the lipolytic system in human myocardium and heart perivascular adipose tissue. Effect of coronary atherosclerosis. Int J Mol Sci 21(3):737
Schissel SL et al (1996) Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest 98(6):1455–1464
Edsfeldt A et al (2016) Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol 36(6):1132–1140
Hojjati MR et al (2005) Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem 280(11):10284–10289
Lee SY et al (2012) Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem 287(22):18429–18439
Abel ED, Litwin SE, Sweeney G (2008) Cardiac remodeling in obesity. Physiol Rev 88(2):389–419
Ji R et al (2017) Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight. https://doi.org/10.1172/jci.insight.82922
Russo SB et al (2012) Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 122(11):3919–3930
Law BA et al (2018) Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J 32(3):1403–1416
Drosatos K, Schulze PC (2013) Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 10(2):109–121
Battiprolu PK et al (2013) Diabetic cardiomyopathy and metabolic remodeling of the heart. Life Sci 92(11):609–615
Ussher JR (2014) The role of cardiac lipotoxicity in the pathogenesis of diabetic cardiomyopathy. Expert Rev Cardiovasc Ther 12(3):345–358
Riehle C, Abel ED (2016) Insulin signaling and heart failure. Circ Res 118(7):1151–1169
Timper K, Bruning JC (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10(6):679–689
Ruud J, Steculorum SM, Bruning JC (2017) Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun 8:15259
Cai D (2013) Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 24(1):40–47
Kleinridders A et al (2009) MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10(4):249–259
Gao S et al (2011) Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc Natl Acad Sci USA 108(23):9691–9696
Ramirez S et al (2013) Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 62(7):2329–2337
Cruciani-Guglielmacci C et al (2017) Brain ceramide metabolism in the control of energy balance. Front Physiol 8:787
Borg ML et al (2012) Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol 590(17):4377–4389
Car H et al (2012) Ceramide profiles in the brain of rats with diabetes induced by streptozotocin. FEBS J 279(11):1943–1952
Morselli E et al (2014) Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep 9(2):633–645
Gonzalez-Garcia I et al (2018) Estradiol regulates energy balance by ameliorating hypothalamic ceramide-induced ER stress. Cell Rep 25(2):413-423 e5
Xu Y et al (2011) Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14(4):453–465
Gao Y et al (2017) Disruption of lipid uptake in astroglia exacerbates diet-induced obesity. Diabetes 66(10):2555–2563
Lyu P et al (2020) Unveiling the transcriptome alteration of POMC neuron in diet-induced obesity. Exp Cell Res 389(1):111848
Campana M et al (2018) Inhibition of central de novo ceramide synthesis restores insulin signaling in hypothalamus and enhances beta-cell function of obese Zucker rats. Mol Metab 8:23–36
Shi H et al (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116(11):3015–3025
Sergi D et al (2020) Palmitic acid triggers inflammatory responses in N42 cultured hypothalamic cells partially via ceramide synthesis but not via TLR4. Nutr Neurosci 23(4):321–334
Heras V et al (2020) Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab 32(6):951-966 e8
Biro FM, Wien M (2010) Childhood obesity and adult morbidities. Am J Clin Nutr 91(5):1499S-1505S
Gerendai I et al (1998) Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracing technique. Neuroendocrinology 68(4):244–256
Ben-David O, Futerman AH (2010) The role of the ceramide acyl chain length in neurodegeneration: involvement of ceramide synthases. Neuromolecular Med 12(4):341–350
Bergman BC et al (2015) Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 309(4):E398-408
Warshauer JT et al (2015) Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab Res Rev 31(7):734–744
Lankinen M et al (2015) A healthy nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J Nutr 146(4):662–672
Wang DD et al (2017) Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevencion con Dieta Mediterranea). Circulation 135(21):2028–2040
Ng TW et al (2014) Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J Clin Endocrinol Metab 99(11):E2335–E2340
Tarasov K et al (2014) Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 99(1):E45-52
Huang H et al (2011) Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity (Silver Spring) 19(11):2235–2240
Adams TD et al (2017) Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 377(12):1143–1155
Bikman BT, Summers SA (2011) Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121(11):4222–4230
Salaun E et al (2016) Myriocin prevents muscle ceramide accumulation but not muscle fiber atrophy during short-term mechanical unloading. J Appl Physiol (1985) 120(2):178–187
Hojjati MR, Li Z, Jiang XC (2005) Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta 1737(1):44–51
Li Z et al (2016) Liver serine palmitoyltransferase activity deficiency in early life impairs adherens junctions and promotes tumorigenesis. Hepatology 64(6):2089–2102
Li Z et al (2018) Sphingolipid de novo biosynthesis is essential for intestine cell survival and barrier function. Cell Death Dis 9(2):173
Dolgin V et al (2019) DEGS1 variant causes neurological disorder. Eur J Hum Genet 27(11):1668–1676
Karsai G et al (2019) DEGS1-associated aberrant sphingolipid metabolism impairs nervous system function in humans. J Clin Invest 129(3):1229–1239
Pant DC et al (2019) Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J Clin Invest 129(3):1240–1256
Summers SA (2020) Ceramides: Nutrient signals that drive hepatosteatosis. J Lipid Atheroscler 9(1):50–65
Raichur S (2020) Ceramide synthases are attractive drug targets for treating metabolic diseases. Front Endocrinol (Lausanne) 11:483
Lyu K et al (2020) A membrane-bound diacylglycerol species induces PKCϵ-mediated hepatic insulin resistance. Cell Metab 32(4):654–664.e655
D’Mello NP et al (1994) Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J Biol Chem 269(22):15451–15459
Guillas I et al (2001) C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J 20(11):2655–2665
Bauer R et al (2009) Schlank, a member of the ceramide synthase family controls growth and body fat in Drosophila. EMBO J 28(23):3706–3716
Menuz V et al (2009) Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 324(5925):381–384
Pewzner-Jung Y, Ben-Dor S, Futerman AH (2006) When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem 281(35):25001–25005
Tidhar R et al (2018) Eleven residues determine the acyl chain specificity of ceramide synthases. J Biol Chem 293(25):9912–9921
Laviad EL et al (2008) Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 283(9):5677–5684
Mizutani Y et al (2009) Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 91(6):784–790
Mizutani Y, Kihara A, Igarashi Y (2005) Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J 390(Pt 1):263–271
Gosejacob D et al (2016) Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J Biol Chem 291(13):6989–7003
Wegner MS et al (2016) The enigma of ceramide synthase regulation in mammalian cells. Prog Lipid Res 63:93–119
Kim DW et al (2019) Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179(3):713-728 e17
Sociale M et al (2018) Ceramide synthase schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Rep 22(4):967–978
Voelzmann A et al (2016) Nuclear Drosophila CerS Schlank regulates lipid homeostasis via the homeodomain, independent of the lag1p motif. FEBS Lett 590(7):971–981
Spassieva SD et al (2016) Ectopic expression of ceramide synthase 2 in neurons suppresses neurodegeneration induced by ceramide synthase 1 deficiency. Proc Natl Acad Sci USA 113(21):5928–5933
Acknowledgements
The selection of literature reviewed here does not claim completeness. We apologize to the colleagues in the field whose important contributions we could not include to stay within the scope of this article. We would like to thank Dr. Nasim Biglari for critically reading the manuscript. All authors read and approved the final version of the manuscript. Figures 1, 2, 3, 4 were created with BioRender.com.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD; funded by the DFG within the Excellence Initiative by German Federal and State Governments) and the Federal Ministry of Education and Science (BMBF) through collaboration in Deutsche Zentrum für Diabetesforschung e.V. (DZD, FKZ 82DZD00502 and 82DZD05D03). J.C.B.’s research on the topic discussed here was supported in part by Sanofi-Aventis, Deutschland GmbH, through a cooperation agreement.
Author information
Authors and Affiliations
Contributions
PH and JCB contributed to conception of this work. PH drafted the manuscript and prepared figures using BioRender.com. JCB edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J.C.B. has received research funding through collaborations with Sanofi Aventis and Novo Nordisk Inc., which did not affect the content of this article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hammerschmidt, P., Brüning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci. 79, 395 (2022). https://doi.org/10.1007/s00018-022-04401-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04401-3