Abstract
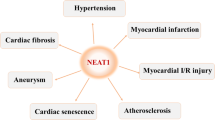
The initial identification of long non-coding RNA myocardial infarction associated transcript (MIAT) as a genetic risk factor of myocardial infarction has made this lncRNA (designated as lncR-MIAT here) a focus of intensive studies worldwide. Emerging evidence supports that lncR-MIAT is susceptible in its expression to multiple deleterious factors like angiotensin II, isoproterenol, hypoxia, and infection and is anomaly overexpressed in serum, plasma, blood cells and myocardial tissues under a variety of cardiovascular conditions including myocardial infarction, cardiac hypertrophy, diabetic cardiomyopathy, dilated cardiomyopathy, sepsis cardiomyopathy, atrial fibrillation and microvascular dysfunction. Experimental results consistently demonstrated that upregulation of lncR-MIAT plays active roles in the pathological processes of the cardiovascular system and knockdown of this lncRNA effectively ameliorates the adverse conditions. The available data revealed that lncR-MIAT acts through multiple mechanisms such as competitive endogenous RNA, natural antisense RNA and RNA/protein interactions. Moreover, the functional domains of lncR-MIAT accounting for certain specific cellular functions of the full-length transcript have been identified and characterized. These insights will not only tremendously advance our understanding of lncRNA biology and pathophysiology, but also offer good opportunities for more innovative and precise design of agents that have the potential to be developed into new drugs for better therapy of cardiovascular diseases (CVDs) in the future. Herein, we provide an overview of lncR-MIAT, focusing on its roles in cardiovascular diseases, underline the unique cellular/molecular mechanisms for its actions, and speculate the perspectives about the translational studies on the potential diagnostic and therapeutic applications of lncR-MIAT.


Similar content being viewed by others
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Batista PJ, Chang HY (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152:1298–1307. https://doi.org/10.1016/j.cell.2013.02.012
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10:155–159. https://doi.org/10.1038/nrg2521
Hobuss L, Bar C, Thum T (2019) Long Non-coding RNAs: at the heart of cardiac dysfunction? Front Physiol 10:30. https://doi.org/10.3389/fphys.2019.00030
Lozano-Vidal N, Bink DI, Boon RA (2019) Long noncoding RNA in cardiac aging and disease. J Mol Cell Biol 11:860–867. https://doi.org/10.1093/jmcb/mjz046
Maass PG, Luft FC, Bahring S (2014) Long non-coding RNA in health and disease. J Mol Med (Berl) 92:337–346. https://doi.org/10.1007/s00109-014-1131-8
Schonrock N, Harvey RP, Mattick JS (2012) Long noncoding RNAs in cardiac development and pathophysiology. Circ Res 111:1349–1362. https://doi.org/10.1161/CIRCRESAHA.112.268953
Uchida S, Dimmeler S (2015) Long noncoding RNAs in cardiovascular diseases. Circ Res 116:737–750. https://doi.org/10.1161/CIRCRESAHA.116.302521
Zhang Y, Du W, Yang B (2019) Long non-coding RNAs as new regulators of cardiac electrophysiology and arrhythmias: molecular mechanisms, therapeutic implications and challenges. Pharmacol Ther 203:107389. https://doi.org/10.1016/j.pharmthera.2019.06.011
Gast M, Rauch BH, Haghikia A, Nakagawa S, Haas J, Stroux A, Schmidt D, Schumann P, Weiss S, Jensen L, Kratzer A, Kraenkel N, Muller C, Bornigen D, Hirose T, Blankenberg S, Escher F, Kuhl AA, Kuss AW, Meder B, Landmesser U, Zeller T, Poller W (2019) Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res 115:1886–1906. https://doi.org/10.1093/cvr/cvz085
Bar C, Chatterjee S, Falcao Pires I, Rodrigues P, Sluijter JPG, Boon RA, Nevado RM, Andres V, Sansonetti M, de Windt L, Ciccarelli M, Hamdani N, Heymans S, Figuinha Videira R, Tocchetti CG, Giacca M, Zacchigna S, Engelhardt S, Dimmeler S, Madonna R, Thum T (2020) Non-coding RNAs: update on mechanisms and therapeutic targets from the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc Res 116:1805–1819. https://doi.org/10.1093/cvr/cvaa195
Bar C, Chatterjee S, Thum T (2016) Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 134:1484–1499. https://doi.org/10.1161/CIRCULATIONAHA.116.023686
Ishii N, Ozaki K, Sato H, Mizuno H, Susumu S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Satoshi S, Nakamura Y, Tanaka T (2006) Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51:1087–1099. https://doi.org/10.1007/s10038-006-0070-9
Vausort M, Wagner DR, Devaux Y (2014) Long noncoding RNAs in patients with acute myocardial infarction. Circ Res 115:668–677. https://doi.org/10.1161/CIRCRESAHA.115.303836
Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q (2015) lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res 116:1143–1156. https://doi.org/10.1161/CIRCRESAHA.116.305510
Zhu XH, Yuan YX, Rao SL, Wang P (2016) LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci 20:3653–3660
Qu X, Du Y, Shu Y, Gao M, Sun F, Luo S, Yang T, Zhan L, Yuan Y, Chu W, Pan Z, Wang Z, Yang B, Lu Y (2017) MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep 7:42657. https://doi.org/10.1038/srep42657
Zhou X, Zhang W, Jin M, Chen J, Xu W, Kong X (2017) lncRNA MIAT functions as a competing endogenous RNA to upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy. Cell Death Dis 8:e2929. https://doi.org/10.1038/cddis.2017.321
Sone M, Hayashi T, Tarui H, Agata K, Takeichi M, Nakagawa S (2007) The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J Cell Sci 120:2498–2506. https://doi.org/10.1242/jcs.009357
Rapicavoli NA, Poth EM, Blackshaw S (2010) The long noncoding RNA RNCR2 directs mouse retinal cell specification. BMC Dev Biol 10:49. https://doi.org/10.1186/1471-213X-10-49
Tsuiji H, Yoshimoto R, Hasegawa Y, Furuno M, Yoshida M, Nakagawa S (2011) Competition between a noncoding exon and introns: Gomafu contains tandem UACUAAC repeats and associates with splicing factor-1. Genes Cells 16:479–490. https://doi.org/10.1111/j.1365-2443.2011.01502.x
Galarneau A, Richard S (2005) Target RNA motif and target mRNAs of the Quaking STAR protein. Nat Struct Mol Biol 12:691–698. https://doi.org/10.1038/nsmb963
Azat M, Huojiahemaiti X, Gao R, Peng P (2019) Long noncoding RNA MIAT: a potential role in the diagnosis and mediation of acute myocardial infarction. Mol Med Rep 20:5216–5222. https://doi.org/10.3892/mmr.2019.10768
Eicher JD, Wakabayashi Y, Vitseva O, Esa N, Yang Y, Zhu J, Freedman JE, McManus DD, Johnson AD (2016) Characterization of the platelet transcriptome by RNA sequencing in patients with acute myocardial infarction. Platelets 27:230–239. https://doi.org/10.3109/09537104.2015.1083543
Wang XM, Li XM, Song N, Zhai H, Gao XM, Yang YN (2019) Long non-coding RNAs H19, MALAT1 and MIAT as potential novel biomarkers for diagnosis of acute myocardial infarction. Biomed Pharmacother 118:109208. https://doi.org/10.1016/j.biopha.2019.109208
Tan J, Liu S, Jiang Q, Yu T, Huang K (2019) LncRNA-MIAT increased in patients with coronary atherosclerotic heart disease. Cardiol Res Pract 2019:6280194. https://doi.org/10.1155/2019/6280194
Toraih EA, El-Wazir A, Alghamdi SA, Alhazmi AS, El-Wazir M, Abdel-Daim MM, Fawzy MS (2019) Association of long non-coding RNA MIAT and MALAT1 expression profiles in peripheral blood of coronary artery disease patients with previous cardiac events. Genet Mol Biol 42:509–518. https://doi.org/10.1590/1678-4685-GMB-2018-0185
Zhu M, Li N, Luo P, Jing W, Wen X, Liang C, Tu J (2018) Peripheral blood leukocyte expression of lncrna miat and its diagnostic and prognostic value in ischemic stroke. J Stroke Cerebrovasc Dis 27:326–337. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.09.009
de Gonzalo-Calvo D, Kenneweg F, Bang C, Toro R, van der Meer RW, Rijzewijk LJ, Smit JW, Lamb HJ, Llorente-Cortes V, Thum T (2016) Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep 6:37354. https://doi.org/10.1038/srep37354
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367:1747–1757. https://doi.org/10.1016/S0140-6736(06)68770-9
Reed GW, Rossi JE, Cannon CP (2017) Acute myocardial infarction. Lancet 389:197–210. https://doi.org/10.1016/S0140-6736(16)30677-8
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force Members C, Thygesen K, Alpert JS, White HD, Biomarker S, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Subcommittee ECG, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging S, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification S, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Intervention S, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Trials, Registries S, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Trials, Registries S, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Trials, Registries S, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Trials, Registries S, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Guidelines ESCCfP, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document R, Morais J, Aguiar C, Almahmeed W et al (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60:1581–1598. https://doi.org/10.1016/j.jacc.2012.08.001
Jose Corbalan J, Vatner DE, Vatner SF (2016) Myocardial apoptosis in heart disease: does the emperor have clothes? Basic Res Cardiol 111:31. https://doi.org/10.1007/s00395-016-0549-2
Rodriguez M, Lucchesi BR, Schaper J (2002) Apoptosis in myocardial infarction. Ann Med 34:470–479. https://doi.org/10.1080/078538902321012414
Chen L, Zhang D, Yu L, Dong H (2019) Targeting MIAT reduces apoptosis of cardiomyocytes after ischemia/reperfusion injury. Bioengineered 10:121–132. https://doi.org/10.1080/21655979.2019.1605812
Cong L, Su Y, Wei D, Qian L, Xing D, Pan J, Chen Y, Huang M (2020) Catechin relieves hypoxia/reoxygenation-induced myocardial cell apoptosis via down-regulating lncRNA MIAT. J Cell Mol Med 24:2356–2368. https://doi.org/10.1111/jcmm.14919
Mercer JR (2014) Mitochondrial bioenergetics and therapeutic intervention in cardiovascular disease. Pharmacol Ther 141:13–20. https://doi.org/10.1016/j.pharmthera.2013.07.011
Suen DF, Norris KL, Youle RJ (2008) Mitochondrial dynamics and apoptosis. Genes Dev 22:1577–1590. https://doi.org/10.1101/gad.1658508
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283:1482–1488. https://doi.org/10.1126/science.283.5407.1482
Gatliff J, Campanella M (2012) The 18 kDa translocator protein (TSPO): a new perspective in mitochondrial biology. Curr Mol Med 12:356–368. https://doi.org/10.2174/1566524011207040356
Morin D, Musman J, Pons S, Berdeaux A, Ghaleh B (2016) Mitochondrial translocator protein (TSPO): from physiology to cardioprotection. Biochem Pharmacol 105:1–13. https://doi.org/10.1016/j.bcp.2015.12.003
Motloch LJ, Hu J, Akar FG (2015) The mitochondrial translocator protein and arrhythmogenesis in ischemic heart disease. Oxid Med Cell Longev 2015:234104. https://doi.org/10.1155/2015/234104
Veenman L, Papadopoulos V, Gavish M (2007) Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des 13:2385–2405. https://doi.org/10.2174/138161207781368710
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656. https://doi.org/10.1038/nrc883
Kugler W, Veenman L, Shandalov Y, Leschiner S, Spanier I, Lakomek M, Gavish M (2008) Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine. Cell Oncol 30:435–450. https://doi.org/10.3233/clo-2008-0431
Mendonca-Torres MC, Roberts SS (2013) The translocator protein (TSPO) ligand PK11195 induces apoptosis and cell cycle arrest and sensitizes to chemotherapy treatment in pre- and post-relapse neuroblastoma cell lines. Cancer Biol Ther 14:319–326. https://doi.org/10.4161/cbt.23613
Bai X, Yang C, Jiao L, Diao H, Meng Z, Wang L, Cui H, Sun L, Zhang Y, Yang B (2021) LncRNA MIAT impairs cardiac contractile function by acting on mitochondrial translocator protein TSPO in a mouse model of myocardial infarction. Signal Transduct Target Ther 6:172. https://doi.org/10.1038/s41392-021-00538-y
Bujak M, Frangogiannis NG (2007) The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74:184–195. https://doi.org/10.1016/j.cardiores.2006.10.002
Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM (2005) Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem 53:1245–1256. https://doi.org/10.1369/jhc.4A6560.2005
Frangogiannis NG (2020) Cardiac fibrosis. Cardiovasc Res. https://doi.org/10.1093/cvr/cvaa324
Chuang TD, Ansari A, Yu C, Sakurai R, Harb A, Liu J, Khorram O, Rehan VK (2020) Mechanism underlying increased cardiac extracellular matrix deposition in perinatal nicotine-exposed offspring. Am J Physiol Heart Circ Physiol 319:H651–H660. https://doi.org/10.1152/ajpheart.00021.2020
Xing PC, An P, Hu GY, Wang DL, Zhou MJ (2020) LncRNA MIAT promotes inflammation and oxidative stress in sepsis-induced cardiac injury by targeting miR-330-5p/TRAF6/NF-kappaB Axis. Biochem Genet 58:783–800. https://doi.org/10.1007/s10528-020-09976-9
Mahtta D, Sudhakar D, Koneru S, Silva GV, Alam M, Virani SS, Jneid H (2020) Targeting inflammation after myocardial infarction. Curr Cardiol Rep 22:110. https://doi.org/10.1007/s11886-020-01358-2
Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ (2018) Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 186:73–87. https://doi.org/10.1016/j.pharmthera.2018.01.001
Liu W, Liu Y, Zhang Y, Zhu X, Zhang R, Guan L, Tang Q, Jiang H, Huang C, Huang H (2015) MicroRNA-150 protects against pressure overload-induced cardiac hypertrophy. J Cell Biochem 116:2166–2176. https://doi.org/10.1002/jcb.25057
Li Y, Wang J, Sun L, Zhu S (2018) LncRNA myocardial infarction-associated transcript (MIAT) contributed to cardiac hypertrophy by regulating TLR4 via miR-93. Eur J Pharmacol 818:508–517. https://doi.org/10.1016/j.ejphar.2017.11.031
Li Z, Liu Y, Guo X, Sun G, Ma Q, Dai Y, Zhu G, Sun Y (2018) Long noncoding RNA myocardial infarctionassociated transcript is associated with the microRNA1505p/P300 pathway in cardiac hypertrophy. Int J Mol Med 42:1265–1272. https://doi.org/10.3892/ijmm.2018.3700
Wei JQ, Shehadeh LA, Mitrani JM, Pessanha M, Slepak TI, Webster KA, Bishopric NH (2008) Quantitative control of adaptive cardiac hypertrophy by acetyltransferase p300. Circulation 118:934–946. https://doi.org/10.1161/CIRCULATIONAHA.107.760488
Zeng Z, Pan Y, Wu W, Li L, Wu Z, Zhang Y, Deng B, Wei S, Zhang W, Lin F, Song Y (2019) Myocardial hypertrophy is improved with berberine treatment via long non-coding RNA MIAT-mediated autophagy. J Pharm Pharmacol 71:1822–1831. https://doi.org/10.1111/jphp.13170
Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, Brown SM (2018) Septic cardiomyopathy. Crit Care Med 46:625–634. https://doi.org/10.1097/CCM.0000000000002851
Brieler J, Breeden MA, Tucker J (2017) Cardiomyopathy: an overview. Am Fam Physician 96:640–646
Bugger H, Abel ED (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57:660–671. https://doi.org/10.1007/s00125-014-3171-6
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12:144–153. https://doi.org/10.1038/nrendo.2015.216
Bern C (2015) Chagas’ disease. N Engl J Med 373:456–466. https://doi.org/10.1056/NEJMra1410150
Perez-Molina JA, Molina I (2018) Chagas disease. Lancet 391:82–94. https://doi.org/10.1016/S0140-6736(17)31612-4
Frade AF, Laugier L, Ferreira LR, Baron MA, Benvenuti LA, Teixeira PC, Navarro IC, Cabantous S, Ferreira FM, da Silva CD, Gaiotto FA, Bacal F, Pomerantzeff P, Santos RH, Kalil J, Cunha-Neto E, Chevillard C (2016) Myocardial infarction-associated transcript, a long noncoding RNA, is overexpressed during dilated cardiomyopathy due to chronic Chagas disease. J Infect Dis 214:161–165. https://doi.org/10.1093/infdis/jiw095
Cimolai MC, Alvarez S, Bode C, Bugger H (2015) Mitochondrial mechanisms in septic cardiomyopathy. Int J Mol Sci 16:17763–17778. https://doi.org/10.3390/ijms160817763
Ehrman RR, Sullivan AN, Favot MJ, Sherwin RL, Reynolds CA, Abidov A, Levy PD (2018) Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit Care 22:112. https://doi.org/10.1186/s13054-018-2043-8
Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456. https://doi.org/10.1152/physrev.00014.2006
Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O (2006) Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation 114:2434–2442. https://doi.org/10.1161/CIRCULATIONAHA.106.633735
Lu Y, Zhang Y, Wang N, Pan Z, Gao X, Zhang F, Zhang Y, Shan H, Luo X, Bai Y, Sun L, Song W, Xu C, Wang Z, Yang B (2010) MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation 122:2378–2387. https://doi.org/10.1161/CIRCULATIONAHA.110.958967
Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, Li Y, Gao X, Dong D, Zhang Y, Bai Y, Ai J, Sun L, Lu H, Luo XY, Wang Z, Lu Y, Yang B, Nattel S (2013) MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest 123:1939–1951. https://doi.org/10.1172/JCI62185
Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D, Schoendube F, Hanrath P, Allessie MA (2001) Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 103:691–698. https://doi.org/10.1161/01.cir.103.5.691
Workman AJ, Kane KA, Rankin AC (2001) The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res 52:226–235. https://doi.org/10.1016/s0008-6363(01)00380-7
Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S (1997) Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 81:512–525. https://doi.org/10.1161/01.res.81.4.512
Zhang H, Garratt CJ, Zhu J, Holden AV (2005) Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovasc Res 66:493–502. https://doi.org/10.1016/j.cardiores.2005.01.020
Wang Z, Lu Y, Yang B (2011) MicroRNAs and atrial fibrillation: new fundamentals. Cardiovasc Res 89:710–721. https://doi.org/10.1093/cvr/cvq350
Wang Z, Yue L, White M, Pelletier G, Nattel S (1998) Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation 98:2422–2428. https://doi.org/10.1161/01.cir.98.22.2422
Burstein B, Nattel S (2008) Atrial structural remodeling as an antiarrhythmic target. J Cardiovasc Pharmacol 52:4–10. https://doi.org/10.1097/FJC.0b013e3181668057
Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E (2015) Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol 66:943–959. https://doi.org/10.1016/j.jacc.2015.06.1313
Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM (2017) Pathophysiology of paroxysmal and persistent atrial fibrillation: rotors, foci and fibrosis. Heart Lung Circ 26:887–893. https://doi.org/10.1016/j.hlc.2017.05.119
Yao L, Zhou B, You L, Hu H, Xie R (2020) LncRNA MIAT/miR-133a-3p axis regulates atrial fibrillation and atrial fibrillation-induced myocardial fibrosis. Mol Biol Rep 47:2605–2617. https://doi.org/10.1007/s11033-020-05347-0
Zhang LS JY, Liu YY, Yu YH, Sun X, Tian H, Xu J, Yue E, Lv Y, Dong CR, Wang XY, Liu GQ, Zhang DY, Wang ZG, Bai YL, Yang BF (2020) Long non-coding RNA MIAT governs atrial fibrillation via dual mechanisms as a competitive endogenous RNA and an antisense RNA. Basic Res Cardiol (unpublished data)
Jayprokas CSM (2017) Cancer and noncoding RNAs: antisense RNA and cancer. Elsevier Publisher, Amsterdam
Garcia-Padilla C, Dominguez JN, Aranega AE, Franco D (2019) Differential chamber-specific expression and regulation of long non-coding RNAs during cardiac development. Biochim Biophys Acta Gene Regul Mech 1862:194435. https://doi.org/10.1016/j.bbagrm.2019.194435
Farsangi SJ, Rostamzadeh F, Sheikholeslami M, Jafari E, Karimzadeh M (2020) Modulation of the expression of long non-coding RNAs H19, GAS5, and MIAT by endurance exercise in the hearts of rats with myocardial infarction. Cardiovasc Toxicol. https://doi.org/10.1007/s12012-020-09607-0
Jae N, Dimmeler S (2015) Long noncoding RNAs in diabetic retinopathy. Circ Res 116:1104–1106. https://doi.org/10.1161/CIRCRESAHA.115.306051
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146:353–358. https://doi.org/10.1016/j.cell.2011.07.014
Bosson AD, Zamudio JR, Sharp PA (2014) Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol Cell 56:347–359. https://doi.org/10.1016/j.molcel.2014.09.018
Broderick JA, Zamore PD (2014) Competitive endogenous RNAs cannot alter microRNA function in vivo. Mol Cell 54:711–713. https://doi.org/10.1016/j.molcel.2014.05.023
Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M (2014) Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell 54:766–776. https://doi.org/10.1016/j.molcel.2014.03.045
Jens M, Rajewsky N (2015) Competition between target sites of regulators shapes post-transcriptional gene regulation. Nat Rev Genet 16:113–126. https://doi.org/10.1038/nrg3853
Thomson DW, Dinger ME (2016) Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17:272–283. https://doi.org/10.1038/nrg.2016.20
Cai B, Ma W, Ding F, Zhang L, Huang Q, Wang X, Hua B, Xu J, Li J, Bi C, Guo S, Yang F, Han Z, Li Y, Yan G, Yu Y, Bao Z, Yu M, Li F, Tian Y, Pan Z, Yang B (2018) The long noncoding RNA CAREL controls cardiac regeneration. J Am Coll Cardiol 72:534–550. https://doi.org/10.1016/j.jacc.2018.04.085
Zhang Y, Jiao L, Sun L, Li Y, Gao Y, Xu C, Shao Y, Li M, Li C, Lu Y, Pan Z, Xuan L, Zhang Y, Li Q, Yang R, Zhuang Y, Zhang Y, Yang B (2018) LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca(2+) overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res 122:1354–1368. https://doi.org/10.1161/CIRCRESAHA.117.312117
Delihas N, Rokita SE, Zheng P (1997) Natural antisense RNA/target RNA interactions: possible models for antisense oligonucleotide drug design. Nat Biotechnol 15:751–753. https://doi.org/10.1038/nbt0897-751
Dolnick BJ (1997) Naturally occurring antisense RNA. Pharmacol Ther 75:179–184. https://doi.org/10.1016/s0163-7258(97)00050-8
Knowling S, Morris KV (2011) Non-coding RNA and antisense RNA. Nature’s trash or treasure? Biochimie 93:1922–1927. https://doi.org/10.1016/j.biochi.2011.07.031
Cai B, Ma W, Wang X, Sukhareva N, Hua B, Zhang L, Xu J, Li X, Li S, Liu S, Yu M, Xu Y, Song R, Xu B, Yang F, Han Z, Ding F, Huang Q, Yu Y, Zhao Y, Wang J, Bamba D, Zagidullin N, Li F, Tian Y, Pan Z, Yang B (2020) Targeting LncDACH1 promotes cardiac repair and regeneration after myocardium infarction. Cell Death Differ 27:2158–2175. https://doi.org/10.1038/s41418-020-0492-5
Acknowledgements
The authors greatly thank Prof. Zhiguo Wang for continued support and valuable advice of this manuscript.
Funding
This work was supported by the Funds for National Key Research and Development Program of China (2017YFC1307403 to Baofeng Yang, 2017YFC1702003 to Yong Zhang); the Key Program of National Natural Science Foundation of China (81730012 to Yong Zhang); the National Natural Science Foundation of China (81861128022, 81570301, 81570399 to Yong Zhang); and Qiqihar Academy of Medical Sciences Project (QMSI2020B-04 to Chao Yang).
Author information
Authors and Affiliations
Contributions
BY conceived idea of the article. CY performed the literature search and wrote the manuscript. YZ critically revised the work. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Zhang, Y. & Yang, B. MIAT, a potent CVD-promoting lncRNA. Cell. Mol. Life Sci. 79, 43 (2022). https://doi.org/10.1007/s00018-021-04046-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-021-04046-8