Abstract
The placenta sustains embryonic development and is critical for a successful pregnancy outcome. It provides the site of exchange between the mother and the embryo, has immunological functions and is a vital endocrine organ. To perform these diverse roles, the placenta comprises highly specialized trophoblast cell types, including syncytiotrophoblast and extravillous trophoblast. The coordinated actions of transcription factors (TFs) regulate their emergence during development, subsequent specialization, and identity. These TFs integrate diverse signaling cues, form TF networks, associate with chromatin remodeling and modifying factors, and collectively determine the cell type-specific characteristics. Here, we summarize the general properties of TFs, provide an overview of TFs involved in the development and function of the human trophoblast, and address similarities and differences to their murine orthologs. In addition, we discuss how the recent establishment of human in vitro models combined with -omics approaches propel our knowledge and transform the human trophoblast field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The placenta is the most diverse organ among mammals, and it aids fetal development by facilitating nutrient, metabolite and gas exchange between the mother and the fetus. To fulfill these various functions, the progenitor cells of the placenta differentiate into multiple highly specialized trophoblast cell types. Proper trophoblast differentiation is essential for correct placental and fetal development as aberrant differentiation often results in pregnancy complications, hampering both maternal and fetal health. This review focuses on trophoblast cell identity determined by specific gene expression patterns driven by transcription factors (TFs). They form networks, and together with co-factors, chromatin-modifying and -remodeling complexes govern cell identity and differentiation. The review is divided into three main parts describing (i) the development of the human placenta, (ii) general properties of TFs and (iii) human trophoblast cell identity determined by TFs, where we discuss individual TFs and signaling pathways in trophectoderm (TE), cytotrophoblast (CTB), syncytiotrophoblast (STB) and extravillous trophoblast (EVT) in comparison to mouse placenta development.
Development of the human placenta
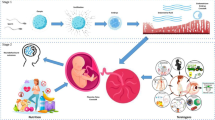
During human development, the first lineage decision segregates the TE and the inner cell mass (ICM), resulting in the blastocyst 4–5 days post fertilization (dpf). While all embryonic cells originate from the ICM, the TE generates the trophoblast compartment of the placenta. The blastocyst implants into the maternal endometrium 6–7 dpf; the TE gives rise to the mononuclear CTB that differentiates into the invasive multinuclear primitive syncytium (PS) (Fig. 1) [1]. Around day 9, vacuoles appear in the PS that fuse and form the lacunar spaces, and eventually breach the maternal capillaries giving rise to the maternal blood sinusoids [2]. Concomitantly, rows of proliferative CTB break through the expanding PS and form primary villi, subsequently invaded by the extraembryonic mesoderm (ExM), which is thought to originate from the ICM. Further proliferation and differentiation result in a villous structure consisting of an ExM-derived core containing fetal capillaries covered by two trophoblast layers: the proliferative villous CTB and its derivative, the multinuclear STB (Fig. 1) [1]. The STB forms a syncytium that provides the exchange site between the maternal and fetal bloodstreams and produces and secretes a plethora of pregnancy hormones. The developing placenta comprises two types of villi: the floating villi and the anchoring villi attached to the decidua through cell columns (Fig. 1). The proximal part of the column is a proliferative progenitor pool that gives rise to the highly invasive and migratory EVT lineage. Two distinct EVT populations exist by 15–16 dpf and safeguard immunological adaptation: the interstitial EVT (iEVT) that invade decidual stroma and the endovascular EVT (eEVT) that colonize and remodel maternal spiral arteries to a high conductance at low-pressure vessels, ensuring optimal blood flow [3]. In addition to the arterial transformation, the eEVT moves down the artery and forms plugs that prevent blood flow and safeguard a hypoxic environment. By the end of the first trimester, the plugs disintegrate, and the three main trophoblast lineages of the human placenta are established: the CTB, the STB and the EVT, providing an outline for further growth and physiological development (Figs. 1 and 2a).
The development of the human placenta. Initiated by fertilization, the zygote undergoes multiple divisions and gives rise to a blastocyst 4–5 days post-fertilization (dpf). The blastocyst consists of the inner cell mass, which gives rise to the embryo proper and the trophectoderm, which gives rise to the trophoblast of the placenta. Upon implantation of the blastocyst into the uterine endometrium, the establishment of the multi-nucleated primitive syncytium (PS) and the cytotrophoblast (CTB) monolayer begins. Around day 9 dpf, lacunae form within the PS, fuse subsequently with uterine capillaries, and establish maternal sinusoids filled with maternal blood by day 13 dpf. Simultaneously, the CTB expands through the PS, extending towards the maternal decidua and forming primary villi. By the end of the third trimester, the main placental structure, the villous tree, is fully established. The villous tree consists of the extraembryonic mesoderm-derived core, fetal capillaries, the CTB monolayer and the multinucleated syncytiotrophoblast (STB) layer, arising from CTB fusion. The floating villi of the villous tree are located in the intervillous space filled with maternal blood, while the anchoring villi extend towards the decidua. At the tip of the anchoring villi, CTB forms a cytotrophoblast cell column, comprising the proliferative progenitor population of invasive extravillous trophoblast (EVT). The EVT can be divided into two subtypes; the endovascular EVT (eEVT), which remodels the maternal spiral arteries and the interstitial EVT (iEVT), which invades the decidua
Comparison of the human and murine placenta. a The main unit of the human placenta is a villous tree, comprising the extraembryonic mesoderm-derived core (ExM-dC), containing fetal capillaries. The ExM-dC is covered by the cytotrophoblast (CTB) monolayer and the multinucleated syncytiotrophoblast (STB) layer that is in contact with maternal blood. The inset shows a cross-section through the villi. At the tip of the villi, the cytotrophoblast cell column forms. It gives rise to the interstitial extravillous trophoblast (iEVT), invading the decidua and the endovascular extravillous trophoblast (eEVT), invading the maternal spiral arteries. b The murine placenta can be divided into the junctional zone and the labyrinth zone. The junctional zone consists of the spongiotrophoblasts and the glycogen cells. The trophoblast giant cells invade the maternal decidua and remodel maternal arteries and thus are considered to correspond to human EVT. In the labyrinth zone, the fetal blood is separated from the maternal blood sinusoids by fetal endothelial cells, two layers of STB (STB-I and STB-II) and sinusoidal giant cells. The labyrinth functionally relates to human villi
While human and murine placentas fulfill analogous functions and both show a hemochorial (maternal blood directly contacts trophoblast cells) type of placentation, they exhibit several morphological and functional differences. The murine placenta comprises the junctional and the labyrinth zones (Fig. 2b). The junctional zone consists of cells derived from the ectoplacental cone (EPC), encompassing the secondary trophoblast giant cells (TGCs), the spongiotrophoblast cells, and the glycogen cells (GlycCs) [4]. The secondary TGCs are thought to correspond to EVTs, as they invade (around E7.5-E9.5) deeply into the decidua and remodel the maternal spiral arteries. The GlycCs are loaded with glycogen and serve as an energy store. The labyrinth zone provides the site of the maternal–fetal exchange and functionally relates to human chorionic villi. During murine development, the TE gives rise to the extraembryonic ectoderm (ExE) and the EPC. The allantois arises from the ExM, and around embryonic day E8.5 fuses with the chorion, a structure of ExE origin, in a process known as the chorioallantoic fusion [5]. This fusion enables the invasion of the ExM-derived blood vessels into the chorionic layer, eventually leading to the establishment of the labyrinth zone. Here, the fetal and the maternal bloodstreams are separated by a trilaminar barrier consisting of the two layers of syncytiotrophoblast and a layer of sinusoidal giant cells lining the maternal blood sinuses [6] (Fig. 2b). Thus, the general functional layout of the murine and human placenta is similar, as in both cases, the fetal vasculature is of ExM origin, and the maternal blood flows in trophoblast-lined sinuses and remains in direct contact with STB; however, species-specific differences do exist. Despite these disparities, the murine placenta has proven to be a very informative animal model, not least due to its amenability to genetic engineering, particularly gene knockout (KO) experiments [7, 8]. In addition, derivation of self-renewing and multipotent mouse trophoblast stem cells (mTSCs), representing the ExE compartment of the mouse embryo [9], contributed enormously to our understanding of the TF networks operating in trophoblast.
Numerous human placental disorders have their pathophysiological roots at the early developmental stages. However, molecular studies of the underlying causes were long hampered by the inaccessibility of placental tissue and the lack of suitable in vitro models. Researchers mainly relied on the scarcely available primary tissue and several transformed and cancer cell lines of restricted potential [5]. While animal models, in particular mice, are widely used to study the development and function of human organs, their ability to model the placenta is limited. As described above, the development of the specific trophoblast lineages and cell types differ between the two species. For instance, various types of the trophoblast giant cells feature prominently in the murine placenta while these are limited to EVT subtypes in humans [4, 10]. The recent establishment of the human trophoblast stem cells (hTSCs) and self-organizing trophoblast organoids (TOs) was a major breakthrough and provided novel, reliable tools to study molecular mechanisms driving trophoblast development and disease (Fig. 3) [11,12,13]. The hTSCs represent the CTB population of the first trimester placenta. In culture conditions activating EGF and WNT and inhibiting TGF-β and ROCK signaling, they self-renew and remain bipotent. Upon induction, hTSCs can differentiate into STB or EVT, providing a versatile in vitro system to follow trophoblast development [11]. TOs require similar signaling inputs to self-organize in 3D into the outer CTB-like layer and the inner STB-like compartment, representing the inside-out model of the human villi [12, 13]. While these models have already proven to be a powerful tool to interrogate TF function in CTB [14,15,16], naive human embryonic stem cells (hESCs) conversion into hTSCs and recently-derived human blastoids can be used to dissect even earlier stages of human trophoblast development [17,18,19,20,21,22,23,24].
Human trophoblast stem cells and trophoblast organoids. The human trophoblast stem cells (hTSC) can be derived from both the blastocyst and the cytotrophoblast (CTB) of the first-trimester placenta. The hTSCs represent the proliferative CTB population and can be differentiated into extravillous trophoblast (EVT) and syncytiotrophoblast (STB) upon defined culture conditions. Moreover, trophoblast organoids (TOs) can be established from the first-trimester CTB. TOs have an STB-like core and CTB-like shell and thus provide an inside-out three-dimensional model. TOs can be further differentiated to form the cytotrophoblast cell column (CCC)-like and EVT-like populations
General properties of TFs controlling gene regulatory networks
Cell identity is determined by the specific TF-driven transcriptional outputs and is coordinated by the signaling inputs [25]. TFs bind DNA in a sequence-specific manner, cooperate with chromatin remodeling and modifying complexes, and other factors to recruit the transcriptional machinery and drive transcription. Specific cell types are usually defined by a particular combination of TFs, as evidenced by cell (re)programming studies. Classical reprogramming experiments demonstrated that transient ectopic expression of Oct4, Sox2, Klf4 and Myc (OSKM) in fibroblasts cultured in murine ESC media resulted in the establishment of self-renewing induced pluripotent stem cells (iPSCs), barely distinguishable from ESCs [26]. Similarly, transient, ectopic expression of Eomes, Tfap2c, Gata3 and optionally Ets2 or Myc in mouse fibroblast coupled to mouse TSC culture conditions (FGF4 and TGF-β) led to the establishment of self-renewing and multipotent induced TSCs (iTSCs) that contributed to the placenta in chimeras [27, 28]. Moreover, a recent report showed reprogramming of human term CTB to iTSCs upon expression of 5 TFs (TFAP2C, TEAD4, CDX2, ELF5 and ETS2) in hTSC culture conditions [29]. The role of TFs in translating the signaling inputs into the transcriptional outputs was demonstrated in the trophoblast context, as constitutive overexpression of Sox2 and Esrrb/Tfap2c was sufficient to confer FGF4-independent self-renewal and sustain multipotency in mTSCs [30].
Importantly, TFs usually do not function as a sum of single players but rather operate as networks at both the transcriptional and protein level [31,32,33,34,35]. Analysis of TFs in mTSCs (e.g., ESRRB, TFAP2C, TEAD4, GATA2/3, SOX2, ELF5, EOMES, CDX2, etc.) revealed that they directly bind their own and each other's genes and drive their expression resulting in the maintenance of the network [30, 36,37,38]. For instance, ESRRB binds itself as well as the Eomes and Elf5 genes and is essential for their expression [36]. Furthermore, in addition to sustaining each other, trophoblast TFs co-bind and co-regulate the expression of downstream gene modules determining the mTSC identity [38]. The co-binding preferentially occurs at the gene regulatory regions referred to as enhancers. Enhancers are densely packed with DNA motifs bound by TFs, are highly enriched for transcriptional cofactors (e.g., p300), exhibit accessible chromatin structure and may cooperate and form regulatory hubs referred to as super-enhancers [39]. A recent study in differentiating mTSCs systematically mapped trophoblast-specific super-enhancers and the associated TFs, of which the vast majority has not been characterized yet in the placental context [38]. Interestingly, no bonafide trophoblast-specific TFs have been identified, as all of the known essential trophoblast TFs are also expressed in a range of embryonic and adult cell types, indicating that cell-type specificity is acquired by their unique combinations. This notion is exemplified by EOMES, CDX2 and SOX2 that function largely separately in the early mesendoderm, late mesoderm, and neuroectoderm lineage derivatives, respectively, but uniquely cooperate in the trophoblast lineage [40,41,42,43]. Another example is provided by the key stem cell/progenitor TF SOX2 that has context-dependent protein interactors and therefore different target gene networks (e.g., OCT4 in mESC, TFAP2C in trophoblast, and OTX2 in neuroectoderm) [30, 34, 44,45,46]. The acquisition of new TF combinations in a network could be driven by the evolution of novel cis-regulatory elements, in agreement with the genetic theory of morphological evolution [47]. It has been reported that endogenous retroviruses function as species-specific placental enhancers bound by CDX2, EOMES and ELF5 TFs [48]. The tissue-specificity of these enhancers relies on the low methylation levels in trophoblast, providing access to otherwise silenced regions (by DNA methylation), which are the source of regulatory variation [48]. Another example is a human long terminal repeat (LTR) element driving placental expression of the corticotropin-releasing hormone (CRH) via the TF DLX3 and thereby regulating gestational length. This LTR element was sufficient to reignite CRH expression in transgenic mice, resulting in prolonged gestational length [49]. In sum, TFs operate as a highly intertwined transcriptional regulatory circuitry, determining trophoblast cell identity.
TF protein networks in trophoblast
TFs also form networks at the protein level. For instance, interactions of MSX2 with both GATA3 and TFAP2C [15] were identified in hTSCs, and mutual interactions for ELF5, TFAP2C and EOMES [37] as well as TFAP2C and SOX2 [30] were reported in mTSCs. Interestingly, the relative abundance of EOMES, ELF5 and TFAP2C determines the outcomes of their interactions. In mTSCs, ELF5 interacts with EOMES and recruits TFAP2C to triply occupied sites at trophoblast-specific genes, driving their expression. In contrast, the interaction of ELF5 and TFAP2C becomes predominant as their protein levels increase. This triggers binding to double- and single-occupancy sites that harbor the cognate Tfap2c motif, causing activation of the associated differentiation-promoting genes [37]. Thus, mouse trophoblast cell identity shifts in line with the stoichiometry of these associations, indicating that specificity is not determined by a single TF but by its interactions. This is further illustrated by the largely cell-type-specific protein interactomes and gene binding patterns of SOX2 and ESRRB in mTSCs vs. mESCs [30].
TFs exert their function in cooperation with transcriptional cofactors, chromatin remodeling complexes and chromatin-modifying enzymes. Transcriptional cofactors provide the link to the general RNA Polymerase II (PolII) machinery and comprise a diverse array of proteins and complexes. For instance, YAP is a TEAD4 cofactor in mTSCs and hTSCs [50,51,52], while the Integrator complex was reported to interact with ESRRB in mTSCs. ESRRB also interacted with components of the Lysine-specific histone demethylase 1 (LSD1), and Nucleosome Remodeling and Deacetylase (NuRD) complexes [36]. We have also recently reported a strong interaction of the TF MSX2 with the canonical BRG1/BRM associated factors (cBAF) complex, a subtype of the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex, in hTSCs. Chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) analyses have demonstrated a substantial binding overlap between MSX2, cBAF components ARID1A and BRG1, and H3K27ac in hTSCs. Intriguingly, depletion of MSX2 resulted in spontaneous STB differentiation, increased cBAF occupancy and elevated levels of H3K27ac. Thus, in hTSCs, MSX2 keeps cBAF “in check”, prevents premature activation of STB genes and reinforces the hTSC identity [15]. These findings provide a paradigm of how the intrinsic connections between TFs, chromatin remodelers and modifiers coordinately determine transcriptional cell fate. In addition to cBAF, also non-canonical (nc)BAF and Polybromo-associated (P)BAF complexes have been shown to operate in hTSCs [15]; however, their precise functions await elucidation. The SWI/SNF complex has also been implicated in the regulation of self-renewal in mTSCs. Its core subunit BRG1 has been shown to cooperate with EOMES and TFAP2C TFs and positively regulate the expression of crucial stemness markers, including Elf5, Eomes and Cdx2 [53]. The SWI/SNF complex is also essential for early trophoblast development as genetic ablation of Brg1, Baf47, and Baf155 subunits resulted in developmental arrest around the blastocyst stage and implantation failure [54,55,56]. Other chromatin regulators involved in trophoblast self-renewal are the chromatin organizers SATB homeobox 1 (SATB1) and SATB2. Their depletion led to a loss of self-renewal and differentiation of rat TSCs [57]. In contrast, the bromodomain BPTF nucleosome remodeling factor NURF301 is necessary for trophoblast differentiation. Its gene KO resulted in diminished expression of Ascl2 and Hand1, reduced/absent ectoplacental cone, and embryonic lethality [58].
Besides the chromatin remodeling status, active (e.g., H3K4me4, H3K27ac, H3K9ac) and repressive (e.g., H3K27me3, H3K9me2/3) histone modifications have been implicated in transcriptional regulation of trophoblast cell states. Genetic ablation of histone-modifying enzymes, including EZH2 (H3K27 methyltransferase), G9A (H3K9 methyltransferase), KDM6 (H3K27 demethylase), LSD1 (H3K4 demethylase), MYST1/MYST2 (H3K14 acetyltransferase) and SUV39H1 (H3K9 methyltransferase) led to severely impaired trophoblast development and embryonic lethality (reviewed in [59]). In contrast to murine, the epigenome of the human trophoblast remains relatively poorly characterized. Recently, Kwak et al. provided some new insights through genome-wide characterization of histone modifications (H3K4me3, H3K9Ac, H3K27Ac and H3K27me3) and RNA Pol II occupancy in CTB and in vitro differentiated STB. These analyses revealed that the transition from CTB to STB is associated with profound gene regulatory and epigenetic changes [60]. Accordingly, pharmacological inhibition and genetic depletion of histone deacetylase 1 and 2 impaired STB differentiation [61].
Together, these examples illustrate how TFs, chromatin remodelers, and modifiers cooperate and coordinately regulate specific transcriptional outputs that determine cell identity. They also highlight the importance of the holistic approach when studying transcriptional regulation of cell fate decisions.
TFs determining trophoblast identity
TFs operating in TE
Human development commences with the fertilization and formation of a zygote. Following several cell divisions, the first lineage specification takes place at the morula stage, with the outer, polar cells giving rise to TE and the inner, apolar cells becoming the ICM. The outer, polar cells, due to the presence of a “free” apical surface, show different localization of aPKC, AMOT, and PARD6B and divergent distribution of the adherens junctions in comparison to the inner cells [52]. As a consequence of this polarization and localized mechanical strain, the HIPPO signaling pathway is differentially activated. In the inner cells, the HIPPO signaling effector YAP1 is phosphorylated and retained in the cytoplasm. In the outer cells, the unphosphorylated YAP1 translocates to the nucleus where, as a cofactor, together with TEAD4 and GATA3 activates the expression of key downstream TFs. As the HIPPO/TEAD4 pathway is required for the expression of Gata3 in the early mouse embryo, it is likely that a similar mechanism operates in the human context [52]. GATA3 and its paralog GATA2 have a redundant function in establishing the TE lineage in the mouse, as only the double KO and not the single KO mice fail at the preimplantation stage [62]. Interestingly, GATA2 is not expressed in the human outer morula cells (as in the mouse) but only later in the TE of both species [52].
In contrast to the mouse morula, where YAP1 together with TEAD4 directly drive expression of Cdx2 in the outer cells, concomitantly with the establishment of TE, [50], human embryos show expression of CDX2 only at the blastocyst stage. While in the mouse, the expression of CDX2 and OCT4 become mutually exclusive before the early blastocyst stage [50, 52], in the human, CDX2 is co-expressed with OCT4 in the TE of the early blastocyst and becomes exclusively expressed only in the TE of the late blastocyst [63, 64]. Similar to OCT4, SOX2 is vital for mouse preimplantation development and becomes restricted in expression to the inner cells of the morula. In humans, SOX2 is initially expressed in all nuclei up to the formation of the early blastocyst and becomes confined to the ICM in the expanding blastocyst [52]. There are other prominent differences in TF expression patterns between the mouse and human TE: EOMES, TFAP2C and ID2 are restricted to the mouse TE. However, they are either absent (EOMES) or unrestricted (TFAP2C, ID2) in the human blastocyst [64].
Collectively, despite the evolutionary conservation of blastocyst formation and the first lineage specifications, important molecular differences in timing and expression and thus regulation of the process exist in mouse and human embryos.
TFs operating in CTB
The molecular mechanisms, particularly the TFs, driving placental development between the blastocyst implantation and the establishment of the villi are poorly understood. Because researchers have restricted access to human blastocysts and first trimester placental tissue and their potential for genetic studies is very limited, the developmental period of TE-CTB transition remains largely unexplored. However, there is a considerable amount of data on the TFs operating in the first-trimester trophoblast (Fig. 4). Depending on the location, the CTB is the progenitor population for STB and EVT lineages and the TFs controlling its identity are intensely studied. Notably, the recent establishment of hTSCs representing CTB provided an excellent, genetically amenable model that will further propel our understanding of these TF networks [11].
Many TFs present in TE continue to be expressed in CTB, including GATA2/3, TEAD4, and TFAP2C. Recent studies have shown that both in human and mouse, TEAD4 promotes TSC self-renewal and stemness by driving the expression of cell cycle genes and prevents STB and invasive EVT differentiation by silencing differentiation markers [14]. The importance of TEAD4 for multipotency was further corroborated by the finding that idiopathic recurrent pregnancy failures were associated with loss of TEAD4 expression in trophoblast progenitors [14]. Similarly, depletion of YAP1, a cofactor of TEAD4, revealed its involvement in driving self-renewal, proliferation and stemness by transcriptional regulation of cell cycle- and STB-related genes [51]. In summary, the HIPPO pathway controls the trophoblast progenitors in vivo and stem cells in vitro at early (TE) and later (CTB) stages of human placental development, highlighting its universal role in maintaining trophoblast multipotency.
As already mentioned, GATA3, GATA2 and TFAP2C are other conserved TFs, readily expressed in TE, CTB and hTSCs. While the molecular mechanisms underlying the GATA2/3 and TFAP2C action in CTB and hTSCs await elucidation, the hESC-based trophoblast differentiation model has already provided some insights. Even though disputed by some [65], this in vitro system was used to demonstrate that the network of TFs GATA2, GATA3, TFAP2A and TFAP2C regulates trophoblast identity and facilitates the exit from pluripotency, and depletion of GATA3 prevents specification of trophoblast identity [66]. The critical roles of GATA2, GATA3 and TFAP2C in the human CTB and hTSCs are expected, as their depletion in the mouse system resulted in impaired trophoblast development resulting in embryonic lethality [62, 67]. Moreover, GATA2 and TFAP2C gained further prominence, as depletion of either impaired reprogramming of fibroblasts to naïve hESCs, which have the potential to adopt the hTSC state in suitable media conditions [68]. In addition, GATA2, GATA3 and TFAP2C act as powerful, pioneering TFs, i.e., they can bind nucleosomal DNA and nucleate the formation of a new enhancer and hence drive cell fate decisions in diverse developmental contexts [69, 70]. CDX2 is another TF present in both human and murine TE. However, in contrast to the mouse, CDX2 expression is gradually lost during the first trimester of human placental development and is absent in hTSCs [11, 71]. These observations raise questions to what extent CDX2 is an actual human trophoblast marker, whether several progenitor trophoblast subpopulations exist during the first trimester, and whether the hTSCs media lacks the necessary signaling inputs to maintain CDX2 expression.
As previously discussed, the TE in humans gives rise to the proliferative progenitor population of CTB. Interestingly, a number of TFs vital for sustaining the progenitor identity are continuously expressed starting in the TE (e.g., GATA2/3, TEAD4, TFAP2C, etc.). In contrast, others commence their expression only in the CTB and ExE. For instance, expression of ELF5 is conserved in human and mouse trophoblast progenitor populations (CTB and ExE, respectively) as well as in mTSCs and hTSCs, and downregulated upon differentiation. Similarly, epigenetic regulation of the Elf5/ELF5 promoter by DNA methylation is preserved between these two species [72, 73]. In mice, ELF5 cooperates with EOMES and TFAP2C and acts as a molecular switch governing the balance between mTSCs proliferation and differentiation [37]. ELF5 is essential for mouse placental development and the maintenance of mTSCs; however, its function in the human trophoblast remains to be elucidated [37, 74].
ESRRB, EOMES and SOX2 are indispensable for mouse placental development and maintenance of mTSCs [75,76,77,78]. They are lowly expressed (ESRRB and SOX2) or absent (EOMES) from both the CTB and hTSCs [11, 71], further highlighting the species-specific differences during post-implantation trophoblast development. Interestingly, we have recently identified MSX2 as a novel and human-specific regulator of trophoblast identity [15]. While MSX2 depletion results in loss of hTSC self-renewal and spontaneous STB differentiation, MSX2 forced expression blocks it. Mechanistically, MSX2 cooperates with the chromatin-remodeling cBAF complex to prevent premature syncytiotrophoblast differentiation and reinforce the stemness of hTSCs. Hence, MSX2 was established as a repressor of the STB lineage, playing a pivotal role in cell fate decisions governing human placental development and disease. Similar to MSX2, TP63 seems to be specific to the human trophoblast. TP63 is commonly expressed in the basal layer of stratified epithelia, where it reinforces self-renewal and restricts premature differentiation [79]. In the trophoblast context, it is highly expressed in CTB and hTSCs, where it drives proliferation and prevents epithelial-to-mesenchymal transition and differentiation [80].
Taken together, several TFs have been identified in human CTB that maintain the progenitor state and prevent premature differentiation; however, the relationships between these factors in a regulatory network are unclear; some are yet likely to be discovered, and their function may differ from that in mice.
TFs operating in STB
As in other cell types, the unique combination of TFs determines the identity of STB Fig. 4). There are two types of human STB: the primitive STB mediates embryonic implantation, and the definitive STB lines chorionic villi from the third week onwards. The exact relationship between these two subtypes is unclear. The villous STB remains in direct contact with maternal blood and ensures optimal pregnancy adaptation. It enables efficient transport of nutrients, gases and metabolites via an enlarged cell surface by microvilli, numerous channels and transporters. It synthesizes and secretes a variety of pregnancy hormones, including human chorionic gonadotropin (hCGA and hCGB), placental lactogen (CSH1), pregnancy-specific glycoproteins (PSGs), placental growth factor (PGF), corticotropin-releasing hormone (CRH) and others [81, 82]. Importantly, STB is devoid of human leukocyte antigen class I (HLA-I) molecules, making it invisible to the potentially reactive T cells [83]. Thus, STB is vital for a functional placenta and successful pregnancy. STB undergoes a tightly regulated turnover and is replenished by the coordinated differentiation and fusion of the underlying CTB. This process is tightly controlled and involves biochemical as well as morphological changes. While the direct signal remains elusive, a prerequisite for differentiation is an exit from the cell cycle [84], followed by repression of genes associated with the progenitor state and activation of genes related to STB function, i.e., involved in nutrient transport, hormone synthesis, and immunomodulation. This differentiation process is coordinated by a subset of TFs and signaling inputs. For instance, hCG promotes syncytialization [85]. Initially, it is secreted by CTB and is thought to induce the formation of the primitive syncytium. During later stages, it is produced by STB and acts as a positive regulator of the syncytialization process. Mechanistically, hCG binding to the luteinizing hormone/choriogonadotropin receptor (LH/CG-R) induces high levels of cAMP and activation of protein kinase A (PKA). In turn, PKA phosphorylates the cAMP response element-binding (CREB) TF, which, together with the CREB-binding protein and histone acetyltransferase P300, promotes expression of fusogenic genes and GCM1 [86,87,88]. GCM1 is of particular importance as it directly drives expression of the critical fusogenic proteins SYNCYTIN-1 and -2, encoded by the human endogenous retroviruses (HERV) HERV-W and HERV-FRD [84, 89,90,91]. SYNCYTIN-1 and -2 represent domesticated versions of an env gene that in viruses encode the envelope glycoprotein mediating infection competency and virus-host membrane fusion. SYNCYTIN-1 and -2 interact with their respective receptors, the sodium-dependent neutral amino acid transporters (ASCT1 and ASCT2) and major facilitator superfamily domain-containing 2a (MFSD2a), respectively. The receptors are localized on the target-cell membrane and induce the cell fusion process that is vital for STB formation [88]. Interestingly, the role of GCM1 in STB morphogenesis is conserved in the murine placenta, as embryos deficient for Gcm1 show a block of branching morphogenesis and lack of STB [92].
Another conserved TF implicated in human STB formation is the peroxisome proliferator-activated receptor gamma (PPARG). Its expression is modulated by EGF signaling via phosphorylation of P38. PPARG forms a heterodimer with retinoid X receptor alpha (RXRA) and promotes syncytialization by driving transcription of STB-related genes, including GCM1 and SYNCYTIN-1 [93, 94]. Accordingly, mTSCs deficient for Pparg fail to differentiate to STB [95], and Pparg KO embryos result in embryonic lethality due to a placental phenotype [96]. The TF distal-less 3 (DLX3) has also been demonstrated to control STB identity. Mouse embryos deficient for Dlx3 show embryonic lethality around E10 and their placentas feature malformations in the labyrinth and spongiotrophoblast compartments [97]. In the human placenta, DLX3 appears to bind and regulate the expression of CSH1, CGA, HSD3B1, and PGF and thus regulates STB differentiation [98]. Additionally, it has been demonstrated that DLX3 physically interacts with GCM1 and inhibits its transactivation activity at the PGF promoter, among others [99]. These findings provide an example of how an interaction between TFs impacts their activity and outcome. The transcription factor activator protein-2 alpha (AP-2α; TFAP2A) was reported as a critical regulator of biochemical but not morphological differentiation of the human STB [100]. While expression of a dominant-negative version of TFAP2A significantly inhibited induction of vital STB markers, (including hCG, PSG family of genes, PGF, and cytochrome P-450 (CYP11A1)), it did not affect cell fusion, demonstrating the uncoupling of the biochemical and morphological stages of STB differentiation [100]. Interestingly, transcription of TFAP2A is regulated by a nuclear receptor subfamily 2, group F, member 2 (NR2F2). Induction of NR2F2 promoted expression of TFAP2A, and conversely, NR2F2 depletion reduced it, leading to impaired STB differentiation [101]. Another TF that is indirectly involved in regulating the STB state is OVOL1. OVOL1 is thought to suppress CTB genes including MYC, ID1, TP63 and ASCL2 to facilitate STB differentiation. Consequently, disruption of OVOL1 resulted in incomplete silencing of these genes and impaired STB differentiation [102]. Recently, a single cell (sc)RNA-seq analysis on human embryos cultured using a peri-implantation in vitro model identified TBX3 as a novel regulator of STB cell fate [103]. Functional validation experiments have demonstrated that depletion of TBX3 in the JEG-3 cell line blocked STB differentiation [103].
Overall, several TFs have been identified to control STB fate and function; however, their STB context-specific genome-wide binding profiles, interacting proteins, TF networks and transcriptional co-factors remain largely unexplored.
TFs operating in EVTs
As stated earlier, the EVT encompasses both the proliferative EVT of stratified cell columns and the invasive EVT. The latter include the eEVT that lines the spiral arteries and the iEVT that migrates through the uterine stroma. The diverse EVT subtypes have unique properties as they perform highly specialized functions and are thus regulated by a subtype-specific set of TFs (Fig. 4). These TFs can be divided into those that promote EVT proliferation and those that promote EVT cell cycle exit, differentiation and invasiveness [104]. Hypoxia has been suggested to promote proliferation of EVT via the hypoxia inducible factor 1 (HIF1) TF, and to inhibit their differentiation into the invasive subtypes, supporting placental growth [105]. However, other studies found the opposite effect: that the hypoxia-HIF pathway stimulates EVT invasiveness [106].
One of the TFs implicated in regulating the EVT identity is achaete-scute family bHLH transcription factor 2 (ASCL2). Mice deficient for Ascl2 are embryonic lethal due to impaired spongiotrophoblast development and an overabundance of giant cells [107, 108]. In the human placenta, ASCL2 is expressed in CTB and proliferative EVT but not in STB and invasive EVT. Recent reports have shown that disruption of ASCL2 impaired EVT differentiation of hTSCs and instead induced STB identity [16]. These observations indicate that ASCL2 acts to promote proliferation and inhibits differentiation. Similarly, the FOS like 1 (FOSL1) and MYC TFs are also expressed in CTB and proliferative EVT cell columns but not in the more differentiated lineages. In agreement with this, it has been demonstrated that depletion of FOSL1 in an immortalized EVT cell line HTR-8/SVneo results in loss of proliferation and gain of invasiveness, indicating that its primary role is also to reinforce the undifferentiated, proliferative state [109]. Interestingly, both MYC and FOSL1 are also required for placental development in mice [110, 111].
While GCM1 is a master regulator of STB formation, it is also involved in the regulation of the EVT cell fate. In this context, GCM1 drives the expression of genes promoting invasion, including HTRA4, a serine protease that facilitates fibronectin cleavage [112]. Accordingly, expression of GCM1 provokes invasiveness of the BeWo cell line, while depletion of GCM1 in explant cultures reduces it [91]. Thus, GCM1 promotes cell cycle exit and context-dependent differentiation of both STB and EVT.
One of the few known TFs specifically expressed in the iEVT and required for differentiation is placenta-specific protein 8 (PLAC8). While depletion of PLAC8 in explant culture and HTR8/SVneo cell line abrogated EVT migration and invasion, ectopic expression of PLAC8 promoted it [113]. These observations indicate that PLAC8 may play an important role in human placental development and disease as a critical regulator of iEVT invasion and migration. In the mouse placenta, Plac8 mRNA localizes to trophoblast giant cells at E6.5 and E8.5, and to spongiotrophoblast at E10.5 and E18.5, suggesting a role for PLAC8 in murine placental development [114]. However, a placental phenotype has not been reported in embryos deficient for Plac8 [115]. Another TF expressed in EVTs is the IKZF1 Ikaros family zinc finger protein 1. The expression of dominant-negative IKZF1 abrogated migration and invasion in an immortalized EVT cell line and IKAROS was demonstrated to promote EVT differentiation [116, 117].
WNT signaling is vital not only for the reinforcement of the progenitor state in CTBs but also plays a role in the determination of EVT identity. Canonical WNT signaling operates via the transcriptional co-activator β-CATENIN that translocates to the nucleus, where it associates with the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of TFs, driving transcription of diverse genes. It has been demonstrated that WNT3A stimulated EVT migration and invasion, while the addition of the WNT signaling inhibitor DKK-1 blocked it [118]. Similarly, depletion of TCF4 in both villous explant cultures and the SGHPL-5 cell line impaired their migratory and invasive potential [119]. Collectively, these findings indicate that WNT/TCF4 signaling promotes EVT motility and invasiveness. WNT signaling is also vital for the development of the murine placenta, as mice deficient for components and mediators of this pathway show defects in labyrinth formation (WNT2, WNT7B and R-SPONDIN, FZD5), impaired chorioallantoic fusion (Tcf1/Lef1 dKO), and faulty STB formation (Bcl9L) [120,121,122,123,124]. In addition, WNT/β-CATENIN signaling has been demonstrated to activate the expression of Gcm1 and promote STB-II cell fate [125]. Thus, while indispensable for mouse and human trophoblast development and function, WNT/β-CATENIN signaling plays context-dependent roles. In humans, it sustains CTB progenitor identity and controls migration and invasiveness of the EVT, whereas, in mice, it drives STB identity and labyrinth formation.
Two other pathways that regulate EVT identity are LIF/STAT3 and NOTCH signaling. Signal transducer and activator of transcription 3 (STAT3) is vital for ESC self-renewal as the critical mediator of the LIF/STAT3 signaling. Upon phosphorylation, it undergoes homo- or heterodimerization and translocates to the nucleus, where it regulates the expression of a plethora of genes. Experiments in the choriocarcinoma JEG-3 cell line showed that LIF/STAT3 signaling promotes cell proliferation and invasiveness [126]. Moreover, the STAT3 DNA-binding activity was increased in invasive cells, including first-trimester trophoblast, collectively suggesting that LIF/STAT3 may regulate EVT invasive properties. This notion is in agreement with observations made in murine placentas, where the LIF/STAT3 and the suppressor of cytokine signaling 3 (SOCS3) are implicated in the regulation of placental development [127]. NOTCH1 is expressed at the base of the proliferative cell columns and upon gamma-secretase cleavage gives rise to the NOTCH1 intracellular domain (N1ICD) that acts as a transcriptional activator. Manipulation of NOTCH1 in primary trophoblast models revealed that N1ICD promoted trophoblast survival and repressed CTB markers including TEAD4 and TP63 and induced the EVT progenitor-specific genes MYC and VE-CADHERIN [128]. These findings place NOTCH1 as a key regulator promoting the development of EVT progenitors in the human placenta.
Taken together, although a number of TFs and signaling pathways have been implicated in EVT specification, differentiation and migration, it is likely that more remain to be discovered. Importantly, it is unclear how these TFs exert their function within context-dependent TF networks and with transcriptional co-factors in the EVT lineage.
Current limitations and outlook
Optimal placental development and function are vital for a successful pregnancy outcome and the wellbeing of the embryo and the mother. These processes require coordinated and dynamic actions of TFs that, in concert with signaling inputs, determine cell identity of specialized trophoblast cell types. Despite their indisputable importance, the molecular mechanisms underlying TF actions in the human trophoblast remain poorly understood. In addition, our knowledge is based on studies performed mainly in transformed trophoblast cell lines and explant cultures, encouraging caution in data interpretation and drawing of conclusion. It will be of great interest to validate these TF studies in more physiologically relevant systems like hTSCs and TOs. The recent establishment of the chemically-defined conditions to culture hTSCs and TOs has been a significant breakthrough, transforming the human trophoblast field. It provided researchers with reliable, well-characterized, and physiologically relevant in vitro models of the human trophoblast. Importantly, these models are scalable, amenable to genetic manipulations and screens, and compatible with various -omics approaches, and thus offer an excellent system to study TFs in the human trophoblast. Indeed, several important findings about TFs have recently been made using this system [14,15,16].
While hTSCs and TOs provide invaluable tools to study the human trophoblast and TF regulation, researchers should also consider particularities of these models, when interpreting results. For instance, in comparable culture conditions (EGF, CH99021, A83-01, ROCKi), TOs consist of co-existing CTB-like and STB-like populations, while hTSCs remain CTB-like and do not undergo spontaneous STB differentiation. STB differentiation of hTSCs requires the strong inducer forskolin instead. These observations may suggest that TOs constitute a better model of chorionic villi as they recapitulate the co-existence of CTB and STB in a dynamic balance. Indeed, recent findings confirm that TOs represent villous CTB and STB, while hTSCs resemble cells at the base of the cell columns from where EVT derives [129]. Interestingly, EVT differentiation of both hTSC and TOs requires specific growth factor stimuli and seems less robust compared to STB differentiation. These observations may suggest that the commonly used hTSC/TO culture conditions introduce a bias favoring the villous CTB/STB identity. An alternative explanation may point toward inefficient EVT differentiation protocols and intensive efforts are made toward their further optimization (Sandra Haider et al., personal communication). In summary, while hTSCs and TOs offer valuable tools to investigate the role of TFs in the human trophoblast, additional studies are required to bettter characterize these systems better and harness their full potential.
Discoveries made in mESCs, hESCs, and in mTSCs proved transformative to our understanding of the TF-driven molecular processes that determine cell identity and control development. Based on this blueprint, future research efforts in the context of the human trophoblast should focus on identifying novel TFs and their functional characterization, elucidating the cooperation between these TFs, chromatin remodelers and modifiers, and decoding the TF networks that control human trophoblast lineage specification. Particularly important for dissecting the role of trophoblast TFs in the future will be the unbiased study of global effects in gain and loss of function experiments since most studies in the past focused on selected marker genes. Similarly, the genome-wide determination of TF-bound regions and the identification of the TF protein interactomes will provide a holistic comprehension of trophoblast network control, instead of the insular focus on single factors. Finally, the growing human trophoblast expression atlas comprising bulk and single-cell transcriptome datasets will be a crucial resource for benchmarking mechanistic in vitro studies [11, 130,131,132].
Overall, the field can look forward to an exciting next decade where a combination of new models and technologies will enable breakthroughs in understanding TF control of human placental development and disease.
Availability of data and materials
Not applicable.
References
Boyd JD, Hamilton WJ (1970) The human placenta. Cambridge (England) Heffer. Palgrave Macmillan, London
Hertig AT, Rock J, Adams EC (1956) A description of 34 human ova within the first 17 days of development. Am J Anat 98:435–493. https://doi.org/10.1002/aja.1000980306
Pijnenborg R, Dixon G, Robertson WB, Brosens I (1980) Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1:3–19. https://doi.org/10.1016/s0143-4004(80)80012-9
Simmons DG, Fortier AL, Cross JC (2007) Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol 304:567–578. https://doi.org/10.1016/j.ydbio.2007.01.009
Hemberger M, Hanna CW, Dean W (2020) Mechanisms of early placental development in mouse and humans. Nat Rev Genet 21:27–43. https://doi.org/10.1038/s41576-019-0169-4
Simmons DG, Natale DRC, Begay V, Hughes M, Leutz A, Cross JC (2008) Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Dev Camb Engl 135:2083–2091. https://doi.org/10.1242/dev.020099
Rossant J, Cross JC (2001) Placental development: lessons from mouse mutants. Nat Rev Genet 2:538–548. https://doi.org/10.1038/35080570
Rossant J (2018) Genetic control of early cell lineages in the mammalian embryo. Annu Rev Genet 52:185–201. https://doi.org/10.1146/annurev-genet-120116-024544
Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science 282:2072–2075. https://doi.org/10.1126/science.282.5396.2072
Velicky P, Meinhardt G, Plessl K, Vondra S, Weiss T, Haslinger P, Lendl T, Aumayr K, Mairhofer M, Zhu X, Schütz B, Hannibal RL, Lindau R, Weil B, Ernerudh J, Neesen J, Egger G, Mikula M, Röhrl C, Urban AE, Baker J, Knöfler M, Pollheimer J (2018) Genome amplification and cellular senescence are hallmarks of human placenta development. PLoS Genet 14:e1007698. https://doi.org/10.1371/journal.pgen.1007698
Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T (2018) Derivation of human trophoblast stem cells. Cell Stem Cell 22:50-63.e6. https://doi.org/10.1016/j.stem.2017.11.004
Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J, Mendjan S, Latos PA, Knöfler M (2018) Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep 11:537–551. https://doi.org/10.1016/j.stemcr.2018.07.004
Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H, Reimann F, Gribble FM, Sharkey A, Marsh SGE, O’Rahilly S, Hemberger M, Burton GJ, Moffett A (2018) Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564:263–267. https://doi.org/10.1038/s41586-018-0753-3
Saha B, Ganguly A, Home P, Bhattacharya B, Ray S, Ghosh A, Rumi MAK, Marsh C, French VA, Gunewardena S, Paul S (2020) TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc Natl Acad Sci USA 117:17864–17875. https://doi.org/10.1073/pnas.2002449117
Hornbachner R, Lackner A, Papuchova H, Haider S, Knöfler M, Mechtler K, Latos PA (2021) MSX2 safeguards syncytiotrophoblast fate of human trophoblast stem cells. Proc Natl Acad Sci USA 118:e2105130118. https://doi.org/10.1073/pnas.2105130118
Varberg KM, Iqbal K, Muto M, Simon ME, Scott RL, Kozai K, Choudhury RH, Aplin JD, Biswell R, Gibson M, Okae H, Arima T, Vivian JL, Grundberg E, Soares MJ (2021) ASCL2 reciprocally controls key trophoblast lineage decisions during hemochorial placenta development. Proc Natl Acad Sci USA 118:e2016517118. https://doi.org/10.1073/pnas.2016517118
Liu X, Tan JP, Schröder J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun YBY, Chen J, Sun G, Zhou Y, Poppe D, Lister R, Clark AT, Rackham OJL, Zenker J, Polo JM (2021) Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591:627–632. https://doi.org/10.1038/s41586-021-03372-y
Yanagida A, Spindlow D, Nichols J, Dattani A, Smith A, Guo G (2021) Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28:1016-1022.e4. https://doi.org/10.1016/j.stem.2021.04.031
Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, Wang L, Wang K, Zhao S, Hon GC, Wu J (2021) Blastocyst-like structures generated from human pluripotent stem cells. Nature 591:620–626. https://doi.org/10.1038/s41586-021-03356-y
Sozen B, Jorgensen V, Weatherbee BAT, Chen S, Zhu M, Zernicka-Goetz M (2021) Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat Commun 12:5550. https://doi.org/10.1038/s41467-021-25853-4
Kagawa H, Javali A, Khoei HH, Sommer TM, Sestini G, Novatchkova M, Scholte Op Reimer Y, Castel G, Bruneau A, Maenhoudt N, Lammers J, Loubersac S, Freour T, Vankelecom H, David L, Rivron N (2021) Human blastoids model blastocyst development and implantation. Nature. https://doi.org/10.1038/s41586-021-04267-8
Dong C, Beltcheva M, Gontarz P, Zhang B, Popli P, Fischer LA, Khan SA, Park K-M, Yoon E-J, Xing X, Kommagani R, Wang T, Solnica-Krezel L, Theunissen TW (2020) Derivation of trophoblast stem cells from naïve human pluripotent stem cells. Elife 9:e52504. https://doi.org/10.7554/eLife.52504
Io S, Kabata M, Iemura Y, Semi K, Morone N, Minagawa A, Wang B, Okamoto I, Nakamura T, Kojima Y, Iwatani C, Tsuchiya H, Kaswandy B, Kondoh E, Kaneko S, Woltjen K, Saitou M, Yamamoto T, Mandai M, Takashima Y (2021) Capturing human trophoblast development with naive pluripotent stem cells in vitro. Cell Stem Cell 28:1023-1039.e13. https://doi.org/10.1016/j.stem.2021.03.013
Guo G, Stirparo GG, Strawbridge SE, Spindlow D, Yang J, Clarke J, Dattani A, Yanagida A, Li MA, Myers S, Özel BN, Nichols J, Smith A (2021) Human naive epiblast cells possess unrestricted lineage potential. Cell Stem Cell 28:1040-1056.e6. https://doi.org/10.1016/j.stem.2021.02.025
Niwa H (2018) The principles that govern transcription factor network functions in stem cells. Dev Camb Engl 145:dev157420. https://doi.org/10.1242/dev.157420
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Kubaczka C, Senner CE, Cierlitza M, Araúzo-Bravo MJ, Kuckenberg P, Peitz M, Hemberger M, Schorle H (2015) Direct induction of trophoblast stem cells from murine fibroblasts. Cell Stem Cell 17:557–568. https://doi.org/10.1016/j.stem.2015.08.005
Benchetrit H, Jaber M, Zayat V, Sebban S, Pushett A, Makedonski K, Zakheim Z, Radwan A, Maoz N, Lasry R, Renous N, Inbar M, Ram O, Kaplan T, Buganim Y (2019) Direct induction of the three pre-implantation blastocyst cell types from fibroblasts. Cell Stem Cell 24:983-994.e7. https://doi.org/10.1016/j.stem.2019.03.018
Bai T, Peng C-Y, Aneas I, Sakabe N, Requena DF, Billstrand C, Nobrega M, Ober C, Parast M, Kessler JA (2021) Establishment of human induced trophoblast stem-like cells from term villous cytotrophoblasts. Stem Cell Res 56:102507. https://doi.org/10.1016/j.scr.2021.102507
Adachi K, Nikaido I, Ohta H, Ohtsuka S, Ura H, Kadota M, Wakayama T, Ueda HR, Niwa H (2013) Context-dependent wiring of Sox2 regulatory networks for self-renewal of embryonic and trophoblast stem cells. Mol Cell 52:380–392. https://doi.org/10.1016/j.molcel.2013.09.002
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122:947–956. https://doi.org/10.1016/j.cell.2005.08.020
Kim J, Chu J, Shen X, Wang J, Orkin SH (2008) An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132:1049–1061. https://doi.org/10.1016/j.cell.2008.02.039
Niwa H, Ogawa K, Shimosato D, Adachi K (2009) A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460:118–122. https://doi.org/10.1038/nature08113
van den Berg DLC, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA (2010) An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell 6:369–381. https://doi.org/10.1016/j.stem.2010.02.014
Lackner A, Sehlke R, Garmhausen M, Giuseppe Stirparo G, Huth M, Titz-Teixeira F, van der Lelij P, Ramesmayer J, Thomas HF, Ralser M, Santini L, Galimberti E, Sarov M, Stewart AF, Smith A, Beyer A, Leeb M (2021) Cooperative genetic networks drive embryonic stem cell transition from naïve to formative pluripotency. EMBO J 40:e105776. https://doi.org/10.15252/embj.2020105776
Latos PA, Goncalves A, Oxley D, Mohammed H, Turro E, Hemberger M (2015) Fgf and Esrrb integrate epigenetic and transcriptional networks that regulate self-renewal of trophoblast stem cells. Nat Commun 6:7776. https://doi.org/10.1038/ncomms8776
Latos PA, Sienerth AR, Murray A, Senner CE, Muto M, Ikawa M, Oxley D, Burge S, Cox BJ, Hemberger M (2015) Elf5-centered transcription factor hub controls trophoblast stem cell self-renewal and differentiation through stoichiometry-sensitive shifts in target gene networks. Genes Dev 29:2435–2448. https://doi.org/10.1101/gad.268821.115
Lee B-K, Jang YJ, Kim M, LeBlanc L, Rhee C, Lee J, Beck S, Shen W, Kim J (2019) Super-enhancer-guided mapping of regulatory networks controlling mouse trophoblast stem cells. Nat Commun 10:4749. https://doi.org/10.1038/s41467-019-12720-6
Gasperini M, Tome JM, Shendure J (2020) Towards a comprehensive catalogue of validated and target-linked human enhancers. Nat Rev Genet 21:292–310. https://doi.org/10.1038/s41576-019-0209-0
Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ (2008) Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Dev Camb Engl 135:501–511. https://doi.org/10.1242/dev.014357
Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F (2004) Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci USA 101:7641–7645. https://doi.org/10.1073/pnas.0401654101
Zhang S, Cui W (2014) Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells 6:305–311. https://doi.org/10.4252/wjsc.v6.i3.305
Latos PA, Hemberger M (2016) From the stem of the placental tree: trophoblast stem cells and their progeny. Dev Camb Engl 143:3650–3660. https://doi.org/10.1242/dev.133462
Reményi A, Lins K, Nissen LJ, Reinbold R, Schöler HR, Wilmanns M (2003) Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev 17:2048–2059. https://doi.org/10.1101/gad.269303
Iwafuchi-Doi M, Matsuda K, Murakami K, Niwa H, Tesar PJ, Aruga J, Matsuo I, Kondoh H (2012) Transcriptional regulatory networks in epiblast cells and during anterior neural plate development as modeled in epiblast stem cells. Dev Camb Engl 139:3926–3937. https://doi.org/10.1242/dev.085936
Danno H, Michiue T, Hitachi K, Yukita A, Ishiura S, Asashima M (2008) Molecular links among the causative genes for ocular malformation: Otx2 and Sox2 coregulate Rax expression. Proc Natl Acad Sci USA 105:5408–5413. https://doi.org/10.1073/pnas.0710954105
Carroll SB (2008) Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134:25–36. https://doi.org/10.1016/j.cell.2008.06.030
Chuong EB, Rumi MAK, Soares MJ, Baker JC (2013) Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat Genet 45:325–329. https://doi.org/10.1038/ng.2553
Dunn-Fletcher CE, Muglia LM, Pavlicev M, Wolf G, Sun M-A, Hu Y-C, Huffman E, Tumukuntala S, Thiele K, Mukherjee A, Zoubovsky S, Zhang X, Swaggart KA, Lamm KYB, Jones H, Macfarlan TS, Muglia LJ (2018) Anthropoid primate-specific retroviral element THE1B controls expression of CRH in placenta and alters gestation length. PLoS Biol 16:e2006337. https://doi.org/10.1371/journal.pbio.2006337
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H (2009) The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16:398–410. https://doi.org/10.1016/j.devcel.2009.02.003
Meinhardt G, Haider S, Kunihs V, Saleh L, Pollheimer J, Fiala C, Hetey S, Feher Z, Szilagyi A, Than NG, Knöfler M (2020) Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta. Proc Natl Acad Sci USA 117:13562–13570. https://doi.org/10.1073/pnas.2002630117
Gerri C, McCarthy A, Alanis-Lobato G, Demtschenko A, Bruneau A, Loubersac S, Fogarty NME, Hampshire D, Elder K, Snell P, Christie L, David L, Van de Velde H, Fouladi-Nashta AA, Niakan KK (2020) Initiation of a conserved trophectoderm program in human, cow and mouse embryos. Nature 587:443–447. https://doi.org/10.1038/s41586-020-2759-x
Kidder BL, Palmer S (2010) Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome Res 20:458–472. https://doi.org/10.1101/gr.101469.109
Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T (2000) A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell 6:1287–1295. https://doi.org/10.1016/s1097-2765(00)00127-1
Guidi CJ, Sands AT, Zambrowicz BP, Turner TK, Demers DA, Webster W, Smith TW, Imbalzano AN, Jones SN (2001) Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol 21:3598–3603. https://doi.org/10.1128/MCB.21.10.3598-3603.2001
Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, Nam JS, Kim H, Chung H, Lee HW, Park SD, Seong RH (2001) Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol 21:7787–7795. https://doi.org/10.1128/MCB.21.22.7787-7795.2001
Asanoma K, Kubota K, Chakraborty D, Renaud SJ, Wake N, Fukushima K, Soares MJ, Rumi MAK (2012) SATB homeobox proteins regulate trophoblast stem cell renewal and differentiation. J Biol Chem 287:2257–2268. https://doi.org/10.1074/jbc.M111.287128
Goller T, Vauti F, Ramasamy S, Arnold H-H (2008) Transcriptional regulator BPTF/FAC1 is essential for trophoblast differentiation during early mouse development. Mol Cell Biol 28:6819–6827. https://doi.org/10.1128/MCB.01058-08
Knott JG, Paul S (2014) Transcriptional regulators of the trophoblast lineage in mammals with hemochorial placentation. Reprod Camb Engl 148:R121-136. https://doi.org/10.1530/REP-14-0072
Kwak Y-T, Muralimanoharan S, Gogate AA, Mendelson CR (2019) Human trophoblast differentiation is associated with profound gene regulatory and epigenetic changes. Endocrinology 160:2189–2203. https://doi.org/10.1210/en.2019-00144
Jaju Bhattad G, Jeyarajah MJ, McGill MG, Dumeaux V, Okae H, Arima T, Lajoie P, Bérubé NG, Renaud SJ (2020) Histone deacetylase 1 and 2 drive differentiation and fusion of progenitor cells in human placental trophoblasts. Cell Death Dis 11:311. https://doi.org/10.1038/s41419-020-2500-6
Home P, Kumar RP, Ganguly A, Saha B, Milano-Foster J, Bhattacharya B, Ray S, Gunewardena S, Paul A, Camper SA, Fields PE, Paul S (2017) Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Dev Camb Engl 144:876–888. https://doi.org/10.1242/dev.145318
Niakan KK, Eggan K (2013) Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev Biol 375:54–64. https://doi.org/10.1016/j.ydbio.2012.12.008
Blakeley P, Fogarty NME, del Valle I, Wamaitha SE, Hu TX, Elder K, Snell P, Christie L, Robson P, Niakan KK (2015) Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Dev Camb Engl 142:3151–3165. https://doi.org/10.1242/dev.123547
Roberts RM, Loh KM, Amita M, Bernardo AS, Adachi K, Alexenko AP, Schust DJ, Schulz LC, Telugu BPVL, Ezashi T, Pedersen RA (2014) Differentiation of trophoblast cells from human embryonic stem cells: to be or not to be? Reprod Camb Engl 147:D1-12. https://doi.org/10.1530/REP-14-0080
Krendl C, Shaposhnikov D, Rishko V, Ori C, Ziegenhain C, Sass S, Simon L, Müller NS, Straub T, Brooks KE, Chavez SL, Enard W, Theis FJ, Drukker M (2017) GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc Natl Acad Sci USA 114:E9579–E9588. https://doi.org/10.1073/pnas.1708341114
Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T (2002) Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Dev Camb Engl 129:2733–2747
Liu X, Ouyang JF, Rossello FJ, Tan JP, Davidson KC, Valdes DS, Schröder J, Sun YBY, Chen J, Knaupp AS, Sun G, Chy HS, Huang Z, Pflueger J, Firas J, Tano V, Buckberry S, Paynter JM, Larcombe MR, Poppe D, Choo XY, O’Brien CM, Pastor WA, Chen D, Leichter AL, Naeem H, Tripathi P, Das PP, Grubman A, Powell DR, Laslett AL, David L, Nilsson SK, Clark AT, Lister R, Nefzger CM, Martelotto LG, Rackham OJL, Polo JM (2020) Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature 586:101–107. https://doi.org/10.1038/s41586-020-2734-6
Tanaka H, Takizawa Y, Takaku M, Kato D, Kumagawa Y, Grimm SA, Wade PA, Kurumizaka H (2020) Interaction of the pioneer transcription factor GATA3 with nucleosomes. Nat Commun 11:4136. https://doi.org/10.1038/s41467-020-17959-y
Rothstein M, Simoes-Costa M (2020) Heterodimerization of TFAP2 pioneer factors drives epigenomic remodeling during neural crest specification. Genome Res 30:35–48. https://doi.org/10.1101/gr.249680.119
Soncin F, Khater M, To C, Pizzo D, Farah O, Wakeland A, Arul Nambi Rajan K, Nelson KK, Chang C-W, Moretto-Zita M, Natale DR, Laurent LC, Parast MM (2018) Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Dev Camb Engl 145:dev156273. https://doi.org/10.1242/dev.156273
Ng RK, Dean W, Dawson C, Lucifero D, Madeja Z, Reik W, Hemberger M (2008) Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat Cell Biol 10:1280–1290. https://doi.org/10.1038/ncb1786
Hemberger M, Udayashankar R, Tesar P, Moore H, Burton GJ (2010) ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet 19:2456–2467. https://doi.org/10.1093/hmg/ddq128
Donnison M, Broadhurst R, Pfeffer PL (2015) Elf5 and Ets2 maintain the mouse extraembryonic ectoderm in a dosage dependent synergistic manner. Dev Biol 397:77–88. https://doi.org/10.1016/j.ydbio.2014.10.011
Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V (1997) Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 388:778–782. https://doi.org/10.1038/42022
Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ (2000) Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 404:95–99. https://doi.org/10.1038/35003601
Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17:126–140. https://doi.org/10.1101/gad.224503
Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguère V (2001) Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev 15:833–838. https://doi.org/10.1101/gad.873401
Romano R-A, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, Sinha S (2012) ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Dev Camb Engl 139:772–782. https://doi.org/10.1242/dev.071191
Li Y, Moretto-Zita M, Leon-Garcia S, Parast MM (2014) p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am J Pathol 184:3332–3343. https://doi.org/10.1016/j.ajpath.2014.08.006
Murphy VE, Smith R, Giles WB, Clifton VL (2006) Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev 27:141–169. https://doi.org/10.1210/er.2005-0011
Jaremek A, Jeyarajah MJ, Jaju Bhattad G, Renaud SJ (2021) Omics approaches to study formation and function of human placental syncytiotrophoblast. Front Cell Dev Biol 9:674162. https://doi.org/10.3389/fcell.2021.674162
Moffett A, Loke C (2006) Immunology of placentation in eutherian mammals. Nat Rev Immunol 6:584–594. https://doi.org/10.1038/nri1897
Lu X, Wang R, Zhu C, Wang H, Lin H-Y, Gu Y, Cross JC, Wang H (2017) Fine-tuned and cell-cycle-restricted expression of fusogenic protein syncytin-2 maintains functional placental syncytia. Cell Rep 21:1150–1159. https://doi.org/10.1016/j.celrep.2017.10.019
Yang M, Lei ZM, Rao CV (2003) The central role of human chorionic gonadotropin in the formation of human placental syncytium. Endocrinology 144:1108–1120. https://doi.org/10.1210/en.2002-220922
Knerr I, Schubert SW, Wich C, Amann K, Aigner T, Vogler T, Jung R, Dötsch J, Rascher W, Hashemolhosseini S (2005) Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett 579:3991–3998. https://doi.org/10.1016/j.febslet.2005.06.029
Pidoux G, Gerbaud P, Dompierre J, Lygren B, Solstad T, Evain-Brion D, Taskén K (2014) A PKA-ezrin-Cx43 signaling complex controls gap junction communication and thereby trophoblast cell fusion. J Cell Sci 127:4172–4185. https://doi.org/10.1242/jcs.149609
Gerbaud P, Pidoux G (2015) Review: An overview of molecular events occurring in human trophoblast fusion. Placenta 36(Suppl 1):S35-42. https://doi.org/10.1016/j.placenta.2014.12.015
Yu C, Shen K, Lin M, Chen P, Lin C, Chang G-D, Chen H (2002) GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem 277:50062–50068. https://doi.org/10.1074/jbc.M209316200
Liang C-Y, Wang L-J, Chen C-P, Chen L-F, Chen Y-H, Chen H (2010) GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol Reprod 83:387–395. https://doi.org/10.1095/biolreprod.110.083915
Baczyk D, Satkunaratnam A, Nait-Oumesmar B, Huppertz B, Cross JC, Kingdom JCP (2004) Complex patterns of GCM1 mRNA and protein in villous and extravillous trophoblast cells of the human placenta. Placenta 25:553–559. https://doi.org/10.1016/j.placenta.2003.12.004
Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC (2000) The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 25:311–314. https://doi.org/10.1038/77076
Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM, Sadovsky Y (2000) Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocrinol Metab 85:3874–3881. https://doi.org/10.1210/jcem.85.10.6885
Tarrade A, Schoonjans K, Guibourdenche J, Bidart JM, Vidaud M, Auwerx J, Rochette-Egly C, Evain-Brion D (2001) PPAR gamma/RXR alpha heterodimers are involved in human CG beta synthesis and human trophoblast differentiation. Endocrinology 142:4504–4514. https://doi.org/10.1210/endo.142.10.8448
Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS (2009) PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS ONE 4:e8055. https://doi.org/10.1371/journal.pone.0008055
Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–595. https://doi.org/10.1016/s1097-2765(00)80209-9
Morasso MI, Grinberg A, Robinson G, Sargent TD, Mahon KA (1999) Placental failure in mice lacking the homeobox gene Dlx3. Proc Natl Acad Sci USA 96:162–167. https://doi.org/10.1073/pnas.96.1.162
Roberson MS, Meermann S, Morasso MI, Mulvaney-Musa JM, Zhang T (2001) A role for the homeobox protein Distal-less 3 in the activation of the glycoprotein hormone alpha subunit gene in choriocarcinoma cells. J Biol Chem 276:10016–10024. https://doi.org/10.1074/jbc.M007481200
Li S, Roberson MS (2017) DLX3 interacts with GCM1 and inhibits its transactivation-stimulating activity in a homeodomain-dependent manner in human trophoblast-derived cells. Sci Rep 7:2009. https://doi.org/10.1038/s41598-017-02120-5
Cheng Y-H, Aronow BJ, Hossain S, Trapnell B, Kong S, Handwerger S (2004) Critical role for transcription factor AP-2alpha in human trophoblast differentiation. Physiol Genom 18:99–107. https://doi.org/10.1152/physiolgenomics.00181.2003
Hubert MA, Sherritt SL, Bachurski CJ, Handwerger S (2010) Involvement of transcription factor NR2F2 in human trophoblast differentiation. PLoS ONE 5:e9417. https://doi.org/10.1371/journal.pone.0009417
Renaud SJ, Chakraborty D, Mason CW, Rumi MAK, Vivian JL, Soares MJ (2015) OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc Natl Acad Sci USA 112:E6175-6184. https://doi.org/10.1073/pnas.1507397112
Lv B, An Q, Zeng Q, Zhang X, Lu P, Wang Y, Zhu X, Ji Y, Fan G, Xue Z (2019) Single-cell RNA sequencing reveals regulatory mechanism for trophoblast cell-fate divergence in human peri-implantation conceptuses. PLoS Biol 17:e3000187. https://doi.org/10.1371/journal.pbio.3000187
Baines KJ, Renaud SJ (2017) Transcription factors that regulate trophoblast development and function. Prog Mol Biol Transl Sci 145:39–88. https://doi.org/10.1016/bs.pmbts.2016.12.003
Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M (2000) Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 105:577–587. https://doi.org/10.1172/JCI8316
Hayashi M, Sakata M, Takeda T, Tahara M, Yamamoto T, Okamoto Y, Minekawa R, Isobe A, Ohmichi M, Tasaka K, Murata Y (2005) Up-regulation of c-met protooncogene product expression through hypoxia-inducible factor-1alpha is involved in trophoblast invasion under low-oxygen tension. Endocrinology 146:4682–4689. https://doi.org/10.1210/en.2005-0416
Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL (1994) Essential role of Mash-2 in extraembryonic development. Nature 371:333–336. https://doi.org/10.1038/371333a0
Tanaka M, Gertsenstein M, Rossant J, Nagy A (1997) Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev Biol 190:55–65. https://doi.org/10.1006/dbio.1997.8685
Renaud SJ, Kubota K, Rumi MAK, Soares MJ (2014) The FOS transcription factor family differentially controls trophoblast migration and invasion. J Biol Chem 289:5025–5039. https://doi.org/10.1074/jbc.M113.523746
Dubois NC, Adolphe C, Ehninger A, Wang RA, Robertson EJ, Trumpp A (2008) Placental rescue reveals a sole requirement for c-Myc in embryonic erythroblast survival and hematopoietic stem cell function. Dev Camb Engl 135:2455–2465. https://doi.org/10.1242/dev.022707
Johnson RS, Spiegelman BM, Papaioannou V (1992) Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71:577–586. https://doi.org/10.1016/0092-8674(92)90592-z
Wang L-J, Cheong M-L, Lee Y-S, Lee M-T, Chen H (2012) High-temperature requirement protein A4 (HtrA4) suppresses the fusogenic activity of syncytin-1 and promotes trophoblast invasion. Mol Cell Biol 32:3707–3717. https://doi.org/10.1128/MCB.00223-12
Chang W-L, Liu Y-W, Dang Y-L, Jiang X-X, Xu H, Huang X, Wang Y-L, Wang H, Zhu C, Xue L-Q, Lin H-Y, Meng W, Wang H (2018) PLAC8, a new marker for human interstitial extravillous trophoblast cells, promotes their invasion and migration. Dev Camb Engl 145:dev148932. https://doi.org/10.1242/dev.148932
Galaviz-Hernandez C, Stagg C, de Ridder G, Tanaka TS, Ko MSH, Schlessinger D, Nagaraja R (2003) Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene 309:81–89. https://doi.org/10.1016/s0378-1119(03)00508-0
Ledford JG, Kovarova M, Koller BH (2007) Impaired host defense in mice lacking ONZIN. J Immunol Baltim Md 178:5132–5143. https://doi.org/10.4049/jimmunol.178.8.5132
Yamamoto E, Ito T, Abe A, Sido F, Ino K, Itakura A, Mizutani S, Dovat S, Nomura S, Kikkawa F (2005) Ikaros is expressed in human extravillous trophoblasts and involved in their migration and invasion. Mol Hum Reprod 11:825–831. https://doi.org/10.1093/molehr/gah239
Ito T, Nomura S, Okada M, Katsumata Y, Kikkawa F, Rogi T, Tsujimoto M, Mizutani S (2002) Ap-2 and Ikaros regulate transcription of human placental leucine aminopeptidase/oxytocinase gene. Biochem Biophys Res Commun 290:1048–1053. https://doi.org/10.1006/bbrc.2001.6325
Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knöfler M (2006) Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol 168:1134–1147. https://doi.org/10.2353/ajpath.2006.050686
Meinhardt G, Haider S, Haslinger P, Proestling K, Fiala C, Pollheimer J, Knöfler M (2014) Wnt-dependent T-cell factor-4 controls human etravillous trophoblast motility. Endocrinology 155:1908–1920. https://doi.org/10.1210/en.2013-2042
Krivega M, Essahib W, Van de Velde H (2015) WNT3 and membrane-associated β-catenin regulate trophectoderm lineage differentiation in human blastocysts. Mol Hum Reprod 21:711–722. https://doi.org/10.1093/molehr/gav036
Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H (2007) R-spondin3 is required for mouse placental development. Dev Biol 301:218–226. https://doi.org/10.1016/j.ydbio.2006.08.018
Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM (2001) Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Dev Camb Engl 128:25–33
Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ (1996) Targeted disruption of the Wnt2 gene results in placentation defects. Dev Camb Engl 122:3343–3353
Galceran J, Fariñas I, Depew MJ, Clevers H, Grosschedl R (1999) Wnt3a-/–like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev 13:709–717. https://doi.org/10.1101/gad.13.6.709
Zhu D, Gong X, Miao L, Fang J, Zhang J (2017) Efficient induction of syncytiotrophoblast layer II cells from trophoblast stem cells by canonical Wnt signaling activation. Stem Cell Rep 9:2034–2049. https://doi.org/10.1016/j.stemcr.2017.10.014
Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, Wiederanders B, Pfitzner E, Markert UR, Friedrich K (2005) Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol 37:2284–2296. https://doi.org/10.1016/j.biocel.2005.02.025
Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS (2001) Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci USA 98:9324–9329. https://doi.org/10.1073/pnas.161271798
Haider S, Meinhardt G, Saleh L, Fiala C, Pollheimer J, Knöfler M (2016) Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc Natl Acad Sci USA 113:E7710–E7719. https://doi.org/10.1073/pnas.1612335113
Sheridan MA, Zhao X, Fernando RC, Gardner L, Perez-Garcia V, Li Q, Marsh SGE, Hamilton R, Moffett A, Turco MY (2021) Characterization of primary models of human trophoblast. Dev Camb Engl 148:dev199749. https://doi.org/10.1242/dev.199749
Tsang JCH, Vong JSL, Ji L, Poon LCY, Jiang P, Lui KO, Ni Y-B, To KF, Cheng YKY, Chiu RWK, Lo YMD (2017) Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proc Natl Acad Sci USA 114:E7786–E7795. https://doi.org/10.1073/pnas.1710470114
Liu Y, Fan X, Wang R, Lu X, Dang Y-L, Wang H, Lin H-Y, Zhu C, Ge H, Cross JC, Wang H (2018) Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res 28:819–832. https://doi.org/10.1038/s41422-018-0066-y
Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park J-E, Stephenson E, Polański K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA (2018) Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563:347–353. https://doi.org/10.1038/s41586-018-0698-6
Acknowledgements
We are grateful to Sasha Mendjan for his insightful comments. The work was supported by the Austrian Science Fund (Grant P-30941) awarded to P.A.L.
Funding
Open access funding provided by the Austrian Science Fund (FWF). The work was supported by the Austrian Science Fund (Grant P-30941) awarded to P.A.L.
Author information
Authors and Affiliations
Contributions
HP and PAL wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papuchova, H., Latos, P.A. Transcription factor networks in trophoblast development. Cell. Mol. Life Sci. 79, 337 (2022). https://doi.org/10.1007/s00018-022-04363-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04363-6